
Systemic therapy for primary and extragonadal germ cell tumors: prognosis and nuances of treatment
Introduction
Although uncommon in the overall population, testicular germ cell tumors are the most common solid tumors in young men between the ages of 20 and 34, with an estimated 9,310 cases diagnosed in the United States in 2018, with approximately 400 deaths (1). In general, testicular germ cell tumors carry an excellent prognosis, with a 5-year survival rate of approximately 95% (1). An increasing incidence of testicular cancers has been observed, for uncertain reasons (2-5). Testicular germ cell tumors account for the vast majority of malignant tumors arising in the testes; these tumors also occasionally arise in extragonadal sites, such as the retroperitoneum and anterior mediastinum (6,7). Risk factors for testicular cancer include cryptorchidism, family history, and prior history of testicular cancer (8,9).
Testicular germ cell tumor is classically divided into two major subtypes: pure seminoma and non-seminoma (10). Non-seminomas are comprised of four major tumor types: embryonal carcinoma, choriocarcinoma, yolk sac tumor, and teratoma (10). Non-seminomas are typically more clinically aggressive, and the presence of any of the four histologic subtypes will define a tumor as a non-seminoma. A well-established standard of care exists for the majority of testicular germ cell tumors, leading to a high overall survival rate. This review seeks to provide an overview of the current standard of care for systemic therapy of testicular germ cell tumors, including nuances of treatment and briefly explores current areas of active research and future directions for therapy.
Staging and risk classification
The American Joint Committee on Cancer (AJCC) staging system for testicular germ cell tumors, most recently in its eighth edition, incorporates tumor (T), node (N), and metastatic (M) status of the tumor as well as post-orchiectomy serum tumor markers (S), which are unique to this tumor type (11). In addition to incorporating tumor markers into staging, testicular germ cell tumor staging is unique insofar as it lacks a “stage IV” designation—tumors are staged from stage 0 to stage III (11) (Table 1, AJCC Staging System). The serum tumor markers of interest in this disease include alpha fetoprotein (AFP), beta-human chorionic gonadotropin (beta-hCG), and lactate dehydrogenase (LDH) (12). These tumor markers are obtained post-orchiectomy, on the first day of the first cycle of chemotherapy, and during therapy for assessment of response.
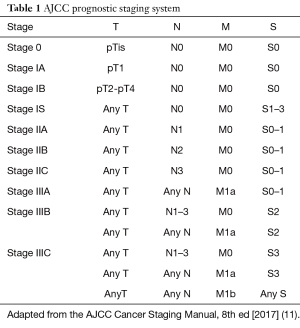
Full table
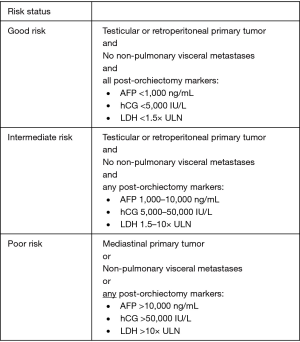
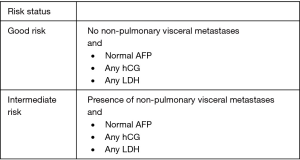
Risk classification criteria were standardized by the International Germ Cell Cancer Consensus Group (IGCCCG) in 1997 on the basis of post-orchiectomy tumor markers and extent of measurable disease (13). Non-seminomas are stratified into good-, intermediate-, and poor-risk status on the basis of location of the primary tumor, presence of non-pulmonary visceral metastases, and levels of post-orchiectomy markers (13) (Figure 1, IGCCCG Risk Stratification for Non-seminoma) (13). Seminomas are stratified solely into good- or intermediate-risk on the basis of non-pulmonary visceral metastases, reflecting the overall favorable prognosis of this disease entity. Tumor markers are not used to assign prognosis in pure seminoma (Figure 2, IGCCCG Risk Stratification for Seminoma) (13).
Pure seminoma
Pure seminoma is staged and risk stratified according to the criteria outlined above. Notably, AFP is not associated with pure seminoma—elevated AFP signifies non-seminoma unless an alternative explanation is present, e.g., liver disease. Tumor marker staging for pure seminoma is on the basis of beta-hCG and LDH. Treatment decisions are typically not made on the basis of an isolated LDH level alone. Stage I seminoma carries an excellent prognosis, with a disease-free survival of 99% with treatment (14). Management options for stage I pure seminoma include active surveillance, chemotherapy with single agent carboplatin, and radiation therapy.
While most patients with stage I pure seminoma are cured with orchiectomy alone, it is estimated that 15% to 20% of patients relapse after initial orchiectomy, which has been demonstrated in prospective trials of surveillance (15-18). In a retrospective analysis of 2,483 patients with stage I germ cell tumors undergoing active surveillance, a relapse rate of 13% was identified among patients with stage I pure seminoma, with a median time to relapse of 14 months, and, as noted above, a 5-year disease-free survival of 99% (19,20). For patients who are unable to or prefer not to pursue a schedule of active surveillance, options include adjuvant carboplatin or radiation therapy.
