
Surgical salvage in patients with advanced testicular cancer: indications, risks and outcomes
Introduction
Germ cell tumors (GCTs), comprising 1% of male cancers overall and 5% of male genitourinary malignancies, are the most common tumors in young men (1). While 70% of patients with advanced GCTs who undergo induction chemotherapy with cisplatin-based chemotherapy will be cured based on serologic and radiographic response, the remaining 30% represent a heterogeneous and challenging group of patients (2,3). For those patients with a residual mass and normal serum tumor markers (STM), a post-chemotherapy retroperitoneal lymph node dissection (PC-RPLND) is the standard treatment approach. After 3 cycles of bleomycin, etoposide and cisplatin (BEP), the expected histologic breakdown of the PC-RPLND specimen is active cancer in 2–9%, teratoma in 64–67% and necrosis in 27–31% (4).
Patients who have persistently elevated STM following induction chemotherapy are presumed to have residual GCT and salvage chemotherapy is the usual option, with PC-RPLND being utilized in select cases. Salvage chemotherapy results in STM normalization and further tumor regression in 25–70% of cases (5). Following salvage chemotherapy, patients who normalize their STM have a higher rate of malignancy in the specimen compared with the post-induction specimens (Table 1). As such, a post-salvage chemotherapy retroperitoneal lymph node dissection (RPLND) is recommended for all patients with a residual mass immediately following second-line therapy, even if tumor markers completely normalize. Even with this approach, the overall long-term results remain poor with 5-year survival rates of 15–40% in patients who have failed induction chemotherapy (6).
Full table
The surgical management of patients with rising or persistently elevated STM after second line chemotherapy has evolved. Several studies have shown benefit as a result of desperation and late relapse (LR) surgical resections (7-10). Elevated STM after salvage 2nd or 3rd line chemotherapy indicates the presence of chemo-refractory disease and long-term survival rates with desperation RPLND have ranged from 33–75% (11). As such, these patients may benefit from surgical resection in selected cases. Based on these results, many investigators questioned whether or not surgery could be applied earlier in the course of disease, potentially obviating the need for salvage chemotherapy altogether.
In this review of the literature, we discuss the indications for and outcomes related to the surgical management of GCTs in the salvage, desperation, and LR settings.
Definitions
When addressing the management of advanced GCT through salvage, desperation, or LR surgery, it is worthwhile to define certain disease specific parameters as well as the surgical categories. The diagnosis of relapsed testicular GCT occurs when there is either a rise in the human chorionic gonadotropin (hCG) or serum alpha fetoprotein (AFP) or radiographic progression following a complete response (CR) or surgical cure (12). Confirming the diagnosis of relapse is crucially important. Early relapse, constituting a majority of relapse occurrences, is defined as relapses which occur within 2 years of initial treatment (13-15). Patients who fail to achieve a CR to induction chemotherapy or who relapse within 6 months have a more unfavorable prognosis (16). While a rising STM should raise concerns for relapsed GCT, there are other widely known causes for mild STM elevations in this setting, and caution should be taken prior to deciding on aggressive intervention. Serum hCG has shown to be elevated in mononucleosis, chronic marijuana use, as well as luteinizing hormone (LH) elevation, due to the cross-reactivity. Additionally, AFP is found to be elevated in benign liver disease and can be mildly elevated due to certain hereditary conditions. According to Rashdan and Einhorn (2016) an AFP of 8–25 ng/mL should never be the only indication for salvage therapy unless it represents a clear and sustained rise. As a separate word of caution, for patients presenting with hCG >50,000 mIU/mL, they may have a rapid decline during the first 2 cycles of chemotherapy but may plateau and may take several months to normalize. As such, in these circumstances, an element of patience is prudent.
