
Minimally invasive therapies for Peyronie’s disease: the current state of the art
Introduction
Peyronie’s disease (PD) is an acquired fibrotic disorder of the tunica albuginea thought to result from trauma to the penis (1). The condition is relatively common, affecting up to 10% of men at some point in their lifetime, with a higher prevalence seen in specific populations such as after radical prostatectomy (2,3). The presentation is highly variable, but patients frequently report penile pain and bothersome penile deformity such as curvature or indentation that frequently prevents satisfactory penetrative intercourse (4). Sexual dysfunction, such as erectile dysfunction (ED) is common as well (5).
Our understanding of the pathophysiologic mechanisms underlying PD continues to evolve. It seems clear that some insult, presumably penile trauma, triggers a fibrotic cascade that ultimately leads to abnormal collagen deposition within the tunical scar or plaque (1,6,7). However, not all penile injuries trigger the PD-process, and there is mounting evidence that a genetic predisposition may be present in some patients (8). In response to tissue injury, fibroblasts undergo differentiation into myofibroblasts (9). These cells have contractile capabilities and also actively alter the surrounding extracellular matrix, thereby contributing to wound healing, or in the case of PD, abnormal scarring (6). Transforming growth factor-β (TGF-β) is one such signaling pathway that has been implicated in this process (10-12). It is also becoming increasingly clear that oxidative stress, including the presence of reactive oxygen species, plays a role in the pathogenesis of PD as well (13,14).
Historically, it was thought that roughly half of all PD cases resolved spontaneously over time. However, it is now clear from several natural history studies that <15% of patients who present with acute-phase PD (within the first 3–12 months of symptom onset) experience deformity resolution in the absence of treatment (4,15). This may be of little clinical consequence in the setting of relatively mild deformity or in the patient who is not sexually active. However, for many patients, PD is a psychologically devastating disease process that can significantly impact emotional well-being for the patient and his partner (16).
Urologists have a multitude of treatment options in their armamentarium. To date, no intervention has higher success rates than surgical straightening, particularly for men who desire the most rapid return of a functionally straight erection capable of penetrative intercourse (17). Yet, many patients have less severe deformity or are not interested in surgical intervention. Moreover, surgery is contraindicated in early-phase PD due to the potential for disease progression during the first year of symptom onset (18). Here we provide a comprehensive review of non-surgical treatment options for PD.
Oral therapy for PD
Oral agents fall into two general categories: anti-fibrotic and anti-oxidant medications. A 2015 survey revealed that over 80% of urologists preferred oral therapy for initial PD management (19). However, data to support their use is limited. The 2015 American Urological Association (AUA) PD Guideline recommended against many oral agents due to a lack of evidence, although anti-inflammatory medications can be used to address penile pain (20). Despite this, oral medications are attractive for many patients and providers due to their non-invasive nature, availability, and perceived lack of side effects. Also, patients may not be psychologically ready for intralesional injections or surgical straightening.
Vitamin E
Vitamin E consists of eight fat-soluble compounds (known as tocopherols and tocotrienols) that are commonly derived from vegetable oils (21). Vitamin E has anti-proliferative and antioxidant properties, making it an excellent candidate for a variety of medical conditions, including cancer (22). The proposed mechanism of action involves a decrease in collagen deposition secondary to free-radical scavenging (23). Due to the wide availability, low cost, and presumed minimal side-effect profile, Vitamin E remains one of the most commonly prescribed oral agents and is used as first-line therapy by as many as 70–80% of surveyed urologists (19,24,25).
Vitamin E was first proposed for treating PD in 1949, and since that time, several studies of varying levels of evidence have been published (26). Overall the data is inconsistent due in large part to significant flaws in study design. Only a single randomized-placebo controlled trial is available. In 2007, Safarinejad and colleagues randomized patients to vitamin E and propionyl-L-carnitine separately or in combination or placebo for 6 months (27). At the end of the study period, there was no significant difference noted in penile pain or curvature improvement. Other studies have evaluated the combination of vitamin E with other therapies such as intralesional injections. For instance, Inal and colleagues reported similar improvements in objective and subjective outcomes in men with acute phase PD who were treated with intralesional interferon (IFN), vitamin E, or the combination (28). In contrast, Paulis and colleagues reported a significantly greater curvature reduction following treatment with combination verapamil + blueberries + propolis + topical diclofenac (VBPD) and vitamin E (12°) vs. VBPD without vitamin E (6°) (29). While a mean difference of 6° may reach statistical significance, this is unlikely to have a meaningful functional impact for most patients. Moreover, this small degree of improvement may be within the margin of error for inter and intra-observer variability.
Carnitine
Carnitine also possesses intrinsic anti-oxidant properties (30). L-carnitine was the subject of a single randomized, placebo-controlled trial by Safarinejad and colleagues (27). The authors randomized patients to vitamin E, propionyl-L-carnitine, combination, or placebo groups. They found no significant differences in penile pain, curvature, or plaque-size between the groups after a 6-month treatment protocol. A comparative study from 2001 by Biagiotti and Cavallini randomized 48 patients with PD (2/3rd with chronic phase) to acetyl-L-carnitine vs. tamoxifen daily for 3 months (31). A significantly greater proportion of patients in the carnitine arm experienced pain resolution (92% vs. 50%). Moreover, there was a mean 7° decrease in penile curvature in the carnitine group, and only 2/24 (8%) experienced curvature progression. In contrast, 54% of patients in the tamoxifen group experienced curvature progression.
L-arginine and L-citrulline
L-arginine is an amino acid and precursor to nitric oxide (NO), a potent vasodilator that acts at the level of cavernosal smooth muscle cells to induce erections (32). NO also has important antioxidant properties that make it an appropriate target candidate for PD-therapies (33). L-arginine, available as an over the counter supplement, has been the subject of few studies, and to date, there are no randomized controlled trials that support or refute its efficacy. However, there is some intriguing basic science evidence that L-arginine may positively impact PD-plaque. Valente and colleagues administered 2.25 g/kg/day into the drinking water of PD-model rats and found that plaque volumes decreased by 80–90% along with a decrease in the collagen/fibroblast ratio (34). When L-arginine was administered concurrently with sildenafil, a phosphodiesterase-5 inhibitor (PDE5I), a decrease in tunical collagen was seen along with increased levels of fibroblast apoptosis. L-arginine has also shown promise in combination with intralesional verapamil +/− penile traction therapy (PTT) although the direct impact of the L-arginine is unclear (35).
While there are rational physiologic mechanisms for using arginine to treat PD, oral arginine supplementation has several drawbacks. For instance, arginine undergoes extensive first-pass metabolism in the liver (approximately 40%), resulting in a lower available circulating concentration (36). Also, side effects, including gastrointestinal (GI) upset and diarrhea, limit use for some patients (37). Citrulline, when administered orally, is converted to arginine in vivo (38). Citrulline does not undergo first-pass metabolism, nor does it have the same propensity for GI-upset. Oral L-citrulline raises circulating L-arginine and NO concentrations and may be more bio-efficient when compared to arginine supplementation itself (39,40). Thus, while supportive data remains sparse, L-arginine and L-citrulline may be considered as non-invasive treatment options, particularly in the setting of combination therapy with other non-surgical options during the active or “inflammatory” phase of PD (35).
Pentoxifylline
Pentoxifylline is a non-specific PDE-inhibitor that has been studied in a variety of conditions including PD (41). Shindel and colleagues showed that pentoxifylline inhibits fibroblast proliferation and attenuates transforming growth factor-β1 mediated elastogenesis and collagen deposition within human tunical PD cells (42,43). Several single-center retrospective series have been published. Smith and colleagues found that more than 90% of patients with calcified PD plaques who treated with Pentoxifylline had stability or even improvement in the degree of calcification compared with 44% in those who did not take pentoxifylline (44). These patients were also more likely to report subjective improvements (63% vs. 25%), although objective outcomes were not reported. In 2014, Alizadeh and colleagues found that 8/30 (27%) and 22/30 (73%) patients treated with oral pentoxifylline had reductions in penile curvature and penile pain, respectively (45). These results were similar to patients who received intralesional verapamil. In another study, intralesional pentoxifylline in combination with antioxidants and topical diclofenac (anti-inflammatory) decreased mean penile curvature by 10° in men with acute phase PD (46). Thus, while there is ample basic science evidence, human clinical data is limited. In this setting, and based on the available literature, Pentoxifylline may be favored in combination with other non-surgical therapies such as PTT and intralesional injections (35).