The initial results of a randomized trial comparing adjuvant carboplatin to radiation therapy for stage I pure seminoma were reported by Oliver et al. in 2005, in which 1,477 patients were randomized to receive either a single cycle of carboplatin (n=560) or radiation therapy (n=885) (21). At a follow-up time point of three years, similar relapse-free survival rates were observed (94.8% in the carboplatin arm versus 95.9% in the radiation therapy arm) (21). The final results of this trial were reported in 2011, and demonstrated a similar 5-year relapse-free survival rate of 96% in the radiation therapy arm and 94.7% in the carboplatin arm (hazard ratio =1.25, P=0.37) (22).
Trials have also explored the use of two cycles of adjuvant chemotherapy. In the 2nd and 3rd Spanish Germ Cell Cancer Cooperative Group Studies, two cycles of adjuvant carboplatin were shown to reduce the rate of relapse for high risk (defined as tumors >4 cm and invasion of the rete testis) stage I seminoma, yielding a 5-year relapse-free survival rate of 96.2% (16,23). The overall survival at five years in these trials was 100% (16,23). Similarly, in the Hellenic Cooperative Oncology Group trial of 138 stage I seminoma patients, two cycles of adjuvant carboplatin demonstrated a 5-year relapse-free survival rate of 96.8% (24). In a head-to-head comparison of surveillance, one cycle of carboplatin, and two cycles of carboplatin in 725 stage I seminoma patients, the relapse rate with one cycle of carboplatin was observed to be 5% versus 1.5% with two cycles of carboplatin at a median follow-up of thirty months (25). The relapse rate was 8.2% with surveillance. Adjuvant radiation therapy is also an acceptable approach for stage I seminoma, albeit with an associated increase risk in secondary malignancies (26,27). A detailed discussion of radiation therapy is beyond the scope of this review. Stage Is is an uncommon form of pure seminoma, in which persistent elevation of tumor markers is observed following orchiectomy. Systemic treatment is not indicated for elevated markers alone, until there is clinical evidence of metastatic disease.
Stage II disease is defined by the presence of lymph node involvement. Clinical N1 (cN1) disease is defined as metastasis with a lymph node mass 2 cm or smaller in the greatest dimension or multiple lymph nodes, with none larger than 2 cm in the greatest dimension. Clinical N2 (cN2) disease includes metastasis with a lymph node mass larger than 2 cm but not larger than 5 cm in the greatest dimension or multiple lymph nodes, with any one mass larger than 2 cm but not larger than 5 cm. Clinical N3 (cN3) disease is defined as metastasis with lymph node mass greater than 5 cm in the largest dimension. On this basis, stage IIA encompasses cN1 disease, while cN2 defines stage IIB and cN3 defines stage IIC. Treatment options for stage IIA and IIB seminoma includes adjuvant radiation therapy or chemotherapy. Standard chemotherapy regimens include bleomycin, etoposide, and cisplatin (BEP) for three cycles or etoposide and cisplatin (EP) for four cycles. Mixed evidence has been observed in comparison with radiation therapy (28-30). In one retrospective study of 1,772 stage II seminoma patients following orchiectomy, 5-year overall survival was higher with radiation therapy compared to chemotherapy for stage IIA patients, however no difference was observed in stage IIB patients (31). Similarly, in a retrospective study of 1,885 stage II seminoma patients receiving either adjuvant chemotherapy or radiation therapy, overall survival was improved with radiation therapy in stage IIA, but not in stage IIB patients (32). These studies are limited by their retrospective and non-randomized nature, however in general practice either chemotherapy or radiation therapy are acceptable for stage II seminoma, with chemotherapy preferred for stage IIB disease.
For stage IIC or stage III seminoma, disease is stratified into good or intermediate risk on the basis of non-pulmonary visceral metastasis. For good risk disease, standard chemotherapy options, as above, include three cycles of BEP or four cycles of EP. Primary mediastinal seminoma is treated by risk status used for gonadal seminoma with three cycles of BEP or four cycles of EP. Equivalency of three cycles versus four cycles of BEP was established by de Wit et al. in a 2001 randomized 2×2 factorial clinical trial, in which patients were randomized to three versus four cycles of BEP in a 5-day or 3-day cycle (33). The trial demonstrated a difference in progression free survival of −1.0%, which met the pre-specified limit for equivalence of 5% (33). Four cycles of EP were established as an acceptable treatment regimen in a 2005, single arm clinical trial of 289 patients with good risk metastatic germ cell tumor, in which a relapse rate of 6% was observed, and 3% death at a median follow-up of 7.7 years (34). Of note, this trial included both pure seminoma and non-seminomatous germ cell tumors. In a retrospective analysis of 223 patients with good risk disease treated between 1985 and 2011 with four cycles of EP versus three cycles of BEP, the 10-year overall survival rate was 91% vs. 98% respectively (P<0.01) (35). The adjusted risk of death, however, did not reach statistical significance (35). Although these two regimens (four cycles of EP versus three cycles of BEP) are commonly held to be equivalent, there is active debate regarding the exclusion of bleomycin, which is explored below.
