The laser of the future: reality and expectations about the new thulium fiber laser—a systematic review
Introduction
More than 30 years have passed since laser lithotripsy became a reality (1-3). It quickly found widespread acceptance among urologists in the late 1980s (4-6), and in less than 10 years, the Holmium:yttrium-aluminum-garnet laser (Ho:YAG) rapidly became the gold-standard for endoscopic laser lithotripsy (7-11), remaining the uncontested reference in this medical field over the last 20 years.
However, there has been increasing interest in a new thulium fiber laser (TFL) lithotripter (12), which has been cleared for clinical use in the Russian Federation and launched in that market in 2018. According to some authors, it seems to be one of the most promising new laser technologies for lithotripsy, being several times more efficient than the current gold-standard Ho:YAG laser, as well as presenting other advantages (13-15). In view of these reports, we decided to review the latest advances in this technology and evaluate the reality and expectations of the new TFL lithotripter, thereby helping the reader to create an objective and evidence-based opinion.
Methods
This review was registered in PROSPERO (16-18), the international database of prospectively registered systematic reviews, with the PROSPERO registration number CRD42019128695. A PubMed search was performed (February 2019) for papers including the terms “holmium” or “thulium” in association with any of the following terms “lithotripsy”, “lithiasis”, “stone(s)”, “calculus”, “calculi”, “lithotripter(s)”, “lithotrite(s)”, “fiber(s)”, “fibre(s)”, “(endo)urology”, (endo)urologic(al)”, or “intrarenal”. The search covered articles published between the years 2015 and 2018, as well as articles already accepted in 2019 but not yet published. The search was deliberately kept as wide-ranging as possible to ensure that any paper about thulium fiber lasers would be found. Furthermore, because of the scarcity of results (see Results section), and in line with another systematic review that we published in the past (19), the medical sections of ScienceDirect, Wiley, SpringerLink, and Mary Ann Liebert publishers as well as Google Scholar were also searched for peer-reviewed abstract presentations published within the previously stated time frame that were not indexed on PubMed. Moreover, key papers and other important studies on the subject were also included and cross-referenced if they were considered noteworthy, despite being published before 2015. The authors adhered to PRISMA guidelines for this review (20). All relevant data was identified and selected, and is summarized below.
Results
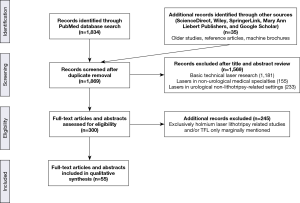
The PubMed search returned 1,834 articles whose abstracts were read. The majority of these articles (1,181 articles) related to basic technical laser research, not directly to medicine. They included research into new laser media, fiber production, soliton and quantum research, proof of concept for new laser systems, communications, imaging, nanotubes, and random bit generators created with lasers; most of these articles were published in journals specializing in the wide-ranging field of optics, and were hence indexed in PubMed. A total of 155 other articles were related to the use of lasers in non-urological medical specialties such as ophthalmology, gastroenterology, ENT, vascular and general surgery, interventional radiology, pneumology, dermatology, and cosmetics.
The remaining 498 articles concerned urology-related fields, with 233 dealing with the use of lasers in a non-lithotripsy-related setting such as holmium laser enucleation of the prostate (HoLEP) and greenlight-laser or other laser ablative techniques. Finally, the last 265 articles were related to urological laser lithotripsy. As expected, the vast majority of these papers were exclusively about holmium lithotripsy. Only 20 papers mentioned the TFL or topics related to it, yet some of these papers were not directly about the TFL, although some generic reviews mentioned it among other themes. Fortunately, the searches of the medical sections of ScienceDirect, Wiley, SpringerLink, Mary Ann Liebert, and Google Scholar which returned many of the papers already picked up in the PubMed search, allowed us to scrutinize the abstract presentations of the major urology congresses over recent years (21-36), and older reference papers, resulting in 35 additional sources of information about the TFL. The source of information selection process is summarized in Figure 1.
