
Fertility considerations in men with testicular cancer
Introduction
Testicular cancer is the most common malignancy in young males and carries a 1 in 250 lifetime risk (1). The peak age of presentation is 25–29 years, a period which directly coincides with the prime age for men interested in conceiving children. Approximately 250,000 testicular cancer survivors currently live in the United States (1). With the significant improvements in treatment modalities for this now highly-curable disease, men who may once have succumbed to the disease can now go on to live long healthy lives with only small decreases in life expectancy (2). Testicular cancer can exert profound detrimental effects on the reproductive health of men. More than half of men with testicular cancer initially present with oligospermia prior to any treatment (3), and only 48% of men receiving cisplatin-based chemotherapy will ultimately successfully father a child (compared with over 90% in the post-orchiectomy surveillance group) (4). Taken as a cohort, 22% of men with a history of testicular cancer who desire a child will require assisted reproductive technology (ART) (4). Despite these well-documented fertility consequences, less than 50% of oncology providers regularly counsel men on fertility preservation prior to initiating treatment (5). Those who do bank prior to initiating treatment demonstrate significantly impaired spermatogenesis with depressed concentration and sperm counts (6). This review will discuss current literature regarding the pathophysiology, diagnosis, and management of fertility related to testicular cancer both before and after treatment.
Mechanism of infertility in testicular cancer
It is now well-documented that men with many types of cancer have pre-treatment impairment of male reproductive health in a variety of ways (7). Testicular cancer has been shown to be the most detrimental of all common malignancies in terms of sperm concentration and total sperm count pre-treatment (3). The mechanistic underpinnings of infertility in testis cancer prior to any treatment toxicity is likely multifactorial in nature. Clearly some of this sub-fertility can be attributed to direct parenchymal damage and replacement by tumor. Other mechanisms underlying these changes are likely both correlative and causative in nature. Correlative etiologies include cryptorchidism, which is associated with increased risk for testicular cancer and—at least in the setting of bilateral cryptorchidism—with infertility (8). While the existence of the syndrome remains controversial, the hypothesized “Testicular Dysgenesis Syndrome” does represent a theoretical entity linking testicular cancer and infertility (9). On the other hand, causative mechanisms linking infertility to testicular cancer vary widely and likely represent the complex physiological process of spermatogenesis. The hypothalamic-pituitary-gonadal (HPG) axis directly controls testicular function, and elevated serum alpha fetoprotein (AFP) or beta human chorionic gonadotropic (beta‐HCG) can interfere with the physiologic feedback mechanism of this axis (10). A disruption in luteinizing hormone (LH), follicle stimulating hormone (FSH) and testosterone levels correlates with spermatogenesis and decreased sperm concentration (11). Testis cancer and metastatic testicular cancer is also associated with elevated inflammatory markers and systemic inflammation, which can significantly impair spermatogenesis (12). Elevated temperature, which can be seen in fever associated with metastatic malignancy or chemotherapy, directly impairs spermatogenesis (13). Disruption of the blood-testis barrier and formation of antisperm antibodies has been associated with testicular cancer (14), though contemporary evidence refutes this to a degree (15). In addition, testicular cancer is associated with elevated oxidative stress and DNA fragmentation, both of which may contribute to reduced fertility (16-18). When combined with the additional toxicity associated with further treatment (particularly chemotherapy), it is clear that men with testicular cancer require aggressive and time-sensitive management to maintain fertility if desired.
Strategies for fertility preservation prior to treatment
Regardless of the underlying etiology of cancer-related infertility, men diagnosed with testicular cancer who desire future fertility should be offered fertility preservation. Cryopreservation of sperm is the primary method of fertility preservation in most post-pubertal male patients. A recent report by Gilbert et al. conclusively demonstrated the cost-effectiveness and efficacy of cryopreservation, which was both the least expensive and also the most efficacious technique of sperm management (19). Cryopreservation has been shown to be robust and reliable and is now widely available (20).
Collection methods vary depending on the specific population being addressed. In most neurologically-intact postpubertal young men, masturbation represents the most simplistic and low-cost approach. Some men, however, are unable to spontaneously provide a sample, and more invasive means should be offered (21). If retrograde ejaculation is present due to alpha blockade [e.g., prazosin in patients with post-traumatic stress disorder (PTSD) or post-retroperitoneal lymph node dissection (RPLND)], a postejaculatory alkalinized urine specimen typically provides sufficient sperm for cryopreservation. In men with spinal cord injuries or young patients unable to provide a specimen via masturbation, penile vibratory stimulation (PVS) with optimized amplitude and frequency represents a minimally invasive approach with little risk (21). This has been used successfully in case reports for young men with newly diagnosed malignancy (22). This method, however, relies on an intact ejaculatory reflex.
