
Commentary: 2-year follow up of pembrolizumab as second-line therapy for advanced urothelial cancer (“KEYNOTE 045”)
Introduction
The publication in 2017 of the KEYNOTE 045 prospective randomized phase 3 trial comparing pembrolizumab anti PD1 antibody with conventional cytotoxic chemotherapy in treatment of recurrent urothelial cancer (1) marked a watershed for immune treatment to displace conventional cytotoxic medications. The latter had dominated first- and second-line treatment planning for urothelial advanced urothelial cancers for decades, dating to the seminal publication on single-agent cisplatin induced regressions by Yagoda and colleagues in 1976 (2). Now with over two years further follow up on the KEYNOTE 045 pivotal trial, presented by Fradet and colleagues (3) several key attributes of the regimen were confirmed.
Updated statistics and response observations
The update shows stability of the estimates in the hazard ratio of the primary endpoint overall survival (OS), which was better for pembrolizumab and the progression free survival (PFS), which was not better, overall. A key observation for the newer report (3) is the now more mature later-time point PFS and OS, for which the tails of the curves are defined much more plainly. The PFS does look better considering only those at the time points past 8 months. At 12 months, the PFS is 18.2% vs. 9.9% and OS 44.2% vs. 29.8%, favoring pembrolizumab. At 24 months, the better PFS (12.4% vs. 3.0%) and better OS (26.9% vs. 14.3%), with the majority (60%) of the latter having received crossover checkpoint inhibitor therapy; the use of crossover PD-L1 in the rest of the chemotherapy treatment population was not reported. This pattern with OS differences much larger than PFS demonstrates, as has been seen in other cancer immunotherapy trials, there is a part of the population getting a survival benefit more than just those with an obviously improved PFS. Table 1 compares several of these summary statistics. The patterns of the observed side effect frequencies also did not change. Additionally, as described in the earlier publication, the time-to-response was consistently about 2 months, with occasionally later responders (either for immunotherapy or for chemotherapy) being the exception. Perhaps the most clinically significant update of the longer-term report is now directly observable duration of response, with the immune therapy’s median not reached [visually in excess of 20 months on the graph (3), range 1.8+ to 30+] and the chemotherapy median 4.4 months (range 1.4+ to 29.9+ months).
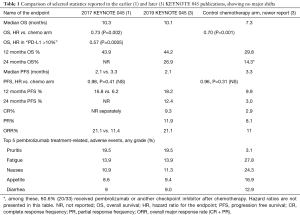
Full table
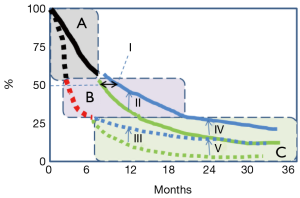
A stylized diagram of OS and PFS curves [from (3)] is shown in Figure 1. The three segments indicate a retrospective segmentation into three key groups. About half the treatment population did not have a significant PFS or OS difference (segment A), about a quarter had more OS difference, but still little PFS difference (segment B), and the remainder (segment C) have both an OS improvement and better PFS at the milestones indicated. Clearly, there has been some OS impact of pembrolizumab in some for whom an early progression was seen, despite that early progression (most obvious in segment B, but may include some of the segment C group), but conversely the part of the population not getting that benefit, or only getting a few months reprieve (segments A and B) is the majority.
This KEYNOTE 045 trial may be contrasted with the experience of the IMvigor 211 randomized phase III trial of atezolizumab (PD-L1) antibody versus chemotherapy (4), in which the PD-L1 high (IC 2/3) prespecified population had OS which was not different from that of the chemotherapy control arm medians of 11.1 vs. 10.6 months, stratified hazard ratio 0.87 (95% CI: 0.63–1.21; P=0.41). In that same prespecified subgroup, the major response frequencies were similar (23% and 22%), although the duration of the immune therapy response [15.9 (95% CI: 10.4 to not estimated) versus 8.3 (5.6–13.2) months] was a pattern that aligns with the pembrolizumab trial reports, but apparently less of a difference. This reminds one of the continued fundamental importance of empiric testing for apparently similar medications in the same populations. The use of a stratified response assessment technique [used in both KEYNOTE 045 and IMvigor 211 (1,3,4)], with the biomarker-defined subset of the population having the initial statistical evaluation is a way to maintain control of the otherwise untenably simultaneous questions of overall efficacy and biomarker relevance.
Continued efforts for response prediction
As illustrated with the separate segments in Figure 1, different parts of the advanced urothelial cancer population get markedly different levels of benefit from this type of treatment. A central question has been how to predict that chance of response. The favorable OS hazard ratio high PD-L1 subset [0.57 (not updated)] was lower than for the total population of KEYNOTE045 (0.73 and 0.70) The IMvigor 210 (phase 2) trial of atezolizumab and bevacizumab in advanced urothelial cancer did show some relationship (5), with a trend to a higher response rate in the “PD-L1 5% or more” subjects ORR =28.1% (95% CI: 13.8–46.8), a little higher than that observed in the lower expression subset, 21.8% (95% CI: 13.7–32.0), but the phase III trial (4) (above) did not bear this out as a definite improvement, with the 23% ORR rate in the atezolizumab arm, IC 2/3 group.