With the present paper’s objective in mind, we wrote an introductory summary about the Ho:YAG laser called “Ho:YAG laser—the current gold-standard” to highlight its importance as the gold-standard over the last 20 years and to better contextualize the results of the present systematic review. The relevant data retrieved from the bibliographic search have been categorized and summarized into a section called “TFL—the theory”, which discusses the concept behind the TFL, while a longer section entitled “TFL—the practice” discusses the practical issues relating to this new technology.
Ho:YAG laser—the current gold-standard
Ho:YAG laser lithotripters can efficiently fragment any stone composition, and can also be used for other applications including soft-tissue ablation, HoLEP, tumor resections, and management of stricture disease (11,37-41). As the majority of Ho:YAG energy is absorbed by water within 0.4 mm of the laser fiber tip, it is also very safe to use in an aqueous environment, i.e., in any endourological setting (41-43).
In the early days, the Ho:YAG laser lithotripter only had two parameters that the urologist could manipulate: the pulse energy in Joules (J) and the pulse repetition rate, commonly referred to as frequency, in Hertz (Hz). With very few parameter combinations possible, the user was limited to low pulse energies (up to 2 J), low frequencies (up to 15 Hz), and low total power levels (15 to 20 Watts maximum) (19,44,45).
Presently, several high-power Ho:YAG lasers are available, and these are capable of attaining much higher pulse energies (up to 6 J), very high pulse frequencies (up to 100 Hz), and higher total power levels (up to 140 W), thereby overcoming past limitations (46-50). These higher frequencies enabled a new laser lithotripsy philosophy, namely, the ability to ‘dust’ urinary stones quickly and efficiently, avoiding the need for capricious and time-consuming maneuvers associated with the removal of residual stone fragments (19,51-54).
The same progress that resulted in more powerful Ho:YAG laser lithotripters also led to the development of a third parameter for the surgeon to manipulate, the ability to control the pulse duration (i.e., pulse length) and pulse shape, with consequent reduction of stone retropulsion and laser fiber-tip degradation (41,55-60). Besides the standard short-pulse mode, one can currently choose medium-, or long-pulse modes, depending on the Ho:YAG model (56,61-64).
Another special feature for further reduction of stone retropulsion and increase in ablation rates has recently become available, and was termed “Moses technology” by the Ho:YAG laser manufacturer Lumenis™ (46). It consists in delivering a short, low energy pulse to create a vapor bubble before delivering the actual ablative energy pulse (65). Other Ho:YAG laser manufacturers also utilize similar techniques but use different terms and tradenames such as “Stabilization Mode” (48), “Vapor Tunnel” (47), or “Virtual Basket Technology” (66). Developments in the technology have not ended, with other customized approaches to laser pulse delivery such as burst laser lithotripsy (67) as well as even more powerful and feature-rich Ho:YAG laser lithotripters on the horizon (49,50).
This gradual and continuous Ho:YAG laser evolution, with the ability to ablate any type of urinary stone, and the capacity to cut, coagulate, ablate, enucleate, and vaporize tissues, as well as the technology’s excellent safety profile, make the Ho:YAG laser the safest, most versatile, and most successful type of laser currently used in urology.
TFL—the theory
The following section provides a review of the theoretical aspects of the TFL, and then compares it to the Ho:YAG laser.
Wavelengths, water absorption coefficient, and ablation thresholds
The pulsed infrared light emitted by current Ho:YAG lasers used in lithotripsy has, according to the manufacturers, a wavelength of approximately 2,100 nm (2,090 to 2,120 nm) (44,46,47,50,68-72). By contrast, the light emitted by TFLs has a wavelength tunable between 1,810 and 2,100 nm by fiber laser design. However, most preclinical research was done with TFLs using 1,908 and 1,940 nm wavelengths, with most of the abstracts and papers published in the last 4 years referring to a pulsed (or super-pulsed) TFL using a 1,940 nm wavelength (73-84). The TFL is not to be confused with another thulium laser, the continuous wave Thulium:YAG laser (about 2,000 nm wavelength), which is used exclusively for prostate ablation and vaporization, and which is unsuitable for lithotripsy (85).