In men with impaired reflex or psychosocial difficulty with masturbation, electroejaculation represents a viable approach. The process involves placement of a rectal probe under anesthesia (typically general anesthesia in neurologically intact patients) and the pelvic floor directly stimulated to achieve emission (21). The process has been validated in the young male cancer population with a 60–70% success rate (23,24). Interestingly this approach is also viable in specific uncommon situations where the patient has a religious or personal objection to masturbation (24). Care must be taken, however, to rule out concomitant rectal lesions as this is a contraindication to the procedure. In cases where nonoperative management fails or is not feasible, surgical sperm retrieval may also be used. Percutaneous aspiration of the epididymis (PESA) or the testicular sperm aspiration (TESA) commonly results in sperm retrieval rates and quantities sufficient for intracytoplasmic sperm injection (ICSI) but not intrauterine insemination (IUI) (25). Open testicular sperm extraction (TESE) can also be performed in select cases using local or general anesthetic.
Despite prompt diagnosis and referral, some men presenting with testicular cancer will have nonobstructive azoospermia (NOA) at the time of diagnosis, rendering noninvasive approaches to sperm retrieval ineffective. Moody et al. established that nearly half of men with significant volume of testicular cancer still harbor islands of spermatogenesis (26), and at least some men with NOA will actually recover spermatogenesis following treatment (even if cytotoxic in nature) (27). In this cohort, concomitant surgical TESE at the time of orchiectomy (termed onco-TESE) is an option if up-front fertility preservation is desired. This technique was first described by Schrader and colleagues (28) with successes in both the ipsilateral (remote to the cancerous lesion) and contralateral testicles. Benefits of this approach include the added benefit of sperm retrieval prior to chemotherapy initiation with very limited additional morbidity. Contemporary series place success rates approaching 70% (29). Further work has described a microscopic approach (30), successful onco-TESE in patients with bilateral testicular tumors (31), and successful paternity following onco-TESE (32).
Prepubertal fertility preservation
Cryopreservation of sperm from the ejaculate is not possible in the pre-pubertal male patient population. In this population, spermatogonial stem cell cryopreservation via testicular tissue extraction may offer a solution, albeit experimental at present. The procedure, first proposed over 20 years ago, involves harvest of these cells prior to initiation of chemotherapy with subsequent delayed autotransplantation in hopes of restoring spermatogenesis (33). Tempering enthusiasm for this, however, is a recent report that testicular tissue harvested from prepubertal boys with cancer has significantly fewer spermatogonia than healthy controls and may limit future autotransplantation (34). Given that this approach would require two invasive procedures in young boys with cancer who have minimal medical literacy and unclear future paternity interest, the ethics of this approach must also be carefully considered. This topic was recently been discussed in depth and the approach was found to be ethically justifiable in most cases (35). Due to the invasive and experimental nature of the procedure, however, this should be determined in a case-by-case basis with assistance from bioethics where needed and available. Despite over two decades of work, no successes have yet been reported, and any robust adoption of this technique is likely still decades from being clinically viable (36).
Barriers to fertility preservation and the development of oncofertility programs
Unfortunately, fertility preservation is offered to only approximately 50% of patients with a new cancer diagnosis. Female providers appear to be more than twice as likely to refer patients for fertility preservation (37). Despite increasing efforts to ensure patients are offered timely fertility preservation, over 70% of men choose to forego banking (38). The reasons provided by cancer patients included cost and the difficulty in decision making overshadowed by the initial shock associated with receiving the cancer diagnosis (38). In response to these challenges, several centers have developed specialized oncofertility programs designed to standardize the fertility preservation process (39-41). These programs include numerous interventions such as increased access and awareness of fertility preservation services, built in prompts within the electronic medical record to alert providers to consider referral, establishment of a hotline for interested patients, implementation of a standardized care pathway to counsel patients and obtain sperm banking, and subsequent follow up care to support future paternity. While more mature data is necessary to prove an increase in paternity and/or patient satisfaction, these programs appear to carry significant potential benefit with minimal downside.
Reproductive toxicities of testicular cancer treatment
Advances in surgery, chemotherapy, and radiotherapy have led to improved quality of life and treatment outcomes in men with testicular cancer (42). The American Society for Reproductive Medicine (ASRM) and American Society of Clinical Oncology (ASCO) have clinical guidelines recommending fertility counseling and preservation to all patients requiring cytotoxic cancer treatment. Unfortunately, these recommendations are not universally adhered to and a critical understanding of the current treatment modalities and their side effects is of utmost importance (10,43).
Orchiectomy
Radical inguinal orchiectomy is the mainstay of treatment for a suspected testicular neoplasm. This procedure requires an inguinal incision to remove the testicle and spermatic cord at the level of the internal inguinal ring. Several studies have demonstrated the impact of radical orchiectomy on semen parameters and hormonal functions (44-46). In patients undergoing radical orchiectomy for malignancy, Petersen and colleagues identified a reduction in sperm concentration, total sperm count, and serum inhibin B levels (44). Serum human luteinizing hormone (hLH) and human follicle stimulating hormone (hFSH) levels were elevated, but there was no significant difference in serum testosterone levels (44). Furthermore, even with the presence of an apparently normal contralateral testis, semen parameters are impaired in up to 85% of patients with an additional 9% of men developing azoospermia after unilateral orchiectomy (44,47). The ASRM advocates counseling patients on obtaining an ejaculated semen specimen prior to orchiectomy or extracting sperm from the testis during orchiectomy (48), as this may be the only opportunity to find viable sperm. However, emerging evidence and National Comprehensive Cancer Network (NCCN) guidelines support sperm cryopreservation before or after radical orchiectomy. While some patients and clinicians may prefer not to delay orchiectomy for the purposes of cryopreservation, patients should have a thorough understanding of their options and risks (49,50).