There is a diverse classification scheme for RPLNDs in the literature. In general, a primary RPLND refers to surgery after orchiectomy for clinical stage I (CSI) or low volume clinical stage II (CSII) non-seminoma germ cell tumour (NSGCT) with normal post-orchiectomy STMs. PC-RPLND refers to a surgery after completion of chemotherapy. Classically, this includes patients who have a residual mass but negative STMs. Residual or growing masses after chemotherapy are the result of either teratoma or viable GCT. While it is impossible to predict the pathology with 100% certainty based on the clinical and serologic history, there are certain hints that can help predict the histology of resected masses. Growing masses that are associated with rising STM typically indicate relapsed or persistent GCT while radiographic progression without a corresponding rise in STM should raise suspicion for growing teratoma. In the absence of radiographic progression in the setting of rising markers, sanctuary sites must be considered to include the brain and contralateral testis, and appropriate investigation of these sites should be undertaken.
Under this “PC-RPLND” heading falls the terms salvage and desperation RPLND. While used interchangeably at some institutions, at Indiana University we define salvage RPLND as an operation performed in the setting of an enlarged or growing mass following salvage chemotherapy. Patients who normalize their tumor marks following salvage chemotherapy and have residual radiographic disease are recommended to undergo a salvage RPLND. Justification for this management strategy was originally supported by Fox et al., who showed that 55% of salvage RPLND specimens harbored active cancer (17). In the era of taxane-based salvage chemotherapy, this rate decreased. The group from Memorial Sloan Kettering Cancer Center showed that 14% of their patients who received taxane based salvage chemotherapy harbored active cancer compared to 42% who received other salvage therapies (18). In spite of the improved rates of active cancer in the specimens, RPLND is still uniformly recommended as the rates of active cancer remain unacceptably high.
The term desperation RPLND, as described by Donohue, refers to a PC-RPLND (either induction or salvage chemotherapy) performed in the setting of elevated or rising STM (3). Patients who experience progressive disease either during or within 4 weeks after completion of cisplatin-based chemotherapy are deemed to have platinum refractory GCT. Historically, patients who relapsed after previously receiving first-line chemotherapy were not considered surgical candidates and were given additional chemotherapy. However, a surgical cure is possible even in the setting of elevated markers after both induction and salvage chemotherapy so long as appropriate patient selection is undertaken.
LR is defined as the experience of relapse >2 years after initial chemotherapy. While it does occur in 1–3% of patients, it is more frequent in those patients who present with disseminated disease. A pooled analysis of 5,900 patients revealed LR in 119/3,700 (3.2%) of NSGCT and 31/2,200 (1.4%) of seminoma patients (19). Most commonly occurring within 10 years of diagnosis, the retroperitoneum is the most common site of relapse. Because a significant fraction of LR occurs in the RP only, with or without elevated AFP, and somatic malignant transformation is common, LR should be managed surgically if achievable. This is often referred to as resection of LR and is defined as a PC-RPLND performed for RP recurrence 24 months or later after CR to primary therapy (which may or may not have included RPLND).
Indications for salvage, desperation and LR RPLND
Assuming normalization of STM’s after salvage chemotherapy, thorough surgical resection of residual disease with curative intent should be considered given the possibility of residual teratoma or viable GCT (18). Up to 50% of patients undergoing salvage resection have viable GCT in the resected specimen. When multiple metastatic sites are present, all residual disease should be resected as there exists histologic discordance between sites in 30% of cases (20). That being said, necrosis found in the RPLND specimen correlates to necrosis found in lung lesions 90% of the time. Some have thus advocated for expectant management of lung lesions if the findings of the RPLND indicate necrosis to minimize the surgical burden and morbidity (21). PC-RPLND following salvage chemotherapy may, in certain cases, obviate the need for further chemotherapy. Donohue et al. evaluated 91 patients who underwent PC-RPLND at Indiana University following salvage chemotherapy. Fifty-three patients were deemed a complete resection, of whom 25 underwent repeat salvage chemo and 28 did not. Overall, 12 patients in each group died of disease and there was no difference in survival between the two groups (22). As such, in the setting of a presumed complete resection, adjuvant salvage chemotherapy is currently not recommended. While there is a higher likelihood of complications and incomplete resection in the salvage setting, this should not deter experienced surgeons from proceeding with surgical extirpation and reconstruction when indicated. These cases more frequently involve concomitant procedures such as vascular resection, bowel resection and the removal of associated visceral, pulmonary and mediastinal disease.