Both the Ho:YAG laser and TFL energies are highly absorbed by water. However, while the holmium laser radiation at the 2,090 nm wavelength has an absorption coefficient of α=31.8 cm−1 at 20 °C (86), thulium laser radiation at the 1,940 nm wavelength is nearer to the absorption peak of water and has an α=129.2 cm−1 (87). These correspond to water optical penetration depths of 0.314 mm for the Ho:YAG lasers and 0.077 mm for the TFL. Thus, the TFL’s optical penetration depth is four-times less than that of the Ho:YAG laser. However, the optical penetration depth is a non-linear measure, being based on exponentiation of the mathematical constant e (e=2.7182), the Euler’s number forming the base of the natural logarithm. This means that the energy of a laser beam passing through water will be reduced by a factor of e (2.78182) for each successive optical penetration depth it passes through (i.e., it will be reduced by ~63%). For example, a Ho:YAG laser energy pulse in water will have been reduced to 37% of its initial energy after traveling a distance corresponding to its optical penetration depth, while a TFL energy pulse will have been reduced to 1.7% over the same distance (Figure 2). At 1 mm, the Ho:YAG pulse will still have 4% of its initial intensity, while the TFL pulse will have almost vanished, with an undetectable 0.00024% of its initial intensity. Thus, although the optical penetration depth of the TFL is only a quarter of that of the Ho:YAG laser, the logarithmical nature of radiation absorption means that for a 1 mm distance, the TFL has been absorbed approximately 16,000 times more than the Ho:YAG laser. In cases where laser energy produces vapor bubbles, these distances are extended for both the TFL and the Ho:YAG laser, changing during every laser pulse due to vapor bubble channel expansion and collapse (see Retropulsion and “Moses” capabilities section below), but the previously described relationship is always maintained.

The higher water absorption coefficient at the TFL wavelength directly translates into lower water-, (water-containing) tissue ablation-, and vapor-channel-initiation thresholds (65,88) than required for the Ho:YAG laser. Incidentally, the same applies for lithotripsy. The ablation thresholds for different urinary stone compositions such as harder calcium oxalate monohydrate or softer uric acid stones are four-times lower for the TFL than for the Ho:YAG laser (15,65,74,89). This has the following implications: first, the TFL is able to ablate a significantly higher stone volume than a Ho:YAG laser at the same lithotripter settings; second, the TFL can use much lower energy settings (approximately four-times less) than any Ho:YAG laser while achieving the same stone ablation results; and third, as an extension of the previous point, the TFL will be less prone to retropulsion because it can achieve the same ablation rates as the Ho:YAG laser, but at lower energy settings.
Thulium fiber laser assembly—what’s inside?
One of the authors of the present paper performed a recent review on the internal construction of the new TFL and compared it with the Ho:YAG laser (90). To summarize briefly, the construction of a Ho:YAG laser requires a flash lamp powered by a high-voltage power supply that “injects” light (i.e., photons) into a several millimeters diameter and several centimeters long laser crystal rod containing holmium ions (the gain medium). This laser crystal rod then emits photons at the desired holmium wavelength of 2,100 nm, i.e., the Ho:YAG laser radiation. This laser radiation then travels back and forth between two reflective mirrors located at each end of the crystal rod (mirrors which must be aligned with extreme precision) to be formed into a straight collimated laser beam. To make this process effective, all of these components are contained inside a reflective cavity, and the flash lamp pulse continuously “pumps” light into the laser rod. The whole assembly of components is called a laser resonator or cavity (91,92). One of the two reflective mirrors allow some of the laser radiation to escape to form the actual laser output beam. This several mm-wide laser beam must then be focused through a system of lenses to fit inside the small core of a surgical laser fiber to be delivered to its final target (stones or tissues).
Differing from the Ho:YAG laser, the TFL uses several simple laser diodes as the energy light source, instead of a flash lamp (89,90,93,94). The gain medium producing the 1,940 nm TFL laser beam is a simple and very long thulium-ion containing active fiber with a very thin fiber core (10–20 µm core diameter) (90,95). Because the laser beam originates inside this small fiber core, the TFL laser beam can be directly coupled to and delivered through another attachable laser fiber to carry the beam to its target (73). Table 1 summarizes most of the technology-related specifications.