Potassium para-aminobenzoate (POTABA)
POTABA inhibits fibroblast activity through increased monoamine oxidase activity. It has been used to treat PD and other conditions such as scleroderma dating back to the 1950s (47). In a retrospective review of 32 patients treated with POTABA for at least 3-months, penile curvature improved in 18/32 (56%) including complete resolution in 8/31 (26%) (48). A 2005 multi-center prospective placebo-controlled trial randomized 103 patients with a median PD duration of 6 months (acute phase) to POTABA vs. placebo for 12 months (49). There was no difference in the percentage of patients who experienced improvements in penile curvature (POTABA: 64% vs. Placebo: 59%; P=0.07), although patients in the placebo group were more likely to experience curvature progression (22% vs. 3%). A recent study found that 68% of patients treated with POTABA discontinued therapy citing side effects, lack of efficacy, and cost as determining factors (50). Side effects include GI upset, pruritus, fever, rash, and on rare-occasions even liver dysfunction (49-51). Moreover, POTABA is dosed 4-times daily, making it burdensome for patients. Given the lack of sound data to support clinically meaningful benefits, significant side effect profile, and dosing schedule, POTABA is rarely used to treat PD in the modern-era.
Colchicine
Colchicine, historically used to treat gouty diathesis, prevents mobilization of inflammatory mediators through microtubule inhibition. However, basic science work with a PD rat-model showed that colchicine also inhibits collagen deposition, thus providing a scientifically-sound rationale for using this agent to treat PD (52,53). One of the earliest studies to evaluate colchicine for PD was a pilot study of 24 patients (54). The authors found that penile curvature improved in 7/19 (37%), and pain relief was reported in 7/9 patients (78%). A subsequent series by Kadioglu and colleagues found that curvature improved in 30% and worsened in 22% of patients treated with colchicine in the acute phase of PD (55). Shorter disease duration, less severe curvature (<30°), and absence of vascular risk factors were associated with improved outcomes.
Prieto Castro and colleagues randomized a cohort of 45 patients with acute-phase PD (duration <6 months) and relatively mild curvature (<30°) to ibuprofen vs. colchicine and vitamin E for 6 months (56). The authors found that a significantly greater percentage of patients in the colchicine/vitamin E group reported improvements in penile curvature (48% vs. 18%), although the quantitative results were not reported. Another randomized, controlled trial comparing colchicine to placebo did not identify any significant differences in objective outcomes such as penile deformity between the treatment and control arms (57). This was true, regardless of the symptom severity. Thus, although retrospective data is mixed, the strongest data does not support a beneficial effect on objective outcomes with colchicine as monotherapy for PD. Importantly, the most common side effects reported with colchicine include GI upset and diarrhea, but severe events, including myelosuppression and even neuromuscular toxicity, have been encountered (58).
Tamoxifen
Tamoxifen, a selective-estrogen receptor modulator, down-regulates TGF-β mediated signaling in myofibroblasts, an important component of the presumed pathophysiology underlying PD (59). Very few studies have evaluated tamoxifen in the context of PD. In one of the earliest published trials, Ralph and colleagues found that pain improved in 80% of patients treated with tamoxifen, and penile deformity improved in 35% (60). Teloken and colleagues randomized 25 patients to tamoxifen vs. placebo for 3 months. There was no significant difference in pain-improvement or objective outcomes, including penile curvature between the treatment and control arms (61). Another study by Biagiotti and Cavallini found that oral L-citrulline more effectively reduced penile pain and prevented disease progression compared with tamoxifen (31). Given the relative paucity of available data, including the lack of efficacy seen in the only placebo-controlled trial, the AUA PD guideline panel recommends against tamoxifen as monotherapy (20). However, combination therapy with tamoxifen and PDE5Is may have a synergistic anti-fibrotic effect, thereby decreasing the presence of myofibroblast transformation and extracellular matrix production (62).
PDE5Is
PDE5Is were first approved by the United States Food and Drug Administration to treat ED over two decades ago and remain the first-line therapy for ED in the absence of medical contraindications (63). Comorbid ED is present in 30–45% of patients with PD (4,5). Whether ED predisposes to PD, or the underlying tunical changes caused by PD prevent adequate rigidity and maintenance of erections, is still up for debate and is beyond the scope of the current review. Early concerns regarding exacerbation of penile deformity with PDE5Is have been debunked. A 2002 report from Levine and Latchemsetty found that >70% of PD patients with ED who utilized sildenafil reported satisfactory erectile function, and no one reported worsening deformity or pain (64). In addition to the beneficial effects on erectile function, it is becoming increasingly clear that PDE5Is possess intrinsic anti-fibrotic properties that may help to modulate the tunical fibrosis that occurs with PD (65). As has been previously discussed, NO is felt to possess inherent anti-fibrotic properties. PDE5Is prevent the degradation of cyclic guanosine monophosphate (cGMP) to GMP, thereby maintaining an increased level of circulating NO (63). Valente and colleagues found that daily sildenafil decreased the collagen/fibroblast ratio and induced apoptosis in a rat model (34). When human PD plaques were exposed to sildenafil and pentoxifylline (a non-specific PDE inhibitor), the authors also found a decrease in collagen and alpha smooth-muscle actin (a marker for myofibroblast activity). Ferrini and colleagues later showed that long-term vardenafil reduced PD collagen and the number of myofibroblasts in a PD-rat model (66).
To date, high-quality human data regarding PDE5I use in PD is lacking. Chung and colleagues evaluated outcomes in a cohort of 65 men with isolated penile septal scarring, including 35 who were treated with daily tadalafil (2.5 mg) (67). This therapy was well tolerated, and not surprisingly, there was a mean 7-point increase in the International Index of Erectile Function (IIEF)-5 score for the tadalafil group. Interestingly, on follow-up penile ultrasonography, resolution of the septal scar was seen in 24/35 patients (69%) who were treated with tadalafil, compared with 3/30 (10%) in the observation group. Improved outcomes with combination therapy using PDE5Is and extracorporeal shock wave therapy and collagenase clostridium histolyticum (CCH) have also been reported, although again it is not clear whether this is due to the antifibrotic properties of the drug or the beneficial impact on erectile function (68,69). Further work is needed, including randomized placebo-controlled trials, to evaluate the efficacy of using PDE5Is for outcomes specific to PD. In the meantime, in the absence of overt medical contraindications, PDE5I-use should be considered as a helpful adjunct for patients with PD, particularly in the presence of diminished erectile rigidity.
Topical therapy
One significant concern with oral medications is the ability to deliver the drug to the target tissues in a high enough quantity to exert their effects—particularly given the lack of significant vascularity within the plaque. Also, oral administration increases the risk of systemic side effects. For these reasons, several authors have explored the possibility of direct topical application.
Verapamil
Verapamil is a calcium-channel blocker that benefits wound healing (70). The proposed mechanism involves decreased proline incorporation within the extracellular matrix of the tunical scar, thereby promoting collagen breakdown (71,72). An industry-sponsored trial from 2007 randomized 57 patients to verapamil, trifluoperazine (calmodulin-blocker) and magnesium sulfate (weak calcium channel blocker) (73). At 3 months, all patients crossed over into the verapamil cohort. Ninety-four percent experienced subjective curvature improvements (mean improvement 61%) and erection quality improved as well. Unfortunately, objective outcomes were not reported. A subsequent report from Martin and colleagues showed that transdermal verapamil fails to accumulate in the target tunica albuginea, thereby weakening the hypothesis that local topical therapy has a meaningful effect on PD outcomes (74).