For intermediate risk disease, treatment options include four cycles of BEP or four cycles of etoposide, ifosfamide (with mesna), and cisplatin (VIP) (36-38). The VIP regimen was studied in the GETUG S99 study, in which 132 patients were included; good risk patients received EP for four cycles, which intermediate risk patients were treated with four cycles of VIP (39). For the 24 patients with intermediate risk disease, a 3-year progression free survival rate of 83% was observed, with a 3-year overall survival rate of 87% (39). As noted above, seminoma does not include a poor risk category.
Non-seminoma
As described above, the category of non-seminoma tumors includes all non-seminomatous tumors, mixed seminoma and non-seminoma tumors, and histologic seminoma tumors with elevated AFP. For stage I non-seminoma, treatment options following orchiectomy include surveillance, surgery with nerve-sparing retroperitoneal lymph node dissection (RPLND), or chemotherapy with one cycle of BEP. With regard to surveillance, an estimated 70–75% of patients with stage I non-seminomatous germ cell tumor is cured by orchiectomy alone, with an estimated death rate of less than three percent with appropriate surveillance (40,41). The comparison of one cycle of BEP to unilateral RPLND for stage I non-seminoma was studied in a randomized trial of 382 patients, which, after a median follow-up of 4.7 years, identified two relapses in the BEP arm, compared to 13 in the surgery arm (P=0.0011) (42). Notably, the choice of unilateral RPLND rather than bilateral nerve sparing RPLND has led to criticism of this study. In the SWENOTECA trial, 745 patients with stage I non-seminoma were stratified to adjuvant BEP versus surveillance based on the presence of lymphovascular tumor invasion (43). A relapse rate of 3.2% was observed in the presence of lymphovascular invasion, versus 1.6% without, at 5 years (43). In the final analysis of the SWENOTECA trial, the 5-year overall survival rate was 100% (44). Other studies have examined the use of two cycles of BEP, with high rates of relapse-free survival exceeding 95%, at the risk of higher rates of toxicity (45-49).
Following RPLND, management options are driven by nodal status. Patient with pN0 disease are managed by surveillance. For pN1 disease, while surveillance is the preferred option, chemotherapy with two cycles of BEP or EP is an acceptable option. For pN2 disease, chemotherapy with two cycles of BEP or EP is preferred, however, surveillance may be considered. For pN3 disease, chemotherapy with three cycles of BEP or four cycles of EP represents the favored treatment approach. In a case series of 40 stage I non-seminoma patients treated with two cycles of BEP all patients were alive at a median follow-up of 113.2 months with the exception of one incidental death, and no relapses were observed, except for one patient with a tumor in the contralateral testicle (49,50). For stage IS patients, the tumors are treated as good risk tumors, with three cycles of BEP or four cycles of EP, as above (51,52). For stage II non-seminoma, management strategies are similar to above, including RPLND, or chemotherapy with three cycles of BEP or four cycles of EP (53-57). In the case of RPLND, for patients with pN2 or pN3 disease, a relapse rate >50% after RPLND has been observed (56,57). The risk of relapse may be reduced to <1% with the addition of adjuvant chemotherapy with EP or BEP (58,59).
In metastatic non-seminoma, treatment is driven by risk stratification as outlined above in Figure 1. Non-seminomas is divided into good-, intermediate-, and poor-risk on the basis of post-orchiectomy tumor markers and sites of disease. Good risk disease is typically treated with three cycles of BEP or four cycles of EP, on the basis of the de Wit trial described above (33,35). For both regimens, a cure rate of approximately 90% has been observed (60,61). As noted above, the therapeutic equivalency of four cycles of EP versus three cycles of BEP has been actively debated and is described in further detail below.
For intermediate-risk non-seminoma, in one head-to-head comparison of four cycles of BEP and four cycles of VIP was studied in a randomized clinical trial of 84 patients (62). No difference was observed in relapse rate or overall survival at a median follow-up of 7.7 years, with a 5-year progression free survival of 85% in the VIP arm and 83% in the BEP arm (HR=0.83) (62). This trial was stopped early. In a randomized trial of 304 patients with advanced germ cell tumors, a similar 2-year overall survival was observed for both the VIP and the BEP arms (63). In both trials, significantly greater hematologic toxicity was observed in the VIP arm. Given the toxicity and efficacy profile of these two regimens, a BEP regimen is typically preferred. Similarly, four cycles of BEP or VIP are acceptable for poor-risk non-seminoma, with a preference for BEP. For these patients, fewer than 50% will achieve long-lasting complete response (61).