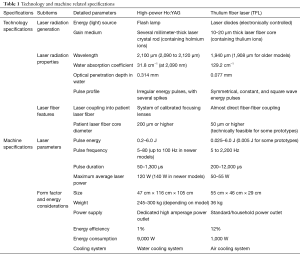
Full table
TFL—the practice
This section deals with the practical aspects of the TFL technology and its capabilities, performance, and potential applications, as well as documenting the technical and safety related issues, i.e., the important and relevant information for surgeons.
Laser setting specifications
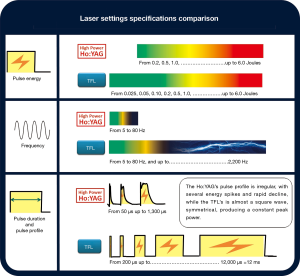
Since the TFL uses electronically-modulated laser diodes (89,92), a comprehensive range of laser lithotripter parameters are available for adjustment, and the frequency, pulse energy, and pulse length/duration ranges are larger and more flexible than those previously available for other types of laser lithotripters (Figure 3).
Pulse frequency
The current TFL medical systems (IRE-Polus, Fryazino, Russia, which is a subsidiary of IPG Photonics Co, Oxford, MA) (12,97,98) are capable of reaching a frequency of up to 2,200 Hz (12,65,76,79,90). This very high frequency contrasts with that of the Ho:YAG lasers, which are physically limited to 30 Hz or lower because of thermal effects and thermo-lensing caused by heat energy from the flash lamp on the laser crystal rod (65,93). Ho:YAG laser manufacturers have overcome this limitation by combining multiple laser resonators (laser cavities) to obtain more powerful lithotripters capable of higher frequencies (80 to 100 Hz) despite the much added expense (15,46,47,49,90); however, the frequencies available are still nowhere near to those achievable with the TFL.
Pulse energy
The TFL is able to reach maximum pulse energies of 6 J, similar to the maximum pulse energies of the most powerful Ho:YAG lasers of today (46,50,68). However, the TFL technology also allows the surgeon to use minimal pulse energies such as 0.05 or 0.025 J (79,80,82,83,90), and sometimes as low as 0.005 J (89), with these being much lower than the usual 0.2 J minimum pulse energy achievable with current Ho:YAG lasers. Such low energies could be of paramount importance, especially when performing a dusting procedure, as they could help obtain the smallest dust particles possible and keep retropulsion to a minimum.
Pulse duration/pulse length
With the TFL, the operator can choose between short-pulse durations (e.g., 200 µs) or longer ones (e.g., 1 ms), as with the current Ho:YAG lasers (46,47,49,50,89), and it is also possible to surpass these values by a factor of 10 and achieve pulse durations of up to 12 ms (65,90). However, unlike the Ho:YAG lasers, the TFL is not able to emit high pulse energies with short-pulse durations (89), which could be considered a limitation, although current trends with Ho:YAG lasers, including technological developments and surgeons’ preferences, are moving towards the use of longer pulse durations.
Total power
The current pulsed TFL systems have a maximum total power of 50–55 W (12,80,81,89). This contrasts with the higher power levels available (100, 120, or even 140 W) for the most recent and powerful Ho:YAG lasers (46,47,49,50). However, power levels higher than 30 W are rarely used for lithotripsy because of the significant risk of collateral tissue damage from the locally high temperatures that can occur with high-power lithotripsy (see Safety profile section) (99-101).
Despite TFL technology being relatively new, attempts have already been made, using several patient cohorts, to determine the optimal settings for lithotripsy using the TFL. Suggested settings include 0.1–0.2 J/15–30 W for the dusting of kidney stones, 0.2–0.5 J/10–15 W for the dusting and fragmentation of ureteric stones, 2–5 J/30–50 W for the fragmentation of large bladder stones (84), 1–1.5 J and 15–30 Hz for percutaneous nephrolithotomy (PCNL) fragmentation, and 0.1–0.3 J and 50–100 Hz for PCNL dusting (78). However, these are very preliminary recommendations, and the ideal TFL lithotripsy settings are far from established, and must be determined in future clinical experience-based studies.
Ablation efficiency, speed, and operating room-time
Initial laboratory studies performed with early TFLs showed disappointing results because the continuous wave TFLs available at that time used significantly lower peak power levels (~110 W), and were thus inappropriate for lithotripsy (93). In comparison, the popular Ho:YAG lasers were shown to be effective lithotripsy tools from the very beginning, being able to reach very high peak powers (102-104).