Electromotive drug administration (EMDA) involves the application of an electrical field to improve the delivery of medication to target tissues (75). This therapy has been extensively studied in the setting of intravesical chemotherapy administration for bladder malignancy. It has also been the subject of several reports within the PD literature. A 2003 study by Levine and colleagues found that 70% of tunical specimens contained measurable levels of verapamil after topical administration with EDMA. A subsequent randomized placebo-controlled study from the same center did not show a significant difference in outcomes, including objective curvature improvement (76). In contrast, Di Stasi and colleagues showed that EMDA with verapamil and dexamethasone improved penile curvature to a greater extent than the lidocaine control (77). EMDA of verapamil and dexamethasone may also decrease erectile pain compared to intralesional injection of verapamil plus dexamethasone (78). Due to the lack of strong data, including small sample sizes and lack of true placebo groups in most cases, further study is warranted (20). Notably, the therapy can be quite tedious, and due to the presence of other treatment options, this modality is rarely utilized in modern clinical practice.
H-100
H-100 is a topical agent designed for treatment during the acute phase of PD. It consists of emu oil, a fatty acid rich transdermal carrier agent, as well as nicardipine and superoxide dismutase. Emu-oil is suggested to improve tissue penetration, theoretically increasing the local concentration of topical therapies (79). The dihydropyridine-type calcium channel blocker nicardipine reduces collagen production and glycosaminoglycan synthesis, while superoxide dismutase is an antioxidant that catalyzes the breakdown of free radicals that are present during an inflammatory process.
The pilot study on H-100 randomized 22 patients to treatment with H-100 or placebo for 3 months, followed by a 3-month cross-over phase (80). Notably, all patients were in the acute phase of PD (<12 months duration). At 6 months, the H-100 cohort experienced a significant improvement in stretched penile length (22.6%), reduction in mean curvature (40.8%), and decrease in mean pain level (85.7%). The placebo arm exhibited a 6.8% improvement in stretched penile length, but no differences were observed in mean curvature or pain. Following the crossover period, patients initially in the placebo cohort showed significant improvement in stretched penile length (17.5%), mean curvature (37.1%), and decreased mean pain level (85.7%). H-100 was well tolerated with a self-limited rash observed in three patients. While interesting, these early findings will require further corroborating evidence before considering H-100 as first-line therapy for the acute phase of PD.
Intralesional therapy
IFN-α2b
IFN are cytokines that have a wide array of medical applications. Specifically concerning PD, in vivo studies of Peyronie’s plaque-derived fibroblasts treated with IFN-α2b resulted in a concentration-dependent inhibition of collagen production and fibroblast proliferation, while upregulating collagenase (81).
Human studies involving the intralesional injection of IFN-α2b in 19 men showed a statistically significant 12° improvement in curvature, along with a significant improvement in penile pain (82). A larger subsequent multi-institutional study comparing IFN-α2b (n=50) to saline (n=53) found a 13.5° improvement in curvature among the IFN-α2b cohort, which was significantly greater than the placebo arm (83). Pain resolved following IFN-α2b in 67.7% of men. Trost and colleagues described a similar improvement in curvature of 5.9° to 11.7° depending on the severity of pre-treatment curvature (84). Hellstrom and colleagues also evaluated intralesional IFN-α2b for ventral plaques and found a 9.3° decrease in penile curvature, which was not significantly different from the 8.7° improvement observed for dorsal curvature (85). Nonetheless, for ventral plaques, tunica albuginea plication is notably more effective than IFN-α2b (46° vs. 9.3° respectively) (86). Adverse effects with IFN-α2b frequently include fevers, chills, arthralgia, sinusitis, and minor penile ecchymosis—these are short-lived and were all managed with non-steroidal anti-inflammatory medications (83).
Verapamil
Intralesional injection of verapamil has been shown to decrease plaque size, penile curvature, collagen and elastin levels in a rat model of PD (87). The earliest peer-reviewed study of intralesional verapamil in humans involved 14 subjects. Patients were administered biweekly injections for 6 months with dose escalation up to 10 mg per injection. All subjects reported plaque softening and improvement in penile narrowing, while 43% noted curvature improvement (88). A follow-up study involved 38 men who completed the full treatment course of 10 mg intralesional injections every other week for a total of 12 injections. Pain resolved in 97%, while 76% had subjective improvement in curvature and 72% reported improvement in the ability to engage in penetrative intercourse. An objective decrease in curvature was seen in 54% (89). These results prompted a larger study of 140 men who also underwent intralesional injection of 10 mg of verapamil. Curvature decreased in 62% by a mean of 17°. Girth, rigidity distal to the plaque and sexual function improved in 83%, 80%, and 71% of men respectively (90). The above studies primarily involved a verapamil dose of 10 mg diluted in 10 mL of injectable solution. However, Cavallini and colleagues later found that 10 mg of verapamil in 20 mL of injectable solution was the optimal concentration to maximize improvements in penile curvature, plaque size, erectile function, and pain (91). Notably, several other studies have not found such robust outcomes with intralesional verapamil (92-94).
CCH
In the early 1980s, Gelbard and colleagues found that purified clostridial collagenase was effective in degrading penile plaques in both in vitro and in vivo settings (95,96). Nearly three decades later, the Investigation for Maximal Peyronie’s Reduction Efficacy and Safety Studies (IMPRESS) I and II trials were published (97). These were phase 3, randomized, placebo-controlled, double-blinded studies involving 417 and 415 subjects in each study, respectively. Patients were in the stable phase of the disease and underwent 4 treatment cycles separated by 6 weeks. Each cycle involved 2 injections (separated by 24–72 hours) of CCH (0.25 mL, 0.58 mg) via a single pass of a 27-gauge needle. Manual modeling was performed 24–72 hours after the second injection. Patients with ventral curvature and extensive plaque calcification were excluded.
Improvement in penile curvature was significantly greater among the CCH cohort relative to placebo [mean improvement 17° (34%) vs. 9.3° (18.2%) respectively]. Symptom bother score was also significantly improved following CCH. Side effects, including bruising and hematoma, were common, and there were 3 incidences of corporal rupture requiring surgical repair (97). Ultimately, the results of these trials led to the FDA approval of CCH in late 2013 for dorsal and dorsolateral curves measuring 30°–90° (98).
Following FDA approval, a retrospective single-institution study noted a greater improvement in mean curvature of 23°, which represented a 38% overall improvement. Subjectively, 57% of men felt that surgery was no longer necessary after CCH injections, and 52% reported restored ability to participate in penetrative intercourse (99). This data has been corroborated by several post-approval studies as well (100,101). In contrast, a retrospective review at another high volume institution found less promising results, with a mean improvement in primary curvature of 5° (10% improvement) (102). Dissatisfaction was reported in 82% of patients, and tunical rupture occurred in 4%.
While CCH appears to be an effective treatment option, there are several issues to consider. First, there may be a discrepancy between subjective and objective findings, as the modest absolute improvement in curvature does not necessarily align with the high levels of patient satisfaction. Perhaps softening of the plaque allows for greater malleability of the erect penis allowing for improved penetrative ability or there may be a positive psychological effect of receiving non-surgical therapy for PD (103). Second, the proportion of men experiencing tunical rupture is a concern. A recent survey of Sexual Medicine Society of North America members that use CCH found that one in three members had at least one patient that experienced a tunical rupture (104). Management for this entity is unclear, and future study is needed to determine whether an intervention such as surgical repair is necessary. Finally, CCH is an expensive treatment modality. A cost analysis study showed that a course of CCH for moderate PD was $25,856, which increased to $26,375 for severe PD (105). This is over 8 times more expensive than penile plication. Given this notable cost, an abbreviated protocol of 3 injections involving 0.9 mg of CCH was introduced (106). The mean reduction in curvature was 17° (32%), which was comparable to the IMPRESS trials.
In summary, intralesional injections with agents such as IFN-α2b, verapamil, and CCH result in statistically significant improvements in penile curvature. Data suggests that IFN-α2b and verapamil may also improve penile pain (83,90). Although a statistically significant improvement in curvature has been consistently described with intralesional injections, a mean curvature reduction of <20° may not be considered clinically meaningful by many patients, particularly those with more severe deformity. Moreover, the timing of administration (acute vs. chronic phase) and patient characteristics (calcification, direction of curvature, indentation deformity) that optimize outcomes remains unclear (107,108). Overall, surgical therapy may be more appropriate for men with chronic phase-PD who have more severe penile deformity (>60–90 degrees; indentation or hourglass with hinge) or in those men who desire the most rapid and reliable path to a functionally straight penis.