Beyond this technical comparison of response parameters of these three reports of two phase III trials, from which we conclude that the trajectory of that earlier KEYNOTE 045 data cut was relatively stable, the updated KEYNOTE 045 trial results are a basis to take a look again at a few other contemporary issues in medical therapy of urothelial cancer. First is the aggregate experience across five PD-1 and PD-L1 antibody drugs which have US FDA indications for treatment of urothelial cancer, all focused on the same immune pathway as the mechanism. At present, pembrolizumab is among five PD-1 and PD-L1 antibody medications with indications for use in urothelial cancer, either as initial therapy for platinum-ineligible patients or as a post-chemotherapy regimen. Both the comparison of the earlier and later KEYNOTE publications (1,3) and the other trials [cited by (6-8)] of PD-1 and PD-L1 trials with similar medications in similar patient populations together form a relatively consistent base of experience. While the KEYNOTE 045 study is distinctive for having a prospective direct comparison with second line chemotherapy, Rui et al. (7) the observed major response frequency, considering pembrolizumab, nivolumab, atezolizumab, avelumab and durvalumab trials that are dominated by single-arm experiences was computed to have an aggregate estimate of ORR found about 16.9% in first- and later-line urothelial cancer patients. Some predictive value for PD-L1 in (only) the durvalumab treatment series (7).
Lavoie et al. (6) reported on a systematic review of predictive biomarkers, across many studies, including the KEYNOTE 045 data set. A top question was the relevance of PD-L1 in tumor as a predictive biomarker. Among several issues, there is not a standardized antibody or positivity threshold denoting a markedly higher (predictive) chance of response (6). Along the same lines heterogeneity within a single patient of results from analyses of biopsies from different specimens may also impair prediction: Which part of the tumor burden (if any) should impact the treatment decision? (5). In the related question of first-line therapy of cisplatin-ineligible patients (not the KEYNOTE 045 population) early-look review of ongoing randomized phase III trials led to regulatory agency recommendations to not use pembrolizumab nor atezolizumab (which have indications as initial therapy of advanced urothelial cancer) if PD-L1 testing was below threshold. Validation of this specific restriction strategy in the context of the completed trials will certainly be of interest (6).
Taking a more broad view of competence for anticancer response, mRNA signatures of the type used to define TCGA clusters (9), Lavoie and colleagues were more optimistic, but careful to allow that the signatures may be influenced by prior treatment exposures. Another approach focusing on CD8+ T cell infiltration, versus exclusion defines a direct way to make plastic the chance of clinical response with adjunctive medications through the surrogate endpoint of increasing infiltration. The tumor mutational burden (TMB), abstracted from the host immune response was analyzed in the IMvigor 210 and IMvigor 211 cohorts (4,10), with the finding now that the earlier data set (IMvigor 210 phase II) did not have a differential chance of response with high TMB, although the phase III trial did.
Overall, although neither fast nor facile to this point, progress to a functional biomarker may occur through these many research efforts (7). The clinical and economic implications of carving out the fraction for whom the PD-1/PD-L1 strategy has no OS, PFS or response advantage (those destined to have responses in “segment A” of the figure), would be substantial, as well as helpful to focus appropriate development of investigational drugs with other anticancer mechanism. Hussain et al. were more direct: the need for a predictive biomarker is “perhaps the most significant unmet need” in urothelial cancer immune treatment (8).
Shifting contexts for non-immune options
Another change in the two years interval between the two PD-L1 publications has been progress in development of at least three urothelial cancer therapies with very specific biomarker requirements. Erdafitinib was tested clinically in 99 pretreated urothelial cancer patients with specific FGFR2 and FGFR3 mutations or fusions, and a 40% major response rate was a basis for its April 2019 US FDA approval (11). The frequency of the specified abnormalities in the general population is low. Notably, erdafitinib-related mutations may also have lower PD-L1 expression; the clinical implications for sequencing or combination remain to be tested empirically.
Two antibody-drug conjugates with promising early trial progress include enfortumab vedotin and sacituzumab govitecan. The enfortumab target antigen is nectin-4, very widely expressed in urothelial cancer, the payload is an antimicrotubule compound. The report observed a 44% overall response rate among 125 patients in the single arm EV-201 phase II trial, and the median duration of response was 7.6 months (12). The target of sacituzumab is the trophoblastic cell surface antigenTrop2, and they payload is a camptothecin (SN-38) inhibitor of topoisomerase 1. The clinical development in triple negative breast cancer (13) is ahead of the urothelial cancer application is in active development, with response reported in 31% of 45 subjects reported at 2019 GU-ASCO (14,15).