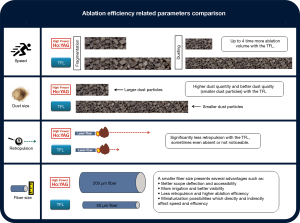
However, TFL technology has evolved dramatically in the last few years, and higher peak powers (~500 W) are now attainable, with pulsed and super-pulsed operation modes (82). The equipment has been shown to be safe and highly efficient, offering improved treatment effectiveness for stones of any compositions, sizes, and locations for both dusting and fragmentation modes, and using retrograde intrarenal surgery, PCNL, micro-PCNL, cystolithotripsy, or other endoscopic techniques (73,75-84,97,104-109). Currently, multiple authors agree that the TFL is more efficient than the Ho:YAG laser, with the most recent studies demonstrating the recent 50 W TFL prototypes to be up to four-times more ablative for dusting and up to two-times more ablative for fragmentation of urinary stones than the current 120 W top-of-the-line Ho:YAG lasers (Figure 4) (65,79,81,108,109). Other studies have shown that the TFL technology has an even higher ablative capacity for both soft uric acid and hard oxalate monohydrate urinary stones, being 5–10 times more efficient than the Ho:YAG laser (13,119).
There are several reasons for the TFLs high efficiency. First, although laser lithotripsy is known to predominantly be a photothermal ablative mechanism (120), the high water absorption of the TFL’s 1,940 nm wavelength laser radiation (see The theory section) brings another mechanism into play: the water trapped in inter-microcrystal space, pores, cracks, and fissures inside the calculi and near the stone’s surface vaporizes suddenly in an explosive fashion, creating very high pressures in a localized region. These high pressures produce mechanical stress waves within the stone, which further ablate the stone, and also remove weakened material from the irradiation site (15,65,89,121). With Ho:YAG lasers, this water-explosive mechanism has not been demonstrated to play a direct role in ablation (120), but it might contribute to the TFL’s four-times lower stone ablation threshold. Second, the individual temporal pulse profiles (pulse shapes in time) of the TFL are symmetrical, and show almost perfect square waves with uniform energy distribution over time and maintain constant peak power; in contrast, the Ho:YAG laser’s pulse profile is asymmetrical, with several initial energy spikes during the same pulse, followed by a rapid decline (Figure 3), with a steady peak power level being currently unattainable (89,96). Third, the TFL produces much lower retropulsion without the constant need to readjust the laser fiber toward the stone (see Retropulsion and “Moses” capabilities section).
A working method or technology may be very effective for performing a certain task, but still take a considerable amount of time to accomplish the task. However, in the case of the TFL, effectiveness and speed go hand in hand, and this high efficiency translates into much swifter procedures and shorter operating room (OR) times than with the current gold-standard Ho:YAG technology (65,109-111,119,122). This presents clear benefits for the individual patient, as well as decreasing the fatigue of the operating surgeon (80).
Dust quantity and quality
The ability to dust urinary stones without the need to remove residual fragments has been recognized by the endourological community as a highly desirable feature of a lithotripter (89). In this regard, high-power multi-cavity (resonators) Ho:YAG lasers allowing higher frequencies have opened a new frontier in dusting capabilities (123,124), although the resulting “dust” is more like smaller fragments than true dust.
The TFL produces three to four-times more dust than a high-power high-frequency Ho:YAG laser at similar power levels, even if the “Moses mode” is turned on (79,109-111). Besides the TFL’s more ablative and efficient dusting settings in terms of primary stone reduction, the resulting dust particle quantity is higher and the particle sizes smaller than with Ho:YAG lasers: the TFL produces more small dust particles under 0.5 mm than the Ho:YAG laser, regardless of the stone composition (96). In addition, the TFL can produce ultra-small stone particles of less than 0.1 mm (65), which are much nearer to real dust in size (89) (Figure 4). Both the larger quantity and better quality of the dust created with the TFL are related to the TFL’s higher water absorption and consequent micro-explosive water vapor-expansion mechanisms, as well as the clearly defined and precise emission of laser energy (89,96,125). With its wide-ranging and flexible parameters, the TFL is also capable of high-frequency dusting, pop-corning, pop-dusting, corn-dusting, and micro-dusting.