Radiotherapy
Superficial, low dose radiotherapy has been utilized for the treatment of PD. The proposed mechanism is an increase in NO synthase along with anti-inflammatory effects (109). However, radiotherapy also carries the risk of corporal fibrosis, as well as end-artery and nerve damage leading to ED (110). Evidence for the use of radiotherapy for PD is primarily based on case series with non-standardized protocols. A review by Mulhall and Hall suggested that radiotherapy may improve painful erections, but did not conclude that there was sufficient evidence to suggest improvement in curvature (111). Further reviews of the literature by Tsambarlis and Mulhall each concluded that the potential penile injury associated with radiotherapy outweighs any potential beneficial effect (103,111).
Shockwave
There are two proposed mechanisms by which shockwave therapy may provide benefit for patients with PD. First, the direct effect of shockwaves on the penile plaque may cause mechanical disruption, thereby inducing remodeling. Second, the inflammatory reaction that results from shockwave heat may recruit macrophages leading to plaque lysis (112). Multiple observational reports have suggested notable improvement in pain and sexual outcomes (via IIEF-5 questionnaires) with a decrease in plaque size and degree of curvature (113-118). However, the results of several randomized studies were not as encouraging. In these studies, a small number of patients were randomized to 4–6 weeks of shockwave therapy (n=16–51) vs. sham treatment (n=20–51). Improvement in curvature was minimal in the treatment arms (0.9°–9°) relative to the sham cohorts (1.1°–5.3°). Similarly, minimal improvements were found with shockwave therapy relative to sham treatments concerning pain (visual analog score pain decrease: 1.1–5.1 vs. 0.8–4.8) and sexual function (IIEF-5: 0.6–9.9 vs. 0.1–6.2) respectively (68,119-121). The risks associated with shockwave are limited and include pain during treatment as well as self-limited local hematoma and skin petechiae (68,119,120).
The AUA and European Association of Urology guidelines conclude that shockwave therapy can be offered to aid in pain control after failure of oral anti-inflammatory agents during the acute phase of PD, but has minimal benefit in the reduction of curvature or plaque size. Furthermore, the pain associated with the acute phase of PD improves spontaneously with time. Therefore, shockwave therapy may represent a costly therapy with limited benefit, and we do not recommend its use for the treatment of PD (20,112).
Vacuum erection devices (VEDs)
VEDs were initially introduced as a treatment for ED. However, the use of VEDs for PD is less well established. A study by Yuan and colleagues that investigated the molecular mechanisms of VED using a rat-model noted upregulation of alpha smooth muscle actin and endothelial NO synthase with downregulation of hypoxia-inducible factor 1-alpha, collagenase, and TGF-β (122). A further study utilizing a rat model for PD showed decreased TGF-β expression as well as a significant improvement in penile curvature following VED therapy (123). In humans, Raheem and colleagues reviewed 31 patients with a mean curvature of 48° that underwent 12 weeks of twice-daily VED therapy for 10 minutes per session (124). Curvature improvement of 5°–25° was observed in 68% of men, while 10% experienced curvature progression. Subsequent surgical correction was required in 48%.
The potential for increased penile length with the use of VEDs has been demonstrated prior to inflatable penile prosthesis placement or in the early period following radical prostatectomy in an effort to limit penile length loss (125,126). However, among PD patients, there is no evidence for length restoration attributable to VED, and further studies are needed regarding the efficacy of VED for curvature improvement.
PTT
PTT refers to the application of mechanical force to the penis in order to induce changes at a cellular level. Chung and colleagues used a strained culture system of PD cells to model the effect of PTT on PD tunical tissue (127). PD cells that underwent traction forces had significantly increased matrix metalloproteinase-8 (MMP-8) expression, which is notable because MMP-8 is involved in collagen degradation. Furthermore, cytokines associated with inflammation and fibroblast replication, including αSMA, Hsp-47, and TGF-β1 were not upregulated.
One of the earliest published investigations of PTT for PD came from Levine and colleagues in 2008 (128). In this retrospective study, 10 men underwent traction therapy for a mean of 4.5 hours per day for 6 months. A 33% measured improvement in curvature (10°–45°) was observed. Additionally, there was an increase of flaccid stretched penile length by 0.5–2.0 cm, while girth increased by 0.5–1.0 cm. Hinge effect significantly improved or resolved in each of the 4 men who presented with penile instability.
A subsequent prospective study compared 55 patients in the acute phase that underwent traction therapy for 6 months to 41 patients that had no intervention (129). Men in the PTT cohort had a mean initial curve of 33°, which significantly improved by a mean of 20° at 9-month follow-up. Pain and erectile function also significantly improved with PTT. Further, penile plaques were no longer identified on ultrasound in 48% of patients.
A recent multi-center controlled study evaluated outcomes in a cohort of men with stable-phase PD who were randomly assigned to PTT with the Penimaster PRO (MSP Concept, Berlin, Germany) vs. no-traction for 12 weeks (130). Patients in the PTT group were instructed to utilize the device for 3–8 hours per day. The PTT cohort experienced a significant mean curvature reduction of 31.2° (41% improvement from baseline) and a length gain of 1.8 cm. There were no significant changes in the non-intervention cohort. Men who underwent PTT for >6 hours per day had a significantly greater improvement in curvature compared to those who utilized the device for <4 hours (51.4% vs. 28.8%). Mild adverse events occurred in 43% and included glans numbness and penile discomfort.
RestoreX, another novel PTT device, was also recently investigated in a randomized, controlled, single-blinded, intention-to-treat study (131). Men with PD (n=110, mean pre-intervention curvature 59.3°) were randomized to 30–90 minutes per day of PTT or no therapy in a 3:1 ratio, respectively. After 3 months, there were significant improvements over placebo with respect to curvature (−11.7° vs. +1.3°) and length gain (1.5 vs. 0 cm). A benefit of this device may be the decreased duration of therapy per day, as studies on other PTT modalities have involved 3–8 hours of use per day. Given the non-invasive nature and lack of significant side effects, PTT may be considered as first-line therapy for men with PD, particularly in those with concerns about penile length loss.
Combination therapy
It is possible that the greatest benefit to be experienced from minimally invasive management of PD lies in a combination of oral, topical, injection, and traction therapies. Abern and colleagues evaluated a combination of oral L-arginine, oral pentoxifylline, and intralesional verapamil injection with or without PTT (35). Mean decrease in curvature was 27° in the combination PTT cohort vs. 21° in the oral and injection therapy alone group. This was not significantly different, but it is worth noting that the duration of PTT was not controlled. When traction was used for >3 hours per day, the PTT combination resulted in a mean penile length gain of 0.6 cm vs. a loss of 0.7 cm with oral and injection therapy. Yafi and colleagues similarly found that traction duration in excess of 3 hours per day resulted in better outcomes in mean utilizing combination therapy with PTT and IFN-α2b (132). In contrast, Ziegelmann and colleagues did not identify any significant difference in curvature or length improvements in men utilizing PTT (mean 10 hours/week) concurrently with CCH (133). However, it should be noted that only 9% of patients consistently performed >3 hours/day of PTT, emphasizing the challenges associated with patient compliance.
Mechanical therapy has also been studied alongside CCH. Ralph and colleagues evaluated CCH and vacuum therapy with and without manual modeling (134). The mean change from baseline curvature was 23° in both cohorts—a result that may have been supplemented in both groups by vacuum therapy. Perhaps traction therapy may be considered in place of vacuum therapy for future research. Overall, these studies are small, and further investigation into combined therapeutic regimens is warranted.
Stem cell therapy
Mesenchymal stem cells (MSC) have been suggested to limit fibrosis, which has prompted interest in evaluating their utility for the treatment of PD. Specifically, adipose-derived stem cells (ADSC), a subtype of MSC, have been studied in rat models of tunica albuginea fibrosis. The first study to evaluate MSC therapy in an animal model for PD involved injection of ADSCs into the tunica albuginea (135). Erectile function improved significantly, while type III collagen and elastin expression were inhibited. In a subsequent study by Gokce and colleagues, ADSCs with and without expression of human IFN-α2b were injected into the tunica albuginea of a rat model for PD (136). Erectile function was significantly improved and PD-like changes were attenuated both with and without IFN-α2b. Despite these encouraging findings in animal models, evidence regarding stem cell therapy for PD in humans is lacking. Therefore, we do not currently recommend its use.