The concept of partial orchiectomy or testis sparing surgery has gained increasing traction in certain scenarios given the potential sequela of radical orchiectomy. In testicular cancer patients, radical orchiectomy is associated with lifelong androgen replacement, infertility, and emotional distress (51,52). Partial orchiectomy for the treatment of a variety of tumor types including prepubertal teratoma, epidermoid cyst, adrenal rest tumors, Leydig and Sertoli cell tumors have been reported in the literature (53,54) Recently, partial orchiectomy has also been used to treat select patients with malignant germ cell tumors. The European guidelines (52) cite partial orchiectomy as a surgical alternative for patients with bilateral testicular neoplasms or neoplasm in a solitary testicle. Careful adherence to oncologically sound principles, close follow-up and compliant patient selection is essential. Therefore, in carefully selected patients and in experienced hands, partial orchiectomy is a burgeoning technique to preserve functional testicular tissue and potentially fertility (55).
Chemotherapy
While chemotherapy has well-recognized gonadotoxic effects, the impact of the various chemotherapeutic drugs or regimens on fertility and spermatogenesis in men with testicular cancer is not completely elucidated. Drug dosing, combination regimens and treatment duration affect sperm parameters and paternity (56,57). However, identifying which testicular cancer patients are most vulnerable to the long-term sequela of chemotherapy is complicated by a lack of strong evidence. This is in part due to inconsistent follow-up of semen analyses and birth-rates and the combinations of drug regimens instituted (58). The most common chemotherapy regimen used for testis cancer is bleomycin, etoposide, and cisplatin (BEP) (59).
Platinum-based chemotherapy agents, which includes cisplatin and carboplatin, are important components of testicular cancer management and pose an intermediate level of risk for permanent azoospermia. The mechanism of action of these analogs is formation of DNA cross-linking. A meta-analysis of testicular cancer patients with normal pretreatment sperm concentration who received platinum-based chemotherapy demonstrated that 48% and 80% recovered spermatogenesis by 2 and 5 years, respectively (60). Patients exposed to carboplatin fared better than those exposed to cisplatin (60).
Alkylating agents, particularly cyclophosphamide and ifosfamide, pose the highest risk for permanent azoospermia. The mechanism of action for these agents is disruption of DNA synthesis and RNA transcription in neoplastic cells. The rapidly dividing spermatocytes in the testicular germinal epithelium are sensitive to alkylating agents and spermatogenesis is frequently affected (57). Several studies of survivors of childhood cancers demonstrate that azoospermia is persistent in 25% of men 5 years after treatment with alkylating agents (61,62).
Vinca alkaloids, such as vinblastine, disrupt mitosis by inhibiting microtubule formation. Vinca alkaloids typically result in temporary azoospermia or oligozoospermia. However, when combined with alkylating agents or platinum analogs, long-term or permanent impairment in spermatogenesis can occur (63,64). Unfortunately, long-term data on many of the other drug classes is limited (Table 1).
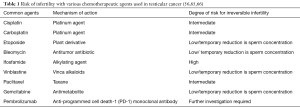
Full table
In a review of 129 patients who received BEP for testicular cancer, spermatogenesis returned 12 months post treatment if two or fewer cycles of BEP was administered. For patients who received three or four courses of BEP or radiotherapy, spermatogenesis returned at 24 months (67). Although survival rates of testicular cancer patients are excellent, continued improvements in risk-adapted treatment protocols aim to improve efficacy while reducing gonadotoxicity. Additionally, improved communication with patients and families will allow for more informed decision making and facilitate identifying barriers to fertility preservation.
Radiotherapy
Radiation therapy (XRT) is a commonly utilized treatment modality for testicular cancer or retroperitoneal metastases. Although the radiosensitive testicles are typically protected by gonadal shielding during XRT, they are still subject to the gonadotoxic effects of scatter radiation (68). With appropriate gonadal shielding, recent data suggests that scatter radiation doses can be as low as 0.28% of the treatment dose, thereby protecting fertility (69). Testicular function is typically affected by XRT in a dose dependent manner. However, hormonal function may also be disrupted by the effect of cranial XRT on the HPG axis (70).