In the setting of relapsed disease or incomplete response to initial therapy, options include conventional chemotherapy or high dose chemotherapy with autologous stem cell rescue. Surgical resection of residual masses is recommended. The VeIP regimen (vinblastine, ifosfamide, cisplatin) was studied in a single arm clinical trial of 135 patients with progressive, disseminated germ cell tumors following treatment with etoposide and platinum-based chemotherapy (64). Among these patients, 49.6% achieved complete remission (64). The TIP regimen (paclitaxel, ifosfamide, cisplatin) has also been studied as a salvage regimen. In a series of 14 patients treated with TIP salvage therapy, five showed a complete or partial response, and with a median follow-up of 41.0 months, the median overall survival was 21.1 months (65). In a study of forty-three patients including both favorable risk and platinum-refractory germ cell tumor patients, one of six patients with cisplatin-refractory disease and five of ten patients with relapsed disease achieved durable complete response (66).
The data in support of high dose chemotherapy and autologous stem cell rescue derive from a retrospective review of 173 patients with metastatic testicular cancer that had progressed after cisplatin-containing chemotherapy, who were given two consecutive courses of high dose carboplatin and etoposide and 11 patients who received one course (67). Among the total 184 patients, 116 experienced complete remission without relapse at a median follow-up of 48 months (67). Of the 135 patients who received this treatment in the second line setting, 94 patients were disease free; in the third line setting, 22 of 49 patients were disease free at the time of follow-up (67).
For patients with relapsed disease after second-line therapy, treatment options include surgical salvage if feasible, and treatment with either conventional or high dose chemotherapy as above, if either regimen has not yet been administered. Pembrolizumab immunotherapy may also be considered in the setting of microsatellite instability or deficient mismatch repair (MSI-H/dMMR) tumors. For patients with platinum-refractory tumors, palliative regimens that have been studied include combinations of gemcitabine with paclitaxel or oxaliplatin, or oral etoposide. In a phase II study of salvage gemcitabine/oxaliplatin in 18 patients with cisplatin-refractory non-seminoma, one patient achieved complete remission, and two patients achieved partial remission, with all three cases characterized by testicular primary embryonal carcinoma (68). In a study of 35 patients who had been pretreated with platinum, an overall response rate of 46% was observed (69). the GEMOX (gemcitabine/oxaliplatin) regimen was also studied in a trial of 29 patients with cisplatin non-seminoma, with 9 patients (32%) achieving complete or partial response (70). For the gemcitabine/paclitaxel combination, in a retrospective review of 31 patients, ten achieved objective response, with six complete remissions (71,72). The combination of gemcitabine, oxaliplatin, and paclitaxel has also been studied, in a trial of 63 patients with refractory germ cell tumor (73). In this trial, an overall response rate of 44% was observed, with complete remissions seen in eight patients, and a median overall survival of 13.3 months (73). Finally, in a phase II study of oral etoposide in 21 evaluable patients, response was observed in 11 patients (74). For all patients with relapsed or refractory disease, referral to a high-volume center with experience treating these tumors as well as enrollment in clinical trial is advisable.
Areas of active investigation
The exclusion of bleomycin is an area of much debate. In the GETUG T93BP trial of 257 patients with good-risk metastatic germ cell tumors, patients were randomized to four cycles of EP versus three cycles of BEP, with equivalency defined as a maximum 10% absolute difference in favorable response (60). A similar favorable response was observed in both groups (95% for three cycles of BEP versus 97% for four cycles of EP), with a non-statistically significant difference in event-free survival (93% for BEP versus 86% for EP, P=0.052), as well as for overall survival (97% for BEP versus 93% for EP) (60). At 4 years, in the intent-to-treat analysis, overall survival was 92% in the EP arm versus 96% in the BEP arm, with no significant differences in pulmonary toxicity observed (60). In light of these differences, the choice of a 10% margin of equivalence is open to debate, and has led some groups to argue in favor of the superiority of the BEP regimen (75). In other clinical trials, a trend towards superiority with the inclusion of BEP has been observed. In a trial of 419 patients with good-risk metastatic non-seminoma, a complete response rate of 95% was observed with four cycles of BEP, compared with 87% with four cycles of EP, with four cancer-related deaths in the BEP arm compared with eight in the EP arm (76). In one study of three cycles of BEP versus three cycles of EP in 171 patients with good risk disease, an overall survival of 95% was observed in the BEP arm versus 86% in the EP arm, without significant difference in toxicity, although it must be noted that only three cycles of EP is not a recommended treatment regimen (77). By contrast, a single-center retrospective study of 944 patients treated with four cycles of EP demonstrated an overall survival outcome of 97.9% (78). Further study on the exclusion of bleomycin for good risk disease is therefore warranted.