Conclusions
A variety of non-surgical therapies have been offered for PD as surgery is contraindicated in the acute phase of PD (Table 1). Data on oral monotherapy does not show reliably effective results. Therefore, low cost and low-side effect profile oral therapies such as L-citrulline and pentoxifylline may be instead given as part of a combination regimen. Intralesional therapy with IFN-α2b, verapamil, and CCH are options for men in both the acute and chronic phases of the disease process as well. Mechanical therapy via PTT offers clinically significant changes in penile length and curvature; however, PTT traditionally requires a motivated patient who is committed to hours of daily therapy. Emerging data with the RestoreX device suggests that shorter duration therapy may still offer clinically meaningful results. The optimal minimally invasive therapy for PD likely involves a combination of non-surgical therapies. Further investigation into particular combination therapies, including oral anti-inflammatory agents, CCH, and PTT, may reveal a synergistic therapeutic effect. Nonetheless, surgery offers the highest success rates for men with chronic-phase PD who desire rapid return of a functionally straight erection.
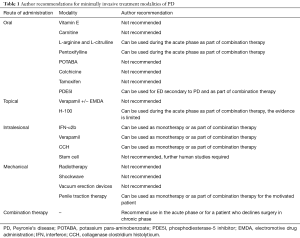
Full table
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Larry I. Lipshultz, Alexander W. Pastuszak) for the focused issue “Contemporary Issues and Controversies in Men’s Health” published in Translational Andrology and Urology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The focused issue “Contemporary Issues and Controversies in Men’s Health” was commissioned by the editorial office without any funding or sponsorship. LA Levine is a speaker for Abbvie and Endo, speaker and consultant for Boston Scientific and Coloplast and officer for Absorption Pharmaceuticals. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Devine CJ Jr, Somers KD, Jordan SG, et al. Proposal: trauma as the cause of the Peyronie's lesion. J Urol 1997;157:285-90. [Crossref] [PubMed]
- Tal R, Heck M, Teloken P, et al. Peyronie's disease following radical prostatectomy: incidence and predictors. J Sex Med 2010;7:1254-61. [Crossref] [PubMed]
- Mulhall JP, Creech SD, Boorjian SA, et al. Subjective and objective analysis of the prevalence of Peyronie's disease in a population of men presenting for prostate cancer screening. J Urol 2004;171:2350-3. [Crossref] [PubMed]
- Mulhall JP, Schiff J, Guhring P. An analysis of the natural history of Peyronie's disease. J Urol 2006;175:2115-8; discussion 2118. [Crossref] [PubMed]
- Burri A, Porst H. The relationship between penile deformity, age, psychological bother, and erectile dysfunction in a sample of men with Peyronie's Disease (PD). Int J Impot Res 2018;30:171-8. [Crossref] [PubMed]
- Milenkovic U, Ilg MM, Cellek S, et al. Pathophysiology and Future Therapeutic Perspectives for Resolving Fibrosis in Peyronie's Disease. Sex Med Rev 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Zargooshi J. Trauma as the cause of Peyronie's disease: penile fracture as a model of trauma. J Urol 2004;172:186-8. [Crossref] [PubMed]
- Herati AS, Pastuszak AW. The Genetic Basis of Peyronie Disease: A Review. Sex Med Rev 2016;4:85-94. [Crossref] [PubMed]
- Sun YB, Qu X, Caruana G, et al. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 2016;92:102-7. [Crossref] [PubMed]
- Haag SM, Hauck EW, Szardening-Kirchner C, et al. Alterations in the transforming growth factor (TGF)-beta pathway as a potential factor in the pathogenesis of Peyronie's disease. Eur Urol 2007;51:255-61. [Crossref] [PubMed]
- El-Sakka AI, Hassoba HM, Pillarisetty RJ, et al. Peyronie's disease is associated with an increase in transforming growth factor-beta protein expression. J Urol 1997;158:1391-4. [Crossref] [PubMed]
- Mateus M, Ilg MM, Stebbeds WJ, et al. Understanding the Role of Adenosine Receptors in the Myofibroblast Transformation in Peyronie's Disease. J Sex Med 2018;15:947-57. [Crossref] [PubMed]
- Vernet D, Ferrini MG, Valente EG, et al. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie's fibrotic plaque and in its rat model. Nitric Oxide 2002;7:262-76. [Crossref] [PubMed]
- Paulis G, Romano G, Paulis L, et al. Recent Pathophysiological Aspects of Peyronie's Disease: Role of Free Radicals, Rationale, and Therapeutic Implications for Antioxidant Treatment-Literature Review. Adv Urol 2017;2017:4653512.
- Gelbard MK, Dorey F, James K. The natural history of Peyronie's disease. J Urol 1990;144:1376-9. [Crossref] [PubMed]
- Terrier JE, Nelson CJ. Psychological aspects of Peyronie's disease. Transl Androl Urol 2016;5:290-5. [Crossref] [PubMed]
- Yafi FA, Diao L, DeLay KJ, et al. Multi-institutional Prospective Analysis of Intralesional Injection of Collagenase Clostridium Histolyticum, Tunical Plication, and Partial Plaque Excision and Grafting for the Management of Peyronie's Disease. Urology 2018;120:138-42. [Crossref] [PubMed]
- Brimley SC, Yafi FA, Greenberg J, et al. Review of Management Options for Active-Phase Peyronie's Disease. Sex Med Rev 2019;7:329-37. [Crossref] [PubMed]
- Sullivan J, Moskovic D, Nelson C, et al. Peyronie's disease: urologist's knowledge base and practice patterns. Andrology 2015;3:260-4. [Crossref] [PubMed]
- Nehra A, Alterowitz R, Culkin DJ, et al. Peyronie's Disease: AUA Guideline. J Urol 2015;194:745-53. [Crossref] [PubMed]
- Szymanska R, Nowicka B, Kruk J., Vitamin E. Occurrence, Biosynthesis by Plants and Functions in Human Nutrition. Mini Rev Med Chem 2017;17:1039-52. [Crossref] [PubMed]
- Jiang Q. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv Nutr 2017;8:850-67. [Crossref] [PubMed]
- Sikka SC, Hellstrom WJ. Role of oxidative stress and antioxidants in Peyronie's disease. Int J Impot Res 2002;14:353-60. [Crossref] [PubMed]
- Ko YH, Moon KH, Lee SW, et al. Urologists' Perceptions and Practice Patterns in Peyronie's Disease: A Korean Nationwide Survey Including Patient Satisfaction. Korean J Urol 2014;55:57-63. [Crossref] [PubMed]
- Shindel AW, Bullock TL, Brandes S. Urologist practice patterns in the management of Peyronie's disease: a nationwide survey. J Sex Med 2008;5:954-64. [Crossref] [PubMed]
- Scardino PL, Scott WW. The use of tocopherols in the treatment of peyronie's disease. Ann N Y Acad Sci 1949;52:390-6. [Crossref]
- Safarinejad MR, Hosseini SY, Kolahi AA. Comparison of vitamin E and propionyl-L-carnitine, separately or in combination, in patients with early chronic Peyronie's disease: a double-blind, placebo controlled, randomized study. J Urol 2007;178:1398-403; discussion 1403. [Crossref] [PubMed]
- Inal T, Tokatli Z, Akand M, et al. Effect of intralesional interferon-alpha 2b combined with oral vitamin E for treatment of early stage Peyronie's disease: a randomized and prospective study. Urology 2006;67:1038-42. [Crossref] [PubMed]
- Paulis G, Brancato T, D'Ascenzo R, et al. Efficacy of vitamin E in the conservative treatment of Peyronie's disease: legend or reality? A controlled study of 70 cases. Andrology 2013;1:120-8. [Crossref] [PubMed]
- Vacante F, Senesi P, Montesano A, et al. L-Carnitine: An Antioxidant Remedy for the Survival of Cardiomyocytes under Hyperglycemic Condition. J Diabetes Res 2018;2018:4028297.