Patients who have persistently elevated or rising STM with surgically resectable disease after chemotherapy should be considered for desperation RPLND. Although the STM are elevated at the time of surgery, 50% of patients have necrosis or teratoma in the final specimen. Chemo-refractory patients who have resectable disease may achieve cure through surgery. In fact, at Indiana University, surgery is the preferred approach for patients with initial localized disease and who experienced relapse in the same location. Murphy et al. (1993) evaluated 48 chemo-refractory patients who underwent desperation surgery between 1977–1990 at Indiana University. In this report, 38/48 patients (79%) were rendered grossly free of disease at the time of surgery and 29/48 (60%) achieved a serologic remission; 10/48 (21%) remained continuously free of disease without the need for additional treatment at a median of 46 months follow-up. Of the 19 patients who relapsed following serologic remission, 6 were ultimately without evidence of disease with further therapy, 4 of which had additional surgery and 2 had high dose chemotherapy (HDCT) with autologous bone marrow transplant (BMT) (23). Therefore, when systemic options either fail or are not available due to patient factors, desperation surgery may be curative in selected patients with presumably resectable, low volume disease (24).
LR should be managed utilizing a multimodal treatment strategy including surgery when complete resection is achievable. Salvage chemotherapy is rarely curative and CRs are often only achieved with the use of a combined approach with surgical resection. Some studies have shown that combining salvage chemotherapy with surgery improves the cure rates (25-28). However, at Indiana University we advocate for upfront surgery with no chemotherapy if the LR appears completely resectable. Even when a complete clinical response is achieved after chemotherapy, investigators from Memorial Sloan Kettering Cancer Center still advocate for consolidative surgery as either teratoma or active cancer is still identified frequently (18).
Pathologic findings at and outcomes following salvage, desperation and LR RPLND
Salvage
At the present time, there is still an absence of completed prospective randomized trials comparing outcomes between conventional dose salvage chemotherapy and HDCT with autologous stem cell transplant. While many Urologists and Oncologists eagerly await the phase III “TIGER” trial comparing conventional-dose chemotherapy (CDCT) and HDCT in the initial salvage setting, the management of the patients after salvage chemotherapy is unlikely to change.
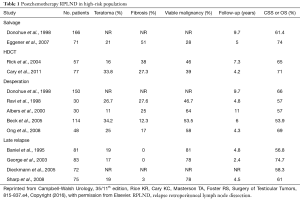
Patients undergoing PC-RPLND after salvage chemotherapy demonstrated higher rates of persistent viable malignancy and worsened survival outcomes compared with patients who received first-line chemotherapy only (see Table 1, obtained from Campbell-Walsh Urology, 35, 815-837.e4, Table 35-6). While overall (OS) and cancer specific (CSS) survival ranged from 60% to 75% in this group, Fox et al. found that patients having received only induction chemotherapy had a CSS of 58.5% compared to those receiving salvage chemotherapy (36.7%) (17,18,29). Regarding surgery after HDCT, Rick et al. observed a 59% recurrence free survival (RFS) and 65% CSS at a median follow-up of 7.3 years in a cohort of 57 patients (30). Supporting this data, Cary et al. reported a 71% OS at a median follow-up of 4.2 years in their cohort of 77 patients (31).
Several authors have evaluated predictive factors at time of salvage RPLND for the presence of active cancer in the resected specimen. Marker trends prior to RPLND appear to have prognostic relevance. Rising STM are associated with both a lower OS as well as a higher risk for finding active cancer (9). The absolute value of the markers additionally has been evaluated.