In addition to chemotherapy selection, another key area of active investigation focuses on mechanisms of chemoresistance. Although a large majority of patients with testicular germ cell tumors are cured, a small group, as described above, develops refractory disease. Intratumoral heterogeneity has been hypothesized to contribute to the emergence of chemoresistance, and was studied in a retrospective study of 275 patients (79). In this study, cases were divided into pure embryonal carcinoma (pure E); mixed embryonal carcinoma, yolk sac tumor, and teratoma (EYT); and mixed embryonal carcinoma, yolk sac tumor, seminoma, and teratoma (EYST) (79). Patients with the EYST phenotype had the highest cancer-specific mortality rate and tended to undergo somatic transformation (79). In a similar retrospective study of 615 patients, stratification of patients into histologic subtype demonstrated that patients with a mixed yolk sac-seminoma phenotype had the poorest clinical outcome (80) (Table 2, Overall survival of non-seminomatous germ cell tumor by stage and histologic subtype). These histologic subtypes therefore have prognostic implications with regards to somatic transformation and drug resistance and may offer an avenue for patient selection and optimization of treatment choice. Novel biomarkers are also under intensive investigation, such as glypican-3 (GPC3), which has been identified in lethal, cisplatin-resistant tumors and represents potential therapeutic target (81).

Full table
Finally, in terms of active clinical studies, the ongoing TIGER trial (NCT02375204) is a randomized, phase III trial comparing conventional-dose chemotherapy using the TIP regimen with high dose chemotherapy plus autologous stem cell transplant in the relapsed, refractory setting (82). A number of novel therapeutic strategies are being investigated in germ cell tumors, including an accelerated scheduled of BEP (NCT02582697), immune checkpoint blockade with durvalumab and tremelimumab (NCT03158064), avelumab (NCT03403777), cabazitaxel (NCT02115165), and the tyrosine kinase inhibitor sorafenib (NCT00772694).
In summary, while testicular germ cell tumors represent a success story of modern medicine in our ability to cure young patients and offer decades of life, many areas of active investigation remain, particularly in the relapsed and refractory setting.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Shanmugalingam T, Soultati A, Chowdhury S, et al. Global incidence and outcome of testicular cancer. Clin Epidemiol 2013;5:417-27. [PubMed]
- Verhoeven RHA, Gondos A, Janssen-Heijnen MLG, et al. Testicular cancer in Europe and the USA: survival still rising among older patients. Ann Oncol 2013;24:508-13. [Crossref] [PubMed]
- Chia VM, Quraishi SM, Devesa SS, et al. International trends in the incidence of testicular cancer, 1973-2002. Cancer Epidemiol Biomarkers Prev 2010;19:1151-9. [Crossref] [PubMed]
- Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol 2003;170:5-11. [Crossref] [PubMed]
- Sarıcı H, Telli O, Eroğlu M. Bilateral testicular germ cell tumors. Turk J Urol 2013;39:249-52. [Crossref] [PubMed]
- Punjani N, Winquist E, Power N. Do retroperitoneal extragonadal germ cell tumours exist? Can Urol Assoc J 2015;9:381-4. [Crossref] [PubMed]
- Greene MH, Kratz CP, Mai PL, et al. Familial testicular germ cell tumors in adults: 2010 summary of genetic risk factors and clinical phenotype. Endocr Relat Cancer 2010;17:R109-21. [Crossref] [PubMed]
- Turnbull C, Rahman N. Genome-wide association studies provide new insights into the genetic basis of testicular germ-cell tumour. Int J Androl 2011;34:e86-96; discussion e96-7.
- Vasdev N, Moon A, Thorpe AC. Classification, epidemiology and therapies for testicular germ cell tumours. Int J Dev Biol 2013;57:133-9. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer International Publishing; 2017.