- Biagiotti G, Cavallini G. Acetyl-L-carnitine vs tamoxifen in the oral therapy of Peyronie's disease: a preliminary report. BJU Int 2001;88:63-7. [Crossref] [PubMed]
- Barassi A, Corsi Romanelli MM, Pezzilli R, et al. Levels of l-arginine and l-citrulline in patients with erectile dysfunction of different etiology. Andrology 2017;5:256-61. [Crossref] [PubMed]
- Paulis G, Brancato T. Inflammatory mechanisms and oxidative stress in Peyronie's disease: therapeutic "rationale" and related emerging treatment strategies. Inflamm Allergy Drug Targets 2012;11:48-57. [Crossref] [PubMed]
- Valente EG, Vernet D, Ferrini MG, et al. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie's fibrotic plaque and related fibroblast cultures. Nitric Oxide 2003;9:229-44. [Crossref] [PubMed]
- Abern MR, Larsen S, Levine LA. Combination of penile traction, intralesional verapamil, and oral therapies for Peyronie's disease. J Sex Med 2012;9:288-95. [Crossref] [PubMed]
- Castillo L, Chapman TE, Yu YM, et al. Dietary arginine uptake by the splanchnic region in adult humans. Am J Physiol 1993;265:E532-9. [PubMed]
- Grimble GK. Adverse gastrointestinal effects of arginine and related amino acids. J Nutr 2007;137:1693S-1701S. [Crossref] [PubMed]
- Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol 1981;241:E473-80. [PubMed]
- Schwedhelm E, Maas R, Freese R, et al. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol 2008;65:51-9. [Crossref] [PubMed]
- Agarwal U, Didelija IC, Yuan Y, et al. Supplemental Citrulline Is More Efficient Than Arginine in Increasing Systemic Arginine Availability in Mice. J Nutr 2017;147:596-602. [Crossref] [PubMed]
- Brant WO, Dean RC, Lue TF. Treatment of Peyronie's disease with oral pentoxifylline. Nat Clin Pract Urol 2006;3:111-5. [Crossref] [PubMed]
- Shindel AW, Lin G, Ning H, et al. Pentoxifylline attenuates transforming growth factor-beta1-stimulated collagen deposition and elastogenesis in human tunica albuginea-derived fibroblasts part 1: impact on extracellular matrix. J Sex Med 2010;7:2077-85. [Crossref] [PubMed]
- Lin G, Shindel AW, Banie L, et al. Pentoxifylline attenuates transforming growth factor-beta1-stimulated elastogenesis in human tunica albuginea-derived fibroblasts part 2: Interference in a TGF-beta1/Smad-dependent mechanism and downregulation of AAT1. J Sex Med 2010;7:1787-97. [Crossref] [PubMed]
- Smith JF, Shindel AW, Huang YC, et al. Pentoxifylline treatment and penile calcifications in men with Peyronie's disease. Asian J Androl 2011;13:322-5. [Crossref] [PubMed]
- Alizadeh M, Karimi F, Fallah MR. Evaluation of verapamil efficacy in Peyronie's disease comparing with pentoxifylline. Glob J Health Sci 2014;6:23-30. [Crossref] [PubMed]
- Paulis G, Barletta D, Turchi P, et al. Efficacy and safety evaluation of pentoxifylline associated with other antioxidants in medical treatment of Peyronie's disease: a case-control study. Res Rep Urol 2015;8:1-10. [Crossref] [PubMed]
- Zarafonetis CJ, Horrax TM. Treatment of Peyronie's disease with potassium para-aminobenzoate (potaba). J Urol 1959;81:770-2. [Crossref] [PubMed]
- Carson CC. Potassium para-aminobenzoate for the treatment of Peyronie's disease: is it effective? Tech Urol 1997;3:135-9. [PubMed]
- Weidner W, Hauck EW, Schnitker J. Potassium paraaminobenzoate (POTABA) in the treatment of Peyronie's disease: a prospective, placebo-controlled, randomized study. Eur Urol 2005;47:530-5; discussion 535-6. [Crossref] [PubMed]
- Park TY, Jeong HG, Park JJ, et al. The Efficacy of Medical Treatment of Peyronie's Disease: Potassium Para-Aminobenzoate Monotherapy vs. Combination Therapy with Tamoxifen, L-Carnitine, and Phosphodiesterase Type 5 Inhibitor. World J Mens Health 2016;34:40-6. [Crossref] [PubMed]
- Al Attar L, Kilgore W. Rare Incidence of Acute Liver Injury with Potassium Para-Aminobenzoate Introduction. Case Rep Gastroenterol 2018;12:230-3. [Crossref] [PubMed]
- Dominguez-Malagon HR, Alfeiran-Ruiz A, Chavarria-Xicotencatl P, et al. Clinical and cellular effects of colchicine in fibromatosis. Cancer 1992;69:2478-83. [Crossref] [PubMed]
- El-Sakka AI, Bakircioglu ME, Bhatnagar RS, et al. The effects of colchicine on a Peyronie's-like condition in an animal model. J Urol 1999;161:1980-3. [Crossref] [PubMed]
- Akkus E, Carrier S, Rehman J, et al. Is colchicine effective in Peyronie's disease? A pilot study. Urology 1994;44:291-5. [Crossref] [PubMed]
- Kadioglu A, Tefekli A, Koksal T, et al. Treatment of Peyronie's disease with oral colchicine: long-term results and predictive parameters of successful outcome. Int J Impot Res 2000;12:169-75. [Crossref] [PubMed]
- Prieto Castro RM, Leva Vallejo ME, Regueiro Lopez JC, et al. Combined treatment with vitamin E and colchicine in the early stages of Peyronie's disease. BJU Int 2003;91:522-4. [Crossref] [PubMed]
- Safarinejad MR. Therapeutic effects of colchicine in the management of Peyronie's disease: a randomized double-blind, placebo-controlled study. Int J Impot Res 2004;16:238-43. [Crossref] [PubMed]
- Cocco G, Chu DC, Pandolfi S. Colchicine in clinical medicine. A guide for internists. Eur J Intern Med 2010;21:503-8. [Crossref] [PubMed]
- Carthy JM, Sundqvist A, Heldin A, et al. Tamoxifen Inhibits TGF-beta-Mediated Activation of Myofibroblasts by Blocking Non-Smad Signaling Through ERK1/2. J Cell Physiol 2015;230:3084-92. [Crossref] [PubMed]
- Ralph DJ, Brooks MD, Bottazzo GF, et al. The treatment of Peyronie's disease with tamoxifen. Br J Urol 1992;70:648-51. [Crossref] [PubMed]
- Teloken C, Rhoden EL, Grazziotin TM, et al. Tamoxifen versus placebo in the treatment of Peyronie's disease. J Urol 1999;162:2003-5. [Crossref] [PubMed]
- Ilg MM, Mateus M, Stebbeds WJ, et al. Antifibrotic Synergy Between Phosphodiesterase Type 5 Inhibitors and Selective Oestrogen Receptor Modulators in Peyronie's Disease Models. Eur Urol 2019;75:329-40. [Crossref] [PubMed]
- Goldstein I, Burnett AL, Rosen RC, et al. The Serendipitous Story of Sildenafil: An Unexpected Oral Therapy for Erectile Dysfunction. Sex Med Rev 2019;7:115-28. [Crossref] [PubMed]
- Levine LA, Latchamsetty KC. Treatment of erectile dysfunction in patients with Peyronie's disease using sildenafil citrate. Int J Impot Res 2002;14:478-82. [Crossref] [PubMed]
- Gonzalez-Cadavid NF, Rajfer J. Treatment of Peyronie's disease with PDE5 inhibitors: an antifibrotic strategy. Nat Rev Urol 2010;7:215-21. [Crossref] [PubMed]
- Ferrini MG, Kovanecz I, Nolazco G, et al. Effects of long-term vardenafil treatment on the development of fibrotic plaques in a rat model of Peyronie's disease. BJU Int 2006;97:625-33. [Crossref] [PubMed]
- Chung E, Deyoung L, Brock GB. The role of PDE5 inhibitors in penile septal scar remodeling: assessment of clinical and radiological outcomes. J Sex Med 2011;8:1472-7. [Crossref] [PubMed]