Semen parameters (concentration and morphology) are impacted by radiation doses as low as 0.1 Gy (71,72). Radiation doses greater than 4 Gy can cause permanent germ cell damage. In men with germ cell neoplasia in situ, radiation doses of 16–20 Gy are commonly administered, resulting in an expectantly high rate of irreversible azoospermia. Fractionated dosing protocols are frequently utilized, but low incremental dosing of radiation increases exposure to radiation scatter and portends a higher risk of gonadotoxicity compared to single, equivalent doses (73).
Complete recovery of spermatogenesis is possible but is dependent on the radiation dose. It may take up to 18 months after radiation doses of less than 1 Gy, 30 months after 2–3 Gy, and more than 5 years for higher than 4 Gy to recover sperm function (72). Compared to the germinal epithelium, Leydig cells are more resistant to the negative impact of XRT. Leydig cells require radiation doses greater than 20–30 Gy in order to impair their function and cause primary hypogonadism (72,74). However, testosterone replacement therapy is ultimately required in 15% to 25% of patients and they should be monitored after radiotherapy for adequate testicular androgen production (75,76). Finally, clinicians should adhere to surveillance protocols after orchiectomy in an attempt to limit the use of unnecessary ionizing radiation (chest X-ray or computed tomography) in reproductive aged men (76).
Retroperitoneal pelvic lymph node dissection (RPLND)
RPLND is performed as a primary or salvage treatment modality for testicular cancer and confers additional risk of infertility. It is imperative that clinicians inform patients of the reproductive implications preoperatively. Men who undergo RPLND are at risk for anejaculation or retrograde ejaculation due to disruption of the retroperitoneal sympathetic nerves or the hypogastric plexus responsible for emission and ejaculation. The incidence of ejaculatory dysfunction has substantially diminished secondary to the utilization of nerve-sparing techniques when oncologically amenable. Utilizing modern primary and post-chemotherapy RPLND techniques and templates, less than 10% of patients experience significant ejaculatory complications (77-79). Non-nerve sparing RPLND has been shown to have a more significant impact on fertility rates in testis cancer survivors. Matos et al. compared men who underwent nerve-sparing and non-nerve sparing RPLND, the authors determined fertility rates to be 62% and 37% respectively (78). This highlights the importance of careful discussion with patients regarding potential complications of surgery and fertility preservation options.
Sexual dysfunction in survivors of testicular cancer
Men with a history of testicular cancer are more likely to experience symptoms of sexual dysfunction (erectile dysfunction, decreased libido, poor body image) when compared to men of the general population (80). The etiology of sexual dysfunction in this population is likely multi-factorial with psychogenic causes playing a primary role. Erectile dysfunction affects roughly between 12% and 40% of testicular cancer survivors, independent of treatment modality (80,81). Pühse and colleagues (82) assessed 539 men who underwent testicular cancer treatment and analyzed various causes of sexual dysfunction. The authors determined that 42% of testicular cancer survivors had decreased sexual activity, 35% had decreased libido, and 32% had erectile dysfunction. Additionally, 85% of men reported a variety of ejaculatory complaints, including retrograde, anejaculation, and premature ejaculation. Overall, 95% of the study population endorsed decreased overall sexual satisfaction (82). Tal et al. studied 76 men who developed erectile dysfunction after testis cancer treatment (83). In men with non-seminomatous germ cell tumor (NSGCT); 79% received chemotherapy, 18% underwent primary RPLND and 20% underwent post-chemotherapy RPLND. In men with seminoma, 66% underwent XRT. In all, 84% of patients complained primarily of loss of erection-sustaining capability. Nearly a quarter of men cited mild erectile dysfunction prior to the diagnosis of testicular cancer. In order to differentiate between vasculogenic and psychogenic erectile dysfunction, penile duplex doppler ultrasonography was performed on the study population. Ultimately, all patients had normal hemodynamics on ultrasound 12 months after testis cancer treatment. The authors assert that erectile dysfunction after treatment for testicular cancer is unlikely vasculogenic but rather psychogenic in nature (83). Clinicians must be aware of sexual dysfunction in this patient population and address these symptoms with appropriate therapies.
Azoospermia after testicular cancer treatment
Advances in testicular cancer treatment modalities have significantly improved the life expectancy and quality of life of pediatric and young adult testicular cancer survivors. While many patients will have recovery of sperm after chemotherapy or radiotherapy, it is difficult to predict which patients will have a return of spermatogenesis (65,84,85). Microdissection TESE (mTESE) and subsequent ICSI has been established as the gold standard technique to treat patients with NOA who desire paternity. While donor sperm and adoption remain viable options, mTESE is the only option for persistently azoospermic men to conceive a biologic child.