Turning from advanced, post-cisplatin treatment as was studied in the KEYNOTE 045 population, another response-prediction dimension is selection of subpopulations for whom chemotherapy may have a particularly higher response rate. Several of these markers are summarized by Tse and colleagues, including a “p-53 like” phenotype—which does not respond to chemotherapy, and mutation of the DNA excision repair protein ERCC1, for which there is a higher response rate to cisplatin treatment (16). The economic savings, and the ability to use markers such as these to focus those likely chemotherapy-refractory patients onto other treatments, would be beneficial to further immune therapy development as well.
Summary
Overall, at this point the longer term, two year term follow up for the KEYNOTE 045 phase III trial comparing pembrolizumab to second line chemotherapy (3) confirms and defines the several key issues in advanced urothelial cancers: On the positive side, the major responses appear at consistent frequency, durability of response for that group who have the lion’s share of the benefit is of obvious clinical significance. On the other hand, the majority of the patients had progression observed at the first evaluation point, typically about 2–3 months, whether on immune therapy or chemotherapy, and the difficulty of defining a predictive marker for PD-1/PD-L1 response, the unmet needs still loom.
The broader category of immune stimulation, and of breaking intratumoral immune resistance pathways whether coordinated with PD-1/PD-L1 pathway treatment or independent of that is the subject of hundreds of research efforts across oncology, and optimistically one can expect that benefits will come to this diagnosis as part of those efforts. Encouraging progress with drugs targeting other proteins and pathways, and with chemotherapy response prediction may be other ways to benefit those patients who had not had impacts from with PD-1 treatments such as pembrolizumab. While the PD-1 breakthrough has been phenomenal, there is much room to grow for advanced urothelial cancer treatment.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has participated in trials sponsored by the manufacturers of pembrolizumab (Merck), nivolumab (BMS), atezolizumab (Roche), durvalumab (AstraZeneca), avelumab (EMD Serono), enfortumab vedotin (Seattle Genetics) lenvatinib (Eisai) and everolimus (Novartis); promotional programs sponsored through EMD Serono, Pfizer, Exelixis. Advisory boards relating to avelumab (Pfizer and EMD Serono), pembrolizumab (Eisai/Merck), enfortumab vedotin (Astellas/SeattleGenetics).
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bellmunt J, de Wit R, Vaughn DJ, et al. KEYNOTE-045 Investigators. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]
- Yagoda A, Watson RC, Gonzalez-Vitale JC, et al. Cis-dichlorodiammineplatinum(II) in advanced bladder cancer. Cancer Treat Rep 1976;60:917-23. [PubMed]
- Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748-57. [Crossref] [PubMed]
- Burgess EF, Livasy C, Hartman A, et al. Discordance of high PD-L1 expression in primary and metastatic urothelial carcinoma lesions. Urol Oncol 2019;37:299.e19-299.e25. [Crossref] [PubMed]
- Lavoie JM, Black PC, Eigl BJ. Predictive Biomarkers for Checkpoint Blockade in Urothelial Cancer: A Systematic Review. J Urol 2019;202:49-56. [Crossref] [PubMed]
- Rui X, Gu TT, Pan HF, Zhang HZ. Evaluation of PD-L1 biomarker for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatments for urothelial carcinoma patients: A meta-analysis. Int Immunopharmacol 2019;67:378-85. [Crossref] [PubMed]
- Hussain SA, Birtle A, Crabb S, et al. From Clinical Trials to Real-life Clinical Practice: The Role of Immunotherapy with PD-1/PD-L1 Inhibitors in Advanced Urothelial Carcinoma. Eur Urol Oncol 2018;1:486-500. [Crossref] [PubMed]
- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017;171:540-556.e25. [Crossref] [PubMed]
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67-76. [Crossref] [PubMed]
- Loriot Y, Necchi A, Park SH, et al. BLC2001 Study Group. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2019;381:338-48. [Crossref] [PubMed]
- Rosenberg JE, O'Donnell PH, Balar AV, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2019;37:2592-600. [Crossref] [PubMed]
- Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med 2019;380:741-51. [Crossref] [PubMed]
- Vlachostergios PJ, Jakubowski CD, Niaz MJ, et al. Antibody-Drug Conjugates in Bladder Cancer. Bladder Cancer 2018;4:247-59. [Crossref] [PubMed]
- Tagawa ST, Faltas BM, Lam ET, et al. Sacituzumab govitecan (IMMU-132) in patients with previously treated metastatic urothelial cancer (mUC): Results from a phase I/II study. J Clin Oncol 2019;37:abstr 354.
- Tse J, Ghandour R, Singla N, et al. Molecular Predictors of Complete Response Following Neoadjuvant Chemotherapy in Urothelial Carcinoma of the Bladder and Upper Tracts. Int J Mol Sci 2019. [Crossref] [PubMed]