- Palmieri A, Imbimbo C, Creta M, et al. Tadalafil once daily and extracorporeal shock wave therapy in the management of patients with Peyronie's disease and erectile dysfunction: results from a prospective randomized trial. Int J Androl 2012;35:190-5. [Crossref] [PubMed]
- Cocci A, Cito G, Urzi D, et al. Sildenafil 25 mg ODT + Collagenase Clostridium hystoliticum vs Collagenase Clostridium hystoliticum Alone for the Management of Peyronie's Disease: A Matched-Pair Comparison Analysis. J Sex Med 2018;15:1472-7. [Crossref] [PubMed]
- Li Z, Jin Z. Comparative effect and safety of verapamil in keloid and hypertrophic scar treatment: a meta-analysis. Ther Clin Risk Manag 2016;12:1635-41. [Crossref] [PubMed]
- Lee RC, Ping JA. Calcium antagonists retard extracellular matrix production in connective tissue equivalent. J Surg Res 1990;49:463-6. [Crossref] [PubMed]
- Aggeler J, Frisch SM, Werb Z. Changes in cell shape correlate with collagenase gene expression in rabbit synovial fibroblasts. J Cell Biol 1984;98:1662-71. [Crossref] [PubMed]
- Fitch WP 3rd, Easterling WJ, Talbert RL, et al. Topical verapamil HCl, topical trifluoperazine, and topical magnesium sulfate for the treatment of Peyronie's disease--a placebo-controlled pilot study. J Sex Med 2007;4:477-84. [Crossref] [PubMed]
- Martin DJ, Badwan K, Parker M, et al. Transdermal application of verapamil gel to the penile shaft fails to infiltrate the tunica albuginea. J Urol 2002;168:2483-5. [Crossref] [PubMed]
- Kos B, Vasquez JL, Miklavcic D, et al. Investigation of the mechanisms of action behind Electromotive Drug Administration (EMDA). PeerJ 2016;4:e2309. [Crossref] [PubMed]
- Greenfield JM, Shah SJ, Levine LA. Verapamil versus saline in electromotive drug administration for Peyronie's disease: a double-blind, placebo controlled trial. J Urol 2007;177:972-5. [Crossref] [PubMed]
- Di Stasi SM, Giannantoni A, Stephen RL, et al. A prospective, randomized study using transdermal electromotive administration of verapamil and dexamethasone for Peyronie's disease. J Urol 2004;171:1605-8. [Crossref] [PubMed]
- Mehrsai AR, Namdari F, Salavati A, et al. Comparison of transdermal electromotive administration of verapamil and dexamethasone versus intra-lesional injection for Peyronie's disease. Andrology 2013;1:129-32. [Crossref] [PubMed]
- Jeengar MK, Rompicharla SV, Shrivastava S, et al. Emu oil based nano-emulgel for topical delivery of curcumin. Int J Pharm 2016;506:222-36. [Crossref] [PubMed]
- Twidwell J, Levine L. Topical treatment for acute phase Peyronie's disease utilizing a new gel, H-100: a randomized, prospective, placebo-controlled pilot study. Int J Impot Res 2016;28:41-5. [Crossref] [PubMed]
- Duncan MR, Berman B, Nseyo UO. Regulation of the proliferation and biosynthetic activities of cultured human Peyronie's disease fibroblasts by interferons-alpha, -beta and -gamma. Scand J Urol Nephrol 1991;25:89-94. [Crossref] [PubMed]
- Kendirci M, Usta MF, Matern RV, et al. The impact of intralesional interferon alpha-2b injection therapy on penile hemodynamics in men with Peyronie's disease. J Sex Med 2005;2:709-15. [Crossref] [PubMed]
- Hellstrom WJ, Kendirci M, Matern R, et al. Single-blind, multicenter, placebo controlled, parallel study to assess the safety and efficacy of intralesional interferon alpha-2B for minimally invasive treatment for Peyronie's disease. J Urol 2006;176:394-8. [Crossref] [PubMed]
- Trost LW, Ates E, Powers M, et al. Outcomes of intralesional interferon-alpha2B for the treatment of Peyronie disease. J Urol 2013;190:2194-9. [Crossref] [PubMed]
- Stewart CA, Yafi FA, Knoedler M, et al. Intralesional Injection of Interferon-alpha2b Improves Penile Curvature in Men with Peyronie's Disease Independent of Plaque Location. J Urol 2015;194:1704-7. [Crossref] [PubMed]
- Yafi FA, Hatzichristodoulou G, Knoedler CJ, et al. Comparative Analysis of Tunical Plication vs. Intralesional Injection Therapy for Ventral Peyronie's Disease. J Sex Med 2015;12:2492-8. [Crossref] [PubMed]
- Chung E, Garcia F, Young LD, et al. A comparative study of the efficacy of intralesional verapamil versus normal saline injection in a novel Peyronie disease animal model: assessment of immunohistopathological changes and erectile function outcome. J Urol 2013;189:380-4. [Crossref] [PubMed]
- Levine LA, Merrick PF, Lee RC. Intralesional verapamil injection for the treatment of Peyronie's disease. J Urol 1994;151:1522-4. [Crossref] [PubMed]
- Levine LA. Treatment of Peyronie's disease with intralesional verapamil injection. J Urol 1997;158:1395-9. [Crossref] [PubMed]
- Levine LA, Goldman KE, Greenfield JM. Experience with intraplaque injection of verapamil for Peyronie's disease. J Urol 2002;168:621-5; discussion 625-6. [Crossref] [PubMed]
- Cavallini G, Modenini F, Vitali G. Open preliminary randomized prospective clinical trial of efficacy and safety of three different verapamil dilutions for intraplaque therapy of Peyronie's disease. Urology 2007;69:950-4. [Crossref] [PubMed]
- Rehman J, Benet A, Melman A. Use of intralesional verapamil to dissolve Peyronie's disease plaque: a long-term single-blind study. Urology 1998;51:620-6. [Crossref] [PubMed]
- Bennett NE, Guhring P, Mulhall JP. Intralesional verapamil prevents the progression of Peyronie's disease. Urology 2007;69:1181-4. [Crossref] [PubMed]
- Shirazi M, Haghpanah AR, Badiee M, et al. Effect of intralesional verapamil for treatment of Peyronie's disease: a randomized single-blind, placebo-controlled study. Int Urol Nephrol 2009;41:467-71. [Crossref] [PubMed]
- Gelbard MK, Walsh R, Kaufman JJ. Collagenase for Peyronie's disease experimental studies. Urol Res 1982;10:135-40. [Crossref] [PubMed]
- Gelbard MK, Lindner A, Kaufman JJ. The use of collagenase in the treatment of Peyronie's disease. J Urol 1985;134:280-3. [Crossref] [PubMed]
- Gelbard M, Goldstein I, Hellstrom WJ, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol 2013;190:199-207. [Crossref] [PubMed]
- Yang KK, Bennett N. The History of Collagenase Clostridium Histolyticum. Sex Med Rev 2015;3:289-97. [Crossref] [PubMed]
- Ziegelmann MJ, Viers BR, McAlvany KL, et al. Restoration of Penile Function and Patient Satisfaction with Intralesional Collagenase Clostridium Histolyticum Injection for Peyronie's Disease. J Urol 2016;195:1051-6. [Crossref] [PubMed]
- Yang KK, Bennett N. Peyronie's Disease and Injectable Collagenase Clostridium histolyticum: Safety, Efficacy, and Improvements in Subjective Symptoms. Urology 2016;94:143-7. [Crossref] [PubMed]
- Hellstrom WJG, Tue Nguyen HM, Alzweri L, et al. Intralesional Collagenase Clostridium histolyticum Causes Meaningful Improvement in Men with Peyronie's Disease: Results of a Multi-Institutional Analysis. J Urol 2019;201:777-82. [Crossref] [PubMed]
- Tsambarlis PN, Yong R, Levine LA. Limited success with clostridium collagenase histolyticum following FDA approval for the treatment of Peyronie's disease. Int J Impot Res 2019;31:15-9. [Crossref] [PubMed]