Hsiao et al. performed mTESE in 73 post-chemotherapy patients with a surgical sperm retrieval rate of 37%. Of note, the average length of time after chemotherapy was 19 years for the study population, which may also play a role in sperm retrieval success. The authors demonstrated a live birth rate of 42% (20 healthy children). Sperm retrieval success was highest in the testicular cancer population, with a sperm retrieval rate of 85%. Patients treated for sarcoma had the lowest retrieval rate (14%), likely due to the use of alkylating chemotherapy agents (85). More recently, Shin et al. (86) demonstrated similar findings after performing mTESE in 66 men post-chemotherapy. The majority of the patients had a diagnosis of testicular cancer (21 patients) and sperm was successful retrieved in 47% (31 patients) of the study population with a live birth rate of 27%. Associated risks include pain, bleeding, infection, epididymal obstruction and testicular injury. Surgically retrieved sperm can only be used for in vitro fertilization or ICSI in order to achieve conception. These results suggest that mTESE is an effective treatment option for men with persistent azoospermia, but cryopreservation of sperm prior to gonadotoxic treatment is preferred (86).
Reproductive outcomes
Successful paternity has been achieved in testicular cancer patients independent of treatment received or decision to cryopreserve. A cross-sectional study of 680 testicular cancer survivors assessed the fecundity of patients post orchiectomy who underwent surveillance, chemotherapy, radiotherapy or chemotherapy plus radiotherapy. Of the men attempting conception after treatment, paternity was achieved naturally in 77% of men or with the use of ART in 23%. Overall, the success rate in the surveillance, chemotherapy and chemotherapy plus radiotherapy groups were 85%, 71% and 67% respectively (55).
While testicular cancer survivors may recover sperm production after undergoing various cancer treatments, the exact timing and return to baseline sperm parameters is inconsistent. Furthermore, the quality of sperm produced during chemotherapy or radiation is not certain (i.e., sperm aneuploidies, oxidative stress and DNA fragmentation), but these effects typically persist for 3 months after treatment (56). Most experts advocate a waiting period of 6 months to 2 years prior to attempting to conceive. Sperm quality may also be affected by the process of cryopreservation and thawing. Fortunately, there is no evidence to suggest utilizing cryopreserved sperm leads to adverse pregnancy outcomes or congenital malformations. However, recent evidence suggests that men with seminomatous germ cell tumor have poorer post thaw total motile sperm count (TMSC) when compared to men with NSGCT. Men with poorer histologic and prognostic features can be advised to sperm bank several times to improve future ART outcomes, but further research in cryopreservation techniques and the relationship to cancer stage is required (87).
Discussing fertility preservation strategies such as sperm cryopreservation prior to surgery, chemotherapy or radiotherapy is imperative. Sperm cryopreservation prior to undergoing chemotherapy or radiotherapy remains the most cost-effective strategy for fertility preservation when compared to mTESE and/or ART (19).
Conclusions
Fertility preservation in oncologic conditions, particularly testicular cancer, can be a complex process. While feasible for many patients, lack of medical knowledge, inadequate resources, cost and the emotional burden of a cancer diagnosis can further obscure the process of fertility preservation. Therefore, early referral for discussion of fertility preservation options is essential to facilitate optimal management. Providers should be familiar with the various fertility preservation options available for pre and post pubertal patients. Understanding of the success rates and limitations of each of the modalities is imperative in order for patients and families to make informed decisions about their future fertility.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71. [Crossref] [PubMed]
- Gilligan T. Testicular Cancer Survivorship. Hematol Oncol Clin North Am 2011;25:627-39. [Crossref] [PubMed]
- Williams DH, Karpman E, Sander JC, et al. Pretreatment Semen Parameters in Men with Cancer. J Urol 2009;181:736-40. [Crossref] [PubMed]
- Brydøy M, Fossa SD, Klepp O, et al. Paternity following treatment for testicular cancer. J Natl Cancer Inst 2005;97:1580-8. [Crossref] [PubMed]
- Schover LR, Brey K, Lichtin A, et al. Oncologists' attitudes and practices regarding banking sperm before cancer treatment. J Clin Oncol 2002;20:1890-7. [Crossref] [PubMed]