Retropulsion and “Moses” capabilities
Laser lithotripsy is known to produce a retropulsion effect that often forces the urologist to chase after stones up the ureter or inside the kidney; it prolongs procedural times and may sometimes make urinary calculi inaccessible (41,112,126).
The TFL has been shown to produce much less retropulsion than the Ho:YAG laser, sometimes even no retropulsion at all when very low pulse energies and frequencies under 150 Hz are used (74,83,113). As the TFL produces a uniform pulse energy profile, “Moses” capabilities occur naturally with the TFL, with it producing a stream of multiple bubbles during a single laser pulse (127), and each bubble growing and collapsing without interference (98). This corresponds to the true “Moses effect” originally described by Isner in 1986 (128-130), who also coined the expression “Moses effect” (128). Because of the lower peak power, the TFL bubble dimensions are four-times smaller and the generated local pressures ten times lower than those of the Ho:YAG laser (127). Even at very high pulse energies (3 J), stone retropulsion is three times lower with the TFL (81).
The TFL has been reported to offer a better endoscopic view during lithotripsy (80). The reduced retropulsion and consequent reduced medium turbulence could possibly be responsible for this, as less fragments and dust particles are swirled up, thereby reducing the “snow storm” effect that is characteristic of Ho:YAG lithotripsy. By producing less retropulsion, the TFL is also more readily operated by less experienced users, reducing the learning curve and the need to constantly adapt to a persistently changing stone position. More impressively, with fine tuning of parameters and laser fiber positioning, it has been demonstrated that the TFL can be used to rapidly and reproducibly pull stone samples with a “suction effect”, thereby offering an additional tool for manipulating urinary stones during laser lithotripsy (131).
µm laser fibers, fiber degradation, and miniaturization opportunities
The Ho:YAG laser technology has a physical limitation that prohibits coupling of high laser powers to small fibers, as overfilling of the input fiber core, laser leakage into the fiber cladding, and damage to the proximal fiber connectors must be prevented (65,132); thus the Ho:YAG laser fibers cannot have fiber cores smaller than 200 µm.
However, as described in the Theory section, as the TFL laser beam is generated inside a special 10–20 µm laser fiber (90,95), substantially higher laser powers can be delivered through small-core laser fibers, without damaging the fibers (13,95). The highly-focused laser beam exiting the tiny fiber core can be directly coupled to another end-stage delivery fiber, which can be as small as 50 µm (65,90,114), and consequently, the energy density (i.e., the amount of energy delivered per stone surface area) can also increase considerably (90). In comparison with a 200 µm core fiber, a 50 µm core fiber has 16 times less cross-sectional area, and will thus deliver a 16 times more-intense laser beam. Similar to the advantages resulting from the TFL’s increased absorption coefficient, the higher energy density of smaller fibers opens a whole new world of possibilities in comparison with Ho:YAG lasers: first, the same settings can be used to accomplish higher ablation volumes; second, lower lithotripter settings can be used to achieve the same results; and third, the ability to use very low pulse energies (e.g., as low as 0.025 J) and still obtain efficient stone ablation results. All these benefits occur simply because of the high energy density of the smaller fibers. Although currently only fibers as small as 150 µm are being used (12), laboratory lithotripsy studies have already been performed using 50 µm, 100 µm, and 150 µm-core fibers and frequencies over 1,000 Hz (73,114,133). Even small diameter ball-tip fibers with a 100 µm-core have been successfully tested with the TFL, and these allowed swift lithotripsy procedures using very high frequencies (300 Hz) (134).
The degradation of laser fibers is also significantly lower with the TFL than with holmium lasers, with less degradation at the proximal (connector) end, as well as less fiber burn-back, which improves the fibers lifetime and lowers costs (13,65,95). Contrary to the Ho:YAG laser, TFL lithotripsy allows 9 mm bending diameters and 50 W high-power settings to be safely used with regular (200 µm) small-core fibers (77), while 50 µm-core laser fibers can sustain extreme bending with a radius as small as 5 mm, while still delivering laser powers of 15 W without breaking (114).