Lakes et al. evaluated 149 men who underwent RPLND in the setting of elevated STM. Of them, 64% had elevated AFP alone, 25% elevated hCG alone and the remaining 11% with elevation of both STMs. Forty-three percent underwent RPLND after induction chemotherapy while 54% after salvage chemotherapy, of whom 59% had received HDCT. Overall active cancer was seen in 36.9%, teratoma in 35.6% and necrosis in 26.8%. In patients who had AFP rise only, active cancer was seen in 42%, compared to 18.4% in hCG alone. In patients with elevation in both hCG and AFP, 50% had active cancer (32). Support for the prognostic value of the STM elevation came from 2 other studies in this space. Habuchi et al. suggested that high hCG levels is a predictor for disease-related death (33), while Wood et al. showed that all patients with elevated hCG levels relapsed after RPLND while only 30% of patients with isolated AFP elevation relapsed (24).
Arguably, the completeness of resection is regarded as the most important prognostic factor relating to patient outcome. While studies have evaluated the effect on mass location (retroperitoneal/mediastinal vs. visceral), ultimately, it was the ability to achieve complete resection than proved more important. In this regard, the ability to achieve a complete resection may be influenced by the residual mass location. Habuchi et al. reported complete resection in 82% of patients with retroperitoneal/mediastinal disease vs. only 57% of patients with visceral disease (33). While the patients with visceral disease do appear to have worse overall outcomes, attempts for resection in selected patients should not be avoided.
The normalization of tumor markers also has long-term prognostic value in regard to outcome. Ong et al. showed that patients with persistently elevated STM after resection had a 5-year survival of 8% compared to 93% for those patients who normalized their markers (34). In combination with the findings of necrosis in the resected specimen, elevated STM after surgery is particularly worrisome as this clearly indicates residual GCT remaining outside the attempted region of resection.
Desperation
Desperation RPLND can have a significant role in the successful management of patients with advanced GCT. Data from multiple case series report long-term survival rates from 33–75%. (11,34). In 2005, Beck et al. reported data from 114 patients who underwent RPLND in the desperation setting. Active cancer was identified in 53.5% of the resected specimens, while 34% revealed teratoma and 12.3% revealed necrosis. The median 5-year OS was 53.9%. Significantly lower OS rates were seen for patients with active cancer in the resected tissue (31%), compared to teratoma (77%) or fibrosis (85%). Additionally, they showed that marker trends prior to RPLND have prognostic relevance. Rising STM are associated with both a lower OS as well as a higher risk for finding active cancer. In the setting of declining STMs, the 5-year OS was 93.3% compared to increasing (22.7%) or stable (60%) markers. As such, when selecting appropriate patients for desperation RPLND with the highest likelihood of oncologic benefit, the authors recommended use of the following criteria: declining or stable markers after chemotherapy, slowly rising markers after prior CR, and resectable disease at <3 sites. Additionally, in patients who have exhausted all chemotherapy options, or are unable to receive additional chemotherapy, it may be offered as a last resort, so long as it is believed the disease is completely resectable (7).
In 2008, Ong et al. evaluated 48 patients who underwent desperation RPLND and broke the patients down by the presence of rising STM vs. stable/downtrending STM. Overall 58% of patients had active cancer, 25% had teratoma and 17% had necrosis. The 5-year OS was 69%. Favorable prognostic factors in their study were elevation of AFP alone, complete resection, histologic finding of differentiated teratoma and a normalization of STM after RPLND. In fact, the normalization of STM was the only prognostic factor that remained robust on multivariable analysis (34).
Cary et al. evaluated 92 patients who had a residual mass after HDCT, describing their histologic findings and the impact on OS. Overall, 76% received HDCT as 1st line salvage while 23% received HDCT as 2nd line or 3rd line, with 24% of patients being platinum refractory. Forty-two percent of the 92 patients underwent PC-RPLND in the desperation setting. Overall, the histologic breakdown was 38% active cancer, 34% teratoma and 26% necrosis. In the subset of patients in the non-desperation setting, the histologic breakdown was 20% active cancer, 41% teratoma and 39% necrosis. Overall, more active cancer was found in patients who received HDCT as ≥2nd line (60%), compared to patients who received HDCT as 1st line salvage (33%). The 5-year OS of the entire cohort was 70%. The most significant predictor of death was PC-RPLND performed in the desperation setting (31).