- Xu R, Centola GM, Tanrikut C. Genitourinary cancer patients have worse baseline semen parameters than healthy sperm bankers. Andrology 2019;7:449-53. [Crossref] [PubMed]
- Dohle GR. Male infertility in cancer patients: Review of the literature. Int J Urol 2010;17:327-31. [Crossref] [PubMed]
- Caroppo E, Niederberger C, Elhanbly S, et al. Effect of cryptorchidism and retractile testes on male factor infertility: A multicenter, retrospective, chart review. Fertil Steril 2005;83:1581-4. [Crossref] [PubMed]
- Akre O, Richiardi L. Does a testicular dysgenesis syndrome exist? Hum Reprod 2009;24:2053-60. [Crossref] [PubMed]
- Coward RM, Kovac JR, Smith RP, et al. Fertility Preservation in Young Men Treated for Malignancies: Options for Precancer Treatment. Sex Med Rev 2013;1:123-34. [Crossref] [PubMed]
- Hansen PV, Trykker H, Anderson J. Germ cell function and hormonal status in patients with testicular cancer. Cancer 1989;64:956-61. [Crossref] [PubMed]
- Fankhauser CD, Sanders S, Roth L, et al. Systemic inflammatory markers have independent prognostic value in patients with metastatic testicular germ cell tumours undergoing first-line chemotherapy. Br J Cancer 2018;118:825-30. [Crossref] [PubMed]
- Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia 2007;39:203-15. [Crossref] [PubMed]
- Haas GG, Cines DB, Schreiber AD. Immunologic infertility: identification of patients with antisperm antibody. N Engl J Med 1980;303:722-7. [Crossref] [PubMed]
- Paoli D, Gilio B, Piroli E, et al. Testicular tumors as a possible cause of antisperm autoimmune response. Fertil Steril 2009;91:414-9. [Crossref] [PubMed]
- Said TM, Tellez S, Evenson DP, et al. Assessment of sperm quality, DNA integrity and cryopreservation protocols in men diagnosed with testicular and systemic malignancies. Andrologia 2009;41:377-82. [Crossref] [PubMed]
- O’Flaherty C, Vaisheva CF, Hales BF, et al. Characterization of sperm chromatin quality in testicular cancer and Hodgkin’s lymphoma patients prior to chemotherapy. Hum Reprod 2008;23:1044-52. [Crossref] [PubMed]
- Kumar K, Lewis S, Vinci S, et al. Evaluation of sperm DNA quality in men presenting with testicular cancer and lymphoma using alkaline and neutral Comet assays. Andrology 2018;6:230-5. [Crossref] [PubMed]
- Gilbert K, Nangia AK, Dupree JM, et al. Fertility preservation for men with testicular cancer: Is sperm cryopreservation cost effective in the era of assisted reproductive technology? Urol Oncol 2018;36:92.e1-92.e9. [Crossref] [PubMed]
- Agarwal A, Ong C, Durairajanayagam D. Contemporary and future insights into fertility preservation in male cancer patients. Transl Androl Urol 2014;3:27-40. [PubMed]
- Fode M, Ohl DA, Sønksen J. A step-wise approach to sperm retrieval in men with neurogenic anejaculation. Nat Rev Urol 2015;12:607-16. [Crossref] [PubMed]
- Stensvold E, Magelssen H, Oskam IC. Fertility-preserving measures for boys and young men with cancer. Tidsskr Nor Laegeforen 2011;131:1433-5. [Crossref] [PubMed]
- Gat I, Toren A, Hourvitz A, et al. Sperm preservation by electroejaculation in adolescent cancer patients. Pediatr Blood Cancer 2014;61:286-90. [Crossref] [PubMed]
- Berookhim BM, Mulhall JP. Outcomes of operative sperm retrieval strategies for fertility preservation among males scheduled to undergo cancer treatment. Fertil Steril 2014;101:805-11. [Crossref] [PubMed]
- Shin DH, Turek PJ. Sperm retrieval techniques. Nat Rev Urol 2013;10:723-30. [Crossref] [PubMed]
- Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer – predictors of spermatogenesis. BJU Int 2018;122:236-42. [Crossref] [PubMed]
- Carmignani L, Gadda F, Paffoni A, et al. Azoospermia and severe oligospermia in testicular cancer. Arch Ital Urol Androl 2009;81:21-3. [PubMed]
- Schrader M, Muller M, Sofikitis N, et al. “Onco-tese”: Testicular sperm extraction in azoospermic cancer patients before chemotherapy - New guidelines? Urology 2003;61:421-5. [Crossref] [PubMed]
- Furuhashi K, Ishikawa T, Hashimoto H, et al. Onco-testicular sperm extraction: Testicular sperm extraction in azoospermic and very severely oligozoospermic cancer patients. Andrologia 2013;45:107-10. [Crossref] [PubMed]
- Carrasquillo R, Sávio LF, Venkatramani V, et al. Using microscope for onco-testicular sperm extraction for bilateral testis tumors. Fertil Steril 2018;109:745. [Crossref] [PubMed]
- Tsutsumi S, Kawahara T, Takeshima T, et al. Onco-testicular sperm extraction (onco-TESE) for bilateral testicular tumors: two case reports. J Med Case Rep 2017;11:139. [Crossref] [PubMed]