- Gilligan TD, Seidenfeld J, Basch EM, et al. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol 2010;28:3388-404. [Crossref] [PubMed]
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997;15:594-603. [Crossref] [PubMed]
- Mead GM, Fossa SD, Oliver RTD, et al. Randomized trials in 2466 patients with stage I seminoma: patterns of relapse and follow-up. J Natl Cancer Inst 2011;103:241-9. [Crossref] [PubMed]
- Groll RJ, Warde P, Jewett MAS. A comprehensive systematic review of testicular germ cell tumor surveillance. Crit Rev Oncol Hematol 2007;64:182-97. [Crossref] [PubMed]
- Aparicio J, Germà JR, García del Muro X, et al. Risk-adapted management for patients with clinical stage I seminoma: the Second Spanish Germ Cell Cancer Cooperative Group study. J Clin Oncol 2005;23:8717-23. [Crossref] [PubMed]
- Chung P, Parker C, Panzarella T, et al. Surveillance in stage I testicular seminoma - risk of late relapse. Can J Urol 2002;9:1637-40. [PubMed]
- Warde P, Specht L, Horwich A, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol 2002;20:4448-52. [Crossref] [PubMed]
- Kollmannsberger C, Tandstad T, Bedard PL, et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol 2015;33:51-7. [Crossref] [PubMed]
- Cohn-Cedermark G, Stahl O, Tandstad T, et al. Surveillance vs. adjuvant therapy of clinical stage I testicular tumors - a review and the SWENOTECA experience. Andrology 2015;3:102-10. [Crossref] [PubMed]
- Oliver RTD, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet 2005;366:293-300. [Crossref] [PubMed]
- Oliver RTD, Mead GM, Rustin GJS, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol 2011;29:957-62. [Crossref] [PubMed]
- Aparicio J, Maroto P, del Muro XG, et al. Risk-adapted treatment in clinical stage I testicular seminoma: the third Spanish Germ Cell Cancer Group study. J Clin Oncol 2011;29:4677-81. [Crossref] [PubMed]
- Koutsoukos K, Tzannis K, Christodoulou C, et al. Two cycles of adjuvant carboplatin in stage I seminoma: 8-year experience by the Hellenic Cooperative Oncology Group (HECOG). World J Urol 2016;34:853-7. [Crossref] [PubMed]
- Dieckmann K-P, Dralle-Filiz I, Matthies C, et al. Testicular seminoma clinical stage 1: treatment outcome on a routine care level. J Cancer Res Clin Oncol 2016;142:1599-607. [Crossref] [PubMed]
- Travis LB, Curtis RE, Storm H, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst 1997;89:1429-39. [Crossref] [PubMed]
- Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst 2005;97:1354-65. [Crossref] [PubMed]
- Classen J, Schmidberger H, Meisner C, et al. Radiotherapy for stages IIA/B testicular seminoma: final report of a prospective multicenter clinical trial. J Clin Oncol 2003;21:1101-6. [Crossref] [PubMed]
- Patterson H, Norman AR, Mitra SS, et al. Combination carboplatin and radiotherapy in the management of stage II testicular seminoma: comparison with radiotherapy treatment alone. Radiother Oncol 2001;59:5-11. [Crossref] [PubMed]
- Detti B, Livi L, Scoccianti S, et al. Management of Stage II testicular seminoma over a period of 40 years. Urol Oncol 2009;27:534-8. [Crossref] [PubMed]
- Glaser SM, Vargo JA, Balasubramani GK, et al. Stage II Testicular Seminoma: Patterns of Care and Survival by Treatment Strategy. Clin Oncol (R Coll Radiol) 2016;28:513-21. [Crossref] [PubMed]
- Paly JJ, Lin CC, Gray PJ, et al. Management and outcomes of clinical stage IIA/B seminoma: Results from the National Cancer Data Base 1998-2012. Pract Radiat Oncol 2016;6:e249-58. [Crossref] [PubMed]
- de Wit R, Roberts JT, Wilkinson PM, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tr. J Clin Oncol 2001;19:1629-40. [Crossref] [PubMed]
- Kondagunta GV, Bacik J, Bajorin D, et al. Etoposide and cisplatin chemotherapy for metastatic good-risk germ cell tumors. J Clin Oncol 2005;23:9290-4. [Crossref] [PubMed]
- Cary C, Jacob JM, Albany C, et al. Long-Term Survival of Good-Risk Germ Cell Tumor Patients After Postchemotherapy Retroperitoneal Lymph Node Dissection: A Comparison of BEP × 3 vs. EP × 4 and Treating Institution. Clin Genitourin Cancer 2018;16:e307-13. [Crossref] [PubMed]
- Kawai K, Akaza H. Current status of chemotherapy in risk-adapted management for metastatic testicular germ cell cancer. Cancer Sci 2010;101:22-8. [Crossref] [PubMed]
- Calabrò F, Albers P, Bokemeyer C, et al. The contemporary role of chemotherapy for advanced testis cancer: a systematic review of the literature. Eur Urol 2012;61:1212-21. [Crossref] [PubMed]
- Feldman DR, Bosl GJ, Sheinfeld J, et al. Medical treatment of advanced testicular cancer. JAMA 2008;299:672-84. [Crossref] [PubMed]