Despite the lower degradation with the TFL, further mechanisms and tools are being explored to minimize fiber degradation and enhance efficiency even further. These include the use of laser fibers with hollow steel tips to reduce distal fiber burn-back (135); disposable laser fiber tips to provide a cost-effective method to customize and exchange fiber tips according to each procedure’s requirements (136); laser fibers with short tapered distal fiber tips to expand the laser beam and reduce fiber-tip damage and burn-back without compromising scope deflection, irrigation, or lithotripsy efficiency (13); a muzzle brake for the laser fiber tip (similar to the ones used in rifles and artillery cannons to reduce recoil and redirect propellant gases sideways), which reduces stone retropulsion by 85% and provides minimal fiber degradation for an efficient stone ablation (19,137); and vibrating laser fiber tips to further enhance dusting efficiency (138).
With the use of laser fibers with core diameters smaller than 200 µm, many other advantages are readily foreseeable for (flexible) endoscopy, including better irrigation, increased instrument deflection, less retropulsion, and even smaller stone fragments. These will all directly and indirectly affect accessibility, visibility, efficiency, surgical time, and the lithotripsy procedure as a whole (Figure 4) (19,41,112,115-117).
Utilization of smaller laser fibers also enables new miniaturization opportunities. Laser fibers with integrated stone baskets are being developed for the TFL to minimize retropulsion, increase flexibility, and reduce laser-induced nitinol wire damage (139). Inside the single working channel of a ureteroscope, a 50 µm core-diameter fiber also consumes approximately 30 times less cross-sectional area than the standard 270 µm-core fiber currently used in the clinic (114). The standard 1.2 mm (3.6 French/Charriere) flexible ureteroscope working channel is probably excessively large for such small diameter fibers, and could undergo further miniaturization (132). With that consideration, miniature distal tip scope designs for the TFL are being developed, allowing ureteroscope diameters of only 4.5 Fr (1.5 mm in diameter) (132), and these should have a positive impact on morbidity and make an already minimally invasive technique even less invasive (140,141).
Tissue applications of the TFL
Despite being outside of the scope of this systematic review, the authors consider it important to mention that the TFL’s higher water absorption directly translates into lower tissue ablation thresholds (65,88); thus it is expected to be better than the Ho:YAG laser for soft-tissue applications, as briefly summarized below.
One such recently developed soft-tissue application is TFL enucleation of the Prostate (ThuFLEP), which is similar to HoLEP. Recent studies have shown ThuFLEP to be a safe and highly efficacious treatment modality for the management of large volume (> 80 cm3) glands in benign prostatic hyperplasia (BPH) (142,143). Even for giant BPH gland management (>200 cm3), ThuFLEP has been demonstrated as a faster and safer treatment option, with faster recovery than retropubic simple prostatectomy (144). ThuFLEP was shown to be as efficacious as transurethral resection of the prostate (TURP), with shorter operative, catheterization, and hospitalization times (145), as well as better preservation of erectile function (146). ThuFLEP has also been demonstrated to be superior to robotic assisted simple prostatectomy, with shorter OR time, less blood loss, and fewer complications (147). In comparison with HoLEP and monopolar enucleation of the prostate (MEP), ThuFLEP was slightly superior, with 25% and 42% faster enucleation rates, respectively, although the differences did not reach statistical significance (the study was probably underpowered) (142). ThuFLEP also seems to be quicker for the operator to learn than other enucleation techniques such as MEP (142,148).
Other soft-tissue applications have also been described, such as TFL en-bloc enucleation of bladder tumors (149). One expert foresaw the potential applications of the TFL and its better suitability for more precise incision of soft tissues, more rapid prostate ablation, and more efficient laser lithotripsy (150). It is logical to presume that any other technique being conducted with the Ho:YAG laser can also be performed at least as efficaciously and safely with the TFL, including ablation and resection of other tissues and tumors such as upper tract urothelial carcinomas (UTUC) and stricture disease management (11,151,152).