- Roque M, Sampaio M, de Oliveira Salles PG, et al. Onco-testicular sperm extraction: Birth of a healthy baby after fertility preservation in synchronous bilateral testicular cancer and azoospermia. Andrologia 2015;47:482-5. [Crossref] [PubMed]
- Nugent D, Meirow D, Brook PF, et al. Transplantation in reproductive medicine: Previous experience, present knowledge and future prospects. Hum Reprod Update 1997;3:267-80. [Crossref] [PubMed]
- Stukenborg JB, Alves-Lopes JP, Kurek M, et al. Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy. Hum Reprod 2018;33:1677-83. [Crossref] [PubMed]
- McDougall RJ, Gillam L, Delany C, et al. Ethics of fertility preservation for prepubertal children: Should clinicians offer procedures where efficacy is largely unproven? J Med Ethics 2018;44:27-31. [Crossref] [PubMed]
- Giudice MG, de Michele F, Poels J, et al. Update on fertility restoration from prepubertal spermatogonial stem cells: How far are we from clinical practice? Stem Cell Res 2017;21:171-7. [Crossref] [PubMed]
- Quinn GP, Vadaparampil ST, Lee JH, et al. Physician referral for fertility preservation in oncology patients: A national study of practice behaviors. J Clin Oncol 2009;27:5952-7. [Crossref] [PubMed]
- Sonnenburg DW, Brames MJ, Case-Eads S, et al. Utilization of sperm banking and barriers to its use in testicular cancer patients. Support Care Cancer 2015;23:2763-8. [Crossref] [PubMed]
- Sheth KR, Sharma V, Helfand BT, et al. Improved fertility preservation care for male patients with cancer after establishment of formalized oncofertility program. J Urol 2012;187:979-86. [Crossref] [PubMed]
- Lopategui DM, Ibrahim E, Aballa TC, et al. Effect of a formal oncofertility program on fertility preservation rates—first year experience. Transl Androl Urol 2018;7:S271-5. [Crossref] [PubMed]
- Moravek MB, Appiah LC, Anazodo A, et al. Development of a Pediatric Fertility Preservation Program: A Report From the Pediatric Initiative Network of the Oncofertility Consortium. J Adolesc Health 2019;64:563-73. [Crossref] [PubMed]
- Kort JD, Eisenberg ML, Millheiser LS, et al. Fertility issues in cancer survivorship. CA Cancer J Clin 2014;64:118-34. [Crossref] [PubMed]
- Magelssen H, Brydøy M, Fosså SD, et al. The effects of cancer and cancer treatments on male reproductive function. Nat Clin Pract Urol 2006;3:312-22. [Crossref] [PubMed]
- Petersen PM, Skakkebaek NE, Rørth M, et al. Semen quality and reproductive hormones before and after orchiectomy in men with testicular cancer. J Urol 1999;161:822-6. [Crossref] [PubMed]
- Lin WW, Kim ED, Quesada ET, et al. Unilateral testicular injury from external trauma: evaluation of semen quality and endocrine parameters. J Urol 1998;159:841-3. [Crossref] [PubMed]
- Arap MA, Vincentini FC, Cocuzza M, et al. Late hormonal levels, semen parameters, and presence of antisperm antibodies in patients treated for testicular torsion. J Androl 2007;28:528-32. [Crossref] [PubMed]
- Liguori G, Trombetta C, Bucci S, et al. Semen quality before and after orchiectomy in men with testicular cancer. Arch Ital Urol Androl 2008;80:99-102. [PubMed]
- Nangia AK, Krieg SA, Kim SS. Clinical guidelines for sperm cryopreservation in cancer patients. Fertil Steril 2013;100:1203-9. [Crossref] [PubMed]
- Ribeiro de Andrade M, Ferigolo P, Antoniassi MP, et al. Optimal time to bank sperm from patients with testicular tumors. Fertil Steril 2015;104:e291. [Crossref]
- Motzer RJ, Jonasch E, Agarwal N, et al. Testicular Cancer, Version 2.2015. J Natl Compr Canc Netw 2015;13:772-99. [Crossref] [PubMed]
- Weissbach L. Organ preserving surgery of malignant germ cell tumors. J Urol 1995;153:90-3. [Crossref] [PubMed]
- Heidenreich A, Weissbach L, Holtl W, et al. Organ sparing surgery for malignant germ cell tumor of the testis. J Urol 2001;166:2161-5. [Crossref] [PubMed]
- Taskinen S, Fagerholm R, Aronniemi J, et al. Testicular tumors in children and adolescents. J Pediatr Urol 2008;4:134-7. [Crossref] [PubMed]
- Ross JH, Kay R. Prepubertal testicular tumors. Urology 2009;74:94. [Crossref] [PubMed]
- Huddart RA, Norman A, Moynihan C, et al. Fertility, gonadal and sexual function in survivors of testicular cancer. Br J Cancer 2005;93:200. [Crossref] [PubMed]
- Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917-31. [Crossref] [PubMed]
- Abram McBride J, Lipshultz LI. Male fertility preservation. Curr Urol Rep 2018;19:49. [Crossref] [PubMed]
- Trottmann M, Becker AJ, Stadler T, et al. Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur Urol 2007;52:355-67. [Crossref] [PubMed]
- Hinton S, Catalana PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup Long-term gonadal toxicity after therapy for Hodgkin's and non-Hodgkin's lymphoma trial. Cancer 2003;97:1869-75. [Crossref] [PubMed]