- Fizazi K, Delva R, Caty A, et al. A risk-adapted study of cisplatin and etoposide, with or without ifosfamide, in patients with metastatic seminoma: results of the GETUG S99 multicenter prospective study. Eur Urol 2014;65:381-6. [Crossref] [PubMed]
- Oliver RTD, Ong J, Shamash J, et al. Long-term follow-up of Anglian Germ Cell Cancer Group surveillance versus patients with Stage 1 nonseminoma treated with adjuvant chemotherapy. Urology 2004;63:556-61. [Crossref] [PubMed]
- Zuniga A, Kakiashvili D, Jewett MAS. Surveillance in stage I nonseminomatous germ cell tumours of the testis. BJU Int 2009;104:1351-6. [Crossref] [PubMed]
- Albers P, Siener R, Krege S, et al. Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I Nonseminomatous testicular germ cell tumors: AUO trial AH 01/94. J Clin Oncol 2008;26:2966-72. [Crossref] [PubMed]
- Tandstad T, Dahl O, Cohn-Cedermark G, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. J Clin Oncol 2009;27:2122-8. [Crossref] [PubMed]
- Tandstad T, Ståhl O, Håkansson U, et al. One course of adjuvant BEP in clinical stage I nonseminoma mature and expanded results from the SWENOTECA group. Ann Oncol 2014;25:2167-72. [Crossref] [PubMed]
- Bamias A, Aravantinos G, Kastriotis I, et al. Report of the long-term efficacy of two cycles of adjuvant bleomycin/etoposide/cisplatin in patients with stage I testicular nonseminomatous germ-cell tumors (NSGCT): a risk adapted protocol of the Hellenic Cooperative Oncology Group. Urol Oncol 2011;29:189-93. [Crossref] [PubMed]
- Guney S, Guney N, Sonmez NC, et al. Risk-adapted management for patients with clinical stage I non-seminomatous germ cell tumour of the testis. Med Oncol 2009;26:136-42. [Crossref] [PubMed]
- Chovanec M, Hanna N, Cary KC, et al. Management of stage I testicular germ cell tumours. Nat Rev Urol 2016;13:663-73. [Crossref] [PubMed]
- Huddart RA, Reid AM. Adjuvant Therapy for Stage IB Germ Cell Tumors: One versus Two Cycles of BEP. Adv Urol 2018;2018:8781698. [Crossref] [PubMed]
- Chevreau C, Mazerolles C, Soulié M, et al. Long-term efficacy of two cycles of BEP regimen in high-risk stage I nonseminomatous testicular germ cell tumors with embryonal carcinoma and/or vascular invasion. Eur Urol 2004;46:209-14; discussion 214-5. [Crossref] [PubMed]
- Böhlen D, Burkhard FC, Mills R, et al. Fertility and sexual function following orchiectomy and 2 cycles of chemotherapy for stage I high risk nonseminomatous germ cell cancer. J Urol 2001;165:441-4. [Crossref] [PubMed]
- Lv ZJ, Wu S, Dong P, et al. Clinical outcomes in patients with stage I non-seminomatous germ cell cancer. Asian J Androl 2013;15:558-63. [Crossref] [PubMed]
- Mezvrishvili Z, Managadze L. Three cycles of etoposide and cisplatin chemotherapy in clinical stage IS nonseminomatous testicular cancer. Int Urol Nephrol 2006;38:621-4. [Crossref] [PubMed]
- Stephenson AJ, Bosl GJ, Motzer RJ, et al. Nonrandomized comparison of primary chemotherapy and retroperitoneal lymph node dissection for clinical stage IIA and IIB nonseminomatous germ cell testicular cancer. J Clin Oncol 2007;25:5597-602. [Crossref] [PubMed]
- Weissbach L, Bussar-Maatz R, Flechtner H, et al. RPLND or primary chemotherapy in clinical stage IIA/B nonseminomatous germ cell tumors? Results of a prospective multicenter trial including quality of life assessment. Eur Urol 2000;37:582-94. [Crossref] [PubMed]
- Rabbani F, Sheinfeld J, Farivar-Mohseni H, et al. Low-volume nodal metastases detected at retroperitoneal lymphadenectomy for testicular cancer: pattern and prognostic factors for relapse. J Clin Oncol 2001;19:2020-5. [Crossref] [PubMed]
- Stephenson AJ, Bosl GJ, Motzer RJ, et al. Retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer: impact of patient selection factors on outcome. J Clin Oncol 2005;23:2781-8. [Crossref] [PubMed]
- Stephenson AJ, Klein EA. Surgical management of low-stage nonseminomatous germ cell testicular cancer. BJU Int 2009;104:1362-8. [Crossref] [PubMed]
- Kondagunta GV, Sheinfeld J, Mazumdar M, et al. Relapse-free and overall survival in patients with pathologic stage II nonseminomatous germ cell cancer treated with etoposide and cisplatin adjuvant chemotherapy. J Clin Oncol 2004;22:464-7. [Crossref] [PubMed]
- Behnia M, Foster R, Einhorn LH, et al. Adjuvant bleomycin, etoposide and cisplatin in pathological stage II non-seminomatous testicular cancer. the Indiana University experience. Eur J Cancer 2000;36:472-5. [Crossref] [PubMed]