The approach to patients with combined retroperitoneal and mediastinal disease following induction chemotherapy is not standardized. While some advocate for staged procedures based on the extent of extra-retroperitoneal disease based on data from Memorial Sloan-Kettering Cancer Center (MSKCC) and widely accepted at high volume centers, combination RPLND and mediastinal surgery is safe and effective (35). Fadel et al. reported a series of 18 patients who underwent a single stage combined retroperitoneal and posterior mediastinal resection. Of these 18 patients, 4 were in the setting of elevated tumor markers. The 5-year OS rate was 92% with a 5-year disease-free survival rate of 87%. Of the 4 patients with elevated STM, 1 was unable to be completely resected, underwent adjuvant chemotherapy and ultimately died of disease. Another patient relapsed to the liver but was able to be further salvaged with chemotherapy and remained NED. The other two had complete serologic responses to surgery and remained NED at last follow-up (36).
Outcomes reported in several retrospective desperation series are listed in Table 1 (8,29,37).
LR
Among patients presenting with LR, 80% contain viable GCT, with yolk sac tumor being the predominant tumor subtype (25). When multiple sites of disease are present, all residual disease should be resected when possible as histologic discordance between sites exists. The appropriate management of LR patients should be individualized, as there exists a wide variety of presentations. In general, marker-negative relapse is consistent with teratoma and should be surgically resected. Typically, a cure can be achieved with complete resection. If, however, the patient presents with marker-positive relapse, this typically indicates viable GCT. These cases have traditionally been managed using either a surgery-first strategy or chemotherapy followed by surgery. Supporting the critical utility of surgery in the LR setting, Baniel et al. evaluated 81 patients treated for LR at Indiana University. A majority of the patients (47/81) presented >5 years after curative treatment of the initial disease. Eighty point four percent of patients were treated with chemotherapy for the LR and only 26% had a CR, with only 3% remaining NED without surgery. Nineteen patients had complete surgical resection of active cancer or teratoma as part of a multimodal treatment strategy and all remained disease free (28). Based on these results, the investigators suggested that LR patients have a very underwhelming response rate to chemotherapy and surgery was the preferred approach.
The patients receiving chemotherapy upfront usually receive this in order to decrease the burden of disease and make surgery more feasible. Ronnen et al. evaluated 29 patients who received salvage chemotherapy in this setting. Their population consisted of patients not considered surgical candidates because of extensive disease or disease in multiple sites. Treatment with paclitaxel, ifosfamide and cisplatin (TIP) followed by surgical resection resulted in CR in 7/14 (50%) while no other salvage regimen resulted in a favorable clinical response, except for one partial response to combine paclitaxel plus ifosfamide followed by high-dose carboplatin plus etoposide (TI-CE) with stem cell rescue. The median survival of the 29 patients was 23.9 months with a median follow-up of 50.6 months (38).