- Lampe H, Horwich A, Norman A, et al. Fertility after chemotherapy for testicular germ cell cancers. J Clin Oncol 1997;15:239. [Crossref] [PubMed]
- Bokemeyer C, Schmoll HJ, van Rhee J, et al. Long-term gonadal toxicity after therapy for Hodgkin's and non-Hodgkin's lymphoma. Ann Hematol 1994;68:105-10. [Crossref] [PubMed]
- Green DM, Liu W, Kutteh WH, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol 2014;15:1215-23. [Crossref] [PubMed]
- Kelleher S, Wishart SM, Liu PY, et al. Long-term outcomes of elective human sperm cryostorage. Hum Reprod 2001;16:2632. [Crossref] [PubMed]
- Brannigan RE. Fertility preservation in adult male cancer patients. Cancer Treat Res 2007;138:28. [Crossref] [PubMed]
- Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol 2010;28:4831. [Crossref] [PubMed]
- Giwercman A, Peterson PM. Cancer and male infertility. Baillieres Best Pract Res Clin Endocrinol Metab 2000;14:453-71. [Crossref] [PubMed]
- Bujan L, Walschaerts M, Moinard N, et al. Impact of chemotherapy and radiotherapy for testicular germ cell tumors on spermatogenesis and sperm DNA: a multicenter prospective study from the CECOS network. Fertil Steril 2013;100:673-80. [Crossref] [PubMed]
- Fraass BA, Kinsella TJ, Harrington FS, et al. Peripheral dose to the testes: the design and clinical use of a practical and effective gonadal shield. Int J Radiat Oncol Biol Phys 1985;11:609-15. [Crossref] [PubMed]
- Singhal MK, Kapoor A, Singh D, et al. Scattered radiation to gonads: role of testicular shielding for para-aortic and homolateral illiac nodal radiotherapy. J Egypt Natl Canc Inst 2014;26:99-101. [Crossref] [PubMed]
- Littley MD, Shalet SM, Beardwell CG, et al. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med 1989;70:145-60. [PubMed]
- Howell S, Shalet S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin North Am 1998;27:927-43. [Crossref] [PubMed]
- Rowley MJ, Leach DR, Warner GA, et al. Effect of graded doses of ionizing radiation on the human testis. Radiat Res 1974;59:665-78. [Crossref] [PubMed]
- Speiser B, Rubin P, Casarett G. Aspermia following lower truncal irradiation in Hodgkin's disease. Cancer 1973;32:692-8. [Crossref] [PubMed]
- Shalet SM, Tsatsoulis A, Whitehead E, et al. Vulnerability of the human Leydig cell to radiation damage is dependent upon age. J Endocrinol 1989;120:161-5. [Crossref] [PubMed]
- Heidenreich WF, Atkinson M, Paretzke HG. Radiation-induced cell inactivation can increase the cancer risk. Radiat Res 2001;155:870-2. [Crossref] [PubMed]
- McDougal WS, Wein AJ, Kavoussi LR, et al. Campbell-Walsh Urology Tenth Edition Review. 1st edition. 2012:849-50.
- Masterson TA, Cary C, Rice KR, et al. The Evolution and Technique of Nerve-Sparing Retroperitoneal Lymphadenectomy. Urol Clin North Am 2015;42:311-20. [Crossref] [PubMed]
- Matos E, Skrbinc B, Zakotnik B. Fertility in patients treated for testicular cancer. J Cancer Surviv 2010;4:274. [Crossref] [PubMed]
- Abdul-Muhsin HM, Chatterjee S, Bansal P, et al. Robot-assisted retroperitoneal lymph node dissection in testicular cancer. J Surg Oncol 2015;112:736-40. [Crossref] [PubMed]
- Wiechno P, Demkow T, Kubiak K, et al. The quality of life and hormonal disturbances in testicular cancer survivors in cisplatin era. Eur Urol 2007;52:1448-54. [Crossref] [PubMed]
- Rossen P, Pederson AF, Zachariae R, et al. Sexuality and body image in long-term survivors of testicular cancer Eur J Cancer 2012;48:571-8. [Crossref] [PubMed]
- Pühse G, Wachsmuth JU, Kemper S, et al. Chronic pain has a negative impact on sexuality in testis cancer survivors. J Androl 2012;33:886-93. [Crossref] [PubMed]
- Tal R, Stember DS, Logmanieh N, et al. Erectile dysfunction in men treated for testicular cancer. BJU Int 2014;113:907-10. [Crossref] [PubMed]
- Meseguer M, Garrido N, Remohí J, et al. Testicular sperm extraction (TESE) and ICSI in patients with permanent azoospermia after chemotherapy. Hum Reprod 2003;18:1281-5. [Crossref] [PubMed]
- Hsiao W, Stahl PJ, Osterberg EC, et al. Successful treatment of postchemotherapy azoospermia with microsurgical testicular sperm extraction: the Weill Cornell experience. J Clin Oncol 2011;29:1607. [Crossref] [PubMed]
- Shin T, Kobayashi T, Shimomura Y, et al. Microdissection testicular sperm extraction in Japanese patients with persistent azoospermia after chemotherapy. Int J Clin Oncol 2016;21:1167-71. [Crossref] [PubMed]
- Hotaling JM, Patel DP, Vendryes C, et al. Predictors of sperm recovery after cryopreservation in testicular cancer. Asian J Androl 2016;18:35-8. [Crossref] [PubMed]