- Tsambarlis P, Levine LA. Nonsurgical management of Peyronie's disease. Nat Rev Urol 2019;16:172-86. [Crossref] [PubMed]
- Yafi FA, Anaissie J, Zurawin J, et al. Results of SMSNA Survey Regarding Complications Following Intralesional Injection Therapy With Collagenase Clostridium Histolyticum for Peyronie's Disease. J Sex Med 2016;13:684-9. [Crossref] [PubMed]
- Cordon BH, Hofer MD, Hutchinson RC, et al. Superior Cost Effectiveness of Penile Plication vs Intralesional Collagenase Injection for Treatment of Peyronie’s Disease Deformities. Urol Pract 2017;4:118-25. [Crossref]
- Abdel Raheem A, Capece M, Kalejaiye O, et al. Safety and effectiveness of collagenase clostridium histolyticum in the treatment of Peyronie's disease using a new modified shortened protocol. BJU Int 2017;120:717-23. [Crossref] [PubMed]
- Nguyen HMT, Anaissie J, DeLay KJ, et al. Safety and Efficacy of Collagenase Clostridium histolyticum in the Treatment of Acute-Phase Peyronie's Disease. J Sex Med 2017;14:1220-5. [Crossref] [PubMed]
- Lipshultz LI, Goldstein I, Seftel AD, et al. Clinical efficacy of collagenase Clostridium histolyticum in the treatment of Peyronie's disease by subgroup: results from two large, double-blind, randomized, placebo-controlled, phase III studies. BJU Int 2015;116:650-6. [Crossref] [PubMed]
- Trott KR, Kamprad F. Radiobiological mechanisms of anti-inflammatory radiotherapy. Radiother Oncol 1999;51:197-203. [Crossref] [PubMed]
- Nolan MW, Marolf AJ, Ehrhart EJ, et al. Pudendal nerve and internal pudendal artery damage may contribute to radiation-induced erectile dysfunction. Int J Radiat Oncol Biol Phys 2015;91:796-806. [Crossref] [PubMed]
- Mulhall JP, Hall M, Broderick GA, et al. Radiation therapy in Peyronie's disease. J Sex Med 2012;9:1435-41. [Crossref] [PubMed]
- Hatzimouratidis K, Eardley I, Giuliano F, et al. EAU guidelines on penile curvature. Eur Urol 2012;62:543-52. [Crossref] [PubMed]
- Manikandan R, Islam W, Srinivasan V, et al. Evaluation of extracorporeal shock wave therapy in Peyronie's disease. Urology 2002;60:795-9; discussion 799-800. [Crossref] [PubMed]
- Hauck EW, Hauptmann A, Bschleipfer T, et al. Questionable efficacy of extracorporeal shock wave therapy for Peyronie's disease: results of a prospective approach. J Urol 2004;171:296-9. [Crossref] [PubMed]
- Shimpi RK, Jain RJ. Role of extracorporeal shock wave therapy in management of Peyronie's disease: A preliminary report. Urol Ann 2016;8:409-17. [Crossref] [PubMed]
- Li PC, Chen X, Zhu XB, et al. Low-intensity extracorporeal shockwave therapy for Peyronie's disease: A preliminary study of 32 cases. Zhonghua Nan Ke Xue 2018;24:340-4. [PubMed]
- Michel MS, Ptaschnyk T, Musial A, et al. Objective and subjective changes in patients with Peyronie's disease after management with shockwave therapy. J Endourol 2003;17:41-4; discussion 44. [Crossref] [PubMed]
- Hamm R, McLarty E, Ashdown J, et al. Peyronie's disease-the Plymouth experience of extracorporeal shockwave treatment. BJU Int 2001;87:849-52. [Crossref] [PubMed]
- Palmieri A, Imbimbo C, Longo N, et al. A first prospective, randomized, double-blind, placebo-controlled clinical trial evaluating extracorporeal shock wave therapy for the treatment of Peyronie's disease. Eur Urol 2009;56:363-9. [Crossref] [PubMed]
- Hatzichristodoulou G, Meisner C, Gschwend JE, et al. Extracorporeal shock wave therapy in Peyronie's disease: results of a placebo-controlled, prospective, randomized, single-blind study. J Sex Med 2013;10:2815-21. [Crossref] [PubMed]
- Chitale S, Morsey M, Swift L, et al. Limited shock wave therapy vs sham treatment in men with Peyronie's disease: results of a prospective randomized controlled double-blind trial. BJU Int 2010;106:1352-6. [Crossref] [PubMed]
- Yuan J, Lin H, Li P, et al. Molecular mechanisms of vacuum therapy in penile rehabilitation: a novel animal study. Eur Urol 2010;58:773-80. [Crossref] [PubMed]
- Lin H, Liu C, Wang R. Effect of Penile Traction and Vacuum Erectile Device for Peyronie's Disease in an Animal Model. J Sex Med 2017;14:1270-6. [Crossref] [PubMed]
- Raheem AA, Garaffa G, Raheem TA, et al. The role of vacuum pump therapy to mechanically straighten the penis in Peyronie's disease. BJU Int 2010;106:1178-80. [Crossref] [PubMed]
- Köhler TS, Pedro R, Hendlin K, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int 2007;100:858-62. [Crossref] [PubMed]
- Canguven O, Talib RA, Campbell J, et al. Is the daily use of vacuum erection device for a month before penile prosthesis implantation beneficial? a randomized controlled trial. Andrology 2017;5:103-6. [Crossref] [PubMed]
- Chung E, De Young L, Solomon M, et al. Peyronie's disease and mechanotransduction: an in vitro analysis of the cellular changes to Peyronie's disease in a cell-culture strain system. J Sex Med 2013;10:1259-67. [Crossref] [PubMed]
- Levine LA, Newell M, Taylor FL. Penile traction therapy for treatment of Peyronie's disease: a single-center pilot study. J Sex Med 2008;5:1468-73. [Crossref] [PubMed]
- Martínez-Salamanca JI, Egui A, Moncada I, et al. Acute phase Peyronie's disease management with traction device: a nonrandomized prospective controlled trial with ultrasound correlation. J Sex Med 2014;11:506-15. [Crossref] [PubMed]
- Moncada I, Krishnappa P, Romero J, et al. Penile traction therapy with the new device 'Penimaster PRO' is effective and safe in the stable phase of Peyronie's disease: a controlled multicentre study. BJU Int 2019;123:694-702. [Crossref] [PubMed]
- Ziegelmann M, Savage J, Toussi A, et al. Outcomes of a Novel Penile Traction Device in Men with Peyronie's Disease: A Randomized, Single-Blind, Controlled Trial. J Urol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Yafi FA, Pinsky MR, Stewart C, et al. The Effect of Duration of Penile Traction Therapy in Patients Undergoing Intralesional Injection Therapy for Peyronie's Disease. J Urol 2015;194:754-8. [Crossref] [PubMed]
- Ziegelmann MJ, Viers BR, Montgomery BD, et al. Clinical Experience With Penile Traction Therapy Among Men Undergoing Collagenase Clostridium histolyticum for Peyronie Disease. Urology 2017;104:102-9. [Crossref] [PubMed]
- Ralph DJ, Abdel Raheem A, Liu G. Treatment of Peyronie's Disease With Collagenase Clostridium histolyticum and Vacuum Therapy: A Randomized, Open-Label Pilot Study. J Sex Med 2017;14:1430-7. [Crossref] [PubMed]
- Castiglione F, Hedlund P, Van der Aa F, et al. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie's disease. Eur Urol 2013;63:551-60. [Crossref] [PubMed]
- Gokce A, Abd Elmageed ZY, Lasker GF, et al. Intratunical Injection of Genetically Modified Adipose Tissue-Derived Stem Cells with Human Interferon alpha-2b for Treatment of Erectile Dysfunction in a Rat Model of Tunica Albugineal Fibrosis. J Sex Med 2015;12:1533-44. [Crossref] [PubMed]