- Culine S, Kerbrat P, Kramar A, et al. Refining the optimal chemotherapy regimen for good-risk metastatic nonseminomatous germ-cell tumors: a randomized trial of the Genito-Urinary Group of the French Federation of Cancer Centers (GETUG T93BP). Ann Oncol 2007;18:917-24. [Crossref] [PubMed]
- Jones RH, Vasey PA. Part II: testicular cancer--management of advanced disease. Lancet Oncol 2003;4:738-47. [Crossref] [PubMed]
- de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer 1998;78:828-32. [Crossref] [PubMed]
- Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol 1998;16:1287-93. [Crossref] [PubMed]
- Loehrer PJ, Gonin R, Nichols CR, et al. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol 1998;16:2500-4. [Crossref] [PubMed]
- Park S, Lee S, Lee J, et al. Salvage chemotherapy with paclitaxel, ifosfamide, and cisplatin (TIP) in relapsed or cisplatin-refractory germ cell tumors. Onkologie 2011;34:416-20. [Crossref] [PubMed]
- Kurobe M, Kawai K, Oikawa T, et al. Paclitaxel, ifosfamide, and cisplatin (TIP) as salvage and consolidation chemotherapy for advanced germ cell tumor. J Cancer Res Clin Oncol 2015;141:127-33. [Crossref] [PubMed]
- Einhorn LH, Williams SD, Chamness A, et al. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med 2007;357:340-8. [Crossref] [PubMed]
- De Giorgi U, Rosti G, Aieta M, et al. Phase II study of oxaliplatin and gemcitabine salvage chemotherapy in patients with cisplatin-refractory nonseminomatous germ cell tumor. Eur Urol 2006;50:1032-8; discussion 1038-9. [Crossref] [PubMed]
- Kollmannsberger C, Beyer J, Liersch R, et al. Combination chemotherapy with gemcitabine plus oxaliplatin in patients with intensively pretreated or refractory germ cell cancer: a study of the German Testicular Cancer Study Group. J Clin Oncol 2004;22:108-14. [Crossref] [PubMed]
- Pectasides D, Pectasides M, Farmakis D, et al. Gemcitabine and oxaliplatin (GEMOX) in patients with cisplatin-refractory germ cell tumors: a phase II study. Ann Oncol 2004;15:493-7. [Crossref] [PubMed]
- Einhorn LH, Brames MJ, Juliar B, et al. Phase II study of paclitaxel plus gemcitabine salvage chemotherapy for germ cell tumors after progression following high-dose chemotherapy with tandem transplant. J Clin Oncol 2007;25:513-6. [Crossref] [PubMed]
- Mulherin BP, Brames MJ, Einhorn LH. Long-term survival with paclitaxel and gemcitabine for germ cell tumors after progression following high-dose chemotherapy with tandem transplant. Am J Clin Oncol 2015;38:373-6. [Crossref] [PubMed]
- Seidel C, Oechsle K, Lorch A, et al. Efficacy and safety of gemcitabine, oxaliplatin, and paclitaxel in cisplatin-refractory germ cell cancer in routine care--Registry data from an outcomes research project of the German Testicular Cancer Study Group. Urol Oncol 2016;34:167.e21-8. [Crossref] [PubMed]
- Miller JC, Einhorn LH. Phase II study of daily oral etoposide in refractory germ cell tumors. Semin Oncol 1990;17:36-9. [PubMed]
- Nichols C, Kollmannsberger C. Alternatives to standard BEP x 3 in good-prognosis germ cell tumors--you bet your life. J Natl Cancer Inst 2010;102:1214-5. [Crossref] [PubMed]
- de Wit R, Stoter G, Kaye SB, et al. Importance of bleomycin in combination chemotherapy for good-prognosis testicular nonseminoma: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group. J Clin Oncol 1997;15:1837-43. [Crossref] [PubMed]
- Loehrer PJ, Johnson D, Elson P, et al. Importance of bleomycin in favorable-prognosis disseminated germ cell tumors: an Eastern Cooperative Oncology Group trial. J Clin Oncol 1995;13:470-6. [Crossref] [PubMed]
- Funt S, McHugh DJ, Tsai S, et al. Etoposide and cisplatin (EP) for metastatic good-risk germ cell tumors (GCTs): The Memorial Sloan-Kettering Cancer Center (MSKCC) experience in 944 patients (pts). J Clin Oncol 2018;36:4550. [Crossref]
- Bilen MA, Hess KR, Campbell MT, et al. Intratumoral heterogeneity and chemoresistance in nonseminomatous germ cell tumor of the testis. Oncotarget 2016;7:86280-9. [Crossref] [PubMed]
- Tu SM, Bilen MA, Hess KR, et al. Intratumoral heterogeneity: Role of differentiation in a potentially lethal phenotype of testicular cancer. Cancer 2016;122:1836-43. [Crossref] [PubMed]
- Chahoud J, Zhang M, Pisters LL, et al. Identification of Glypican-3 (GPC3) Expression in a Lethal Subgroup of Refractory Cisplatin-Resistant Testicular Germ-Cell Tumors. Clin Genitourin Cancer 2018;16:325-7. [Crossref] [PubMed]
- Ongoing Clinical Trials in Testicular Cancer. The TIGER Trial. Oncol Res Treat 2016;39:553-6. [Crossref] [PubMed]