Conclusions
Overall, the management of patients who are not cured by induction chemotherapy alone presents a challenge to even the most meticulous surgeon. Many authors have advocated for the consolidation of surgical care of patients requiring salvage or desperation surgery to centers with significant experience and the availability of an experienced multi-disciplinary surgical team to include hepatobiliary, thoracic and vascular surgeons. While aggressive surgical resection can offer curative treatment in patients in the salvage, desperation and LR setting, the single most important decision is who to operate on. These surgeries are often challenging; however, they can offer long-term survival in a significant percentage of these patients when complete resection can be achieved and thus the benefit is worth the endeavor.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Wu X, Groves FD, McLaughlin CC, et al. Cancer incidence patterns among adolescents and young adults in the United States. Cancer Causes Control 2005;16:309-20. [Crossref] [PubMed]
- International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol 1997;15:594-603. [Crossref] [PubMed]
- Beck SD, Foster RS, Bihrle R, et al. Post chemotherapy RPLND in patients with elevated markers: current concepts and clinical outcome. Urol Clin North Am 2007;34:219-25. [Crossref] [PubMed]
- Cary C, Jacob JM, Albany C, et al. Long-term survival of good-risk germ cell tumor patients after postchemotherapy retroperitoneal lymph node dissection: a comparison of BEP x 3 vs. EP x 4 and treating institution. Clin Genitourin Cancer 2018;16:e307-13. [Crossref] [PubMed]
- Mead GM, Cullen MH, Huddart R, et al. A phase II trial of TIP (paclitaxel, ifosfamide and cisplatin) given as second-line (post-BEP) salvage chemotherapy for patients with metastatic germ cell cancer: a Medical Research Council trial. Br J Cancer 2005;93:178-84. [Crossref] [PubMed]
- Farmakis D, Pectasides M, Pectasides D. Recent advances in conventional-dose salvage chemotherapy in patients with cisplatin-resistant or refractory testicular germ cell tumors. Eur Urol 2005;48:400-7. [Crossref] [PubMed]
- Beck SD, Foster RS, Bihrle R, et al. Outcome analysis for patients with elevated serum tumor markers at postchemotherapy retroperitoneal lymph node dissection. J Clin Oncol 2005;23:6149-56. [Crossref] [PubMed]
- Albers P, Ganz A, Hannig E, et al. Salvage surgery of chemorefractory germ cell tumors with elevated tumor markers. J Urol 2000;164:381-4. [Crossref] [PubMed]
- Coogan CL, Foster RS, Rowland RG, et al. Postchemotherapy retroperitoneal lymph node dissection is effective therapy in selected patients with elevated tumor markers after primary chemotherapy alone. Urology 1997;50:957-62. [Crossref] [PubMed]
- Daneshmand S. Role of surgical resection for refractory germ cell tumors. Urol Oncol 2015;33:370-8. [Crossref] [PubMed]
- Beck SD, Foster RS, Bihrle R, et al. Pathologic findings and therapeutic outcome of desperation post-chemotherapy retroperitoneal lymph node dissection in advanced germ cell cancer. Urol Oncol 2005;23:423-30. [Crossref] [PubMed]
- Rashdan S, Einhorn LH. Salvage Therapy for Patients With Germ Cell Tumor. J Oncol Pract 2016;12:437-43. [Crossref] [PubMed]
- de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer 1998;78:828-32. [Crossref] [PubMed]
- Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol 1998;16:1287-93. [Crossref] [PubMed]
- Michael H, Lucia J, Foster RS, et al. The pathology of late recurrence of testicular germ cell tumors. Am J Surg Pathol 2000;24:257-73. [Crossref] [PubMed]
- Fosså SD, Stenning SP, Gerl A, et al. Prognostic factors in patients progressing after cisplatin-based chemotherapy for malignant non-seminomatous germ cell tumours. Br J Cancer 1999;80:1392-9. [Crossref] [PubMed]
- Fox EP, Weathers TD, Williams SD, et al. Outcome analysis for patients with persistent nonteratomatous germ cell tumor in postchemotherapy retroperitoneal lymph node dissections. J Clin Oncol 1993;11:1294-9. [Crossref] [PubMed]
- Eggener SE, Carver BS, Loeb S, et al. Pathologic findings and clinical outcome of patients undergoing retroperitoneal lymph node dissection after multiple chemotherapy regimens for metastatic testicular germ cell tumors. Cancer 2007;109:528-35. [Crossref] [PubMed]
- Oldenburg J, Wahlqvist R, Fossa SD. Late relapse of germ cell tumors. World J Urol 2009;27:493-500. [Crossref] [PubMed]
- Hartmann JT, Candelaria M, Kuczyk MA, et al. Comparison of histological results from the resection of residual masses at different sites after chemotherapy for metastatic nonseminomatous germ cell tumours. Eur J Cancer 1997;33:843-7. [Crossref] [PubMed]
- Steyerberg EW, Keizer HJ, Messemer JE, et al. Residual pulmonary masses after chemotherapy for metastatic nonseminomatous germ cell tumor. Prediction of histology. ReHiT Study Group. Cancer 1997;79:345-55. [Crossref] [PubMed]
- Donohue JP, Fox EP, Williams SD, et al. Persistent cancer in postchemotherapy retroperitoneal lymph-node dissection: outcome analysis. World J Urol 1994;12:190-5. [Crossref] [PubMed]
- Murphy BR, Breeden ES, Donohue JP, et al. Surgical salvage of chemorefractory germ cell tumors. J Clin Oncol 1993;11:324-9. [Crossref] [PubMed]
- Wood DP Jr, Herr HW, Motzer RJ, et al. Surgical resection of solitary metastases after chemotherapy in patients with nonseminomatous germ cell tumors and elevated serum tumor markers. Cancer 1992;70:2354-7. [Crossref] [PubMed]
- Sharp DS, Carver BS, Eggener SE, et al. Clinical outcome and predictors of survival in late relapse of germ cell tumor. J Clin Oncol 2008;26:5524-9. [Crossref] [PubMed]
- George DW, Foster RS, Hromas RA, et al. Update on late relapse of germ cell tumor: a clinical and molecular analysis. J Clin Oncol 2003;21:113-22. [Crossref] [PubMed]
- Dieckmann KP, Albers P, Classen J, et al. Late relapse of testicular germ cell neoplasms: a descriptive analysis of 122 cases. J Urol 2005;173:824-9. [Crossref] [PubMed]
- Baniel J, Foster RS, Gonin R, et al. Late relapse of testicular cancer. J Clin Oncol 1995;13:1170-6. [Crossref] [PubMed]
- Donohue JP, Leviovitch I, Foster RS, et al. Integration of surgery and systemic therapy: results and principles of integration. Semin Urol Oncol 1998;16:65-71. [PubMed]
- Rick O, Bokemeyer C, Weinknecht S, et al. Residual tumor resection after high-dose chemotherapy in patients with relapsed or refractory germ cell cancer. J Clin Oncol 2004;22:3713-9. [Crossref] [PubMed]
- Cary C, Pedrosa JA, Jacob J, et al. Outcomes of postchemotherapy retroperitoneal lymph node dissection following high-dose chemotherapy with stem cell transplantation. Cancer 2015;121:4369-75. [Crossref] [PubMed]
- Lakes J, Lusch A, Nini A, et al. Retroperitoneal lymph node dissection in the setting of elevated markers. Curr Opin Urol 2018;28:435-9. [Crossref] [PubMed]
- Habuchi T, Kamoto T, Hara I, et al. Factors that influence the results of salvage surgery in patients with chemorefractory germ cell carcinomas with elevated tumor markers. Cancer 2003;98:1635-42. [Crossref] [PubMed]
- Ong TA, Winkler MH, Savage PM, et al. Retroperitoneal lymph node dissection after chemotherapy in patients with elevated tumour markers: indications, histopathology and outcome. BJU Int 2008;102:198-202. [Crossref] [PubMed]
- Brenner PC, Herr HW, Morse MJ, et al. Simultaneous retroperitoneal, thoracic, and cervical resection of post-chemotherapy residual masses in patients with metastatic non-seminomatous germ cell tumors of the testis. J Clin Oncol 1996;14:1765-9. [Crossref] [PubMed]
- Fadel E, Court B, Chapelier AR, et al. One-stage approach for retroperitoneal and mediastinal metastatic testicular tumor resection. Ann Thorac Surg 2000;69:1717-21. [Crossref] [PubMed]
- Ravi R, Ong J, Oliver RT, et al. Surgery as salvage therapy in chemotherapy-resistant nonseminomatous germ cell tumours. Br J Urol 1998;81:884-8. [Crossref] [PubMed]
- Ronnen EA, Kondagunta GV, Bacik J, et al. Incidence of late-relapse germ cell tumor and outcome to salvage chemotherapy. J Clin Oncol 2005;23:6999-7004. [Crossref] [PubMed]