Form factor and environmental impact
Size and weight
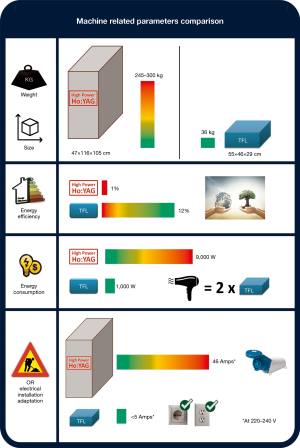
Despite the TFL’s much better performance, the machines are smaller than Ho:YAG laser lithotripters. One of the current 55 W TFL prototypes measures only 55 cm × 46 cm × 29 cm, and weighs only 36 kg with all its components; it uses a simple fan as the cooling mechanism, which helps make it quite compact (12). In comparison with high-power Ho:YAG lasers that measure at least half a cubic meter, weigh 245–300 kg, and use liquid-based chillers to provide cooling water (46,50,68), the TFL is seven times smaller and eight times lighter (Figure 5). In a time when ORs are already cramped with medical equipment, a bulky laser lithotripter only worsens the problem. However, the TFL can fit inside an endoscopy tower/cart along with the other electronic equipment and devices, and the video monitor, thereby saving precious OR space. This convenience means it can sit next to the surgeon ready for use, should the decision to perform a laser procedure be made.
Energy consumption and efficiency
High-power Ho:YAG lasers consume 8,000–10,000 W of electrical power (46,50,68). The TFL consumes almost 10 times less, with a maximum electricity consumption of 800–1,000 W (12), and is therefore more environmentally friendly. To illustrate the point, the electricity consumed by a simple household hairdryer would be enough to power at least two TFL lithotripter machines at the same time. Although lasers are not the most energy efficient machines (153), the wall-plug efficiency (the amount of electrical energy transformed into laser energy) of the TFL is 12%, which is substantially better than that of the Ho:YAG laser, which has an efficiency of only 1–2% (65,153). The remaining 98–99% of the energy is wasted in the form of heat and used in heat-reducing processes (Figure 5). Table 1 summarizes most of the machine-related specifications.
Maintenance and other costs
Because diode-pumped lasers such as the TFL have a higher wall-plug efficiency, the electronically-modulated laser diodes only need small power supplies. As there are almost no moving parts, a simple air-cooling mechanism is sufficient. The lifetimes of laser diodes are very long (often well above 10,000 hours), and there are no lenses or mirrors prone to contamination and misalignment (65,94). It is only logical to conclude that the wear and tear of the machine will be low and the maintenance costs should be minimal. On the contrary, flash lamp-pumped rod lasers such as the Ho:YAG laser require high-voltage power supplies, the lifetime of the flash lamps is limited, they need large and complex water cooling systems with multiple moving parts, and their optical system is fairly complex. Thus, Ho:YAG lasers are much more prone to failure with more wear and tear, and have a higher maintenance cost than TFLs, in addition to their high acquisition costs (15,65,153).
Another cost-related issue concerns the electrical installation of the OR. While the TFL works with a standard power outlet (e.g., 5 Amps at 220 V), high-power Ho:YAG lasers need a dedicated power supply (up to 46 Amps), sometimes even requiring a three-phase power supply (Figure 5). This may require an overhaul of the electrical installation of the OR with its associated costs, as well as creating mobility restrictions inside the OR (12,46,50,68). Additionally, laser fiber-related costs are likely to be reduced with the TFL because the TFL’s uniform beam profile results in lower degradation of fibers (95,114). Following this line of thought, the TFL would probably be a better candidate for the use of reusable fibers than the Ho:YAG laser.
In a time when healthcare-related costs keep on rising, including those for laser lithotripsy, the cost-related issues mentioned previously become even more important (65,154,155).
Safety profile
So far, the TFL has been demonstrated to be a safe alternative to the Ho:YAG laser for lithotripsy for all types of stones in all relevant anatomical regions, as well as for ThuFLEP and en-bloc enucleation of bladder tumors, with significant reduction in the likelihood of intraoperative trauma and postoperative complications (79,84,97,106,107,147,156). Many of the above-mentioned advantages of the TFL (significantly higher efficiency, speed, less retropulsion, smaller fragments, and shorter operating time) also contribute to reduce patient morbidity and complications, some directly and others indirectly. Likewise, the potential to use much smaller laser fibers with the TFL and all the ensuing advantages (better accessibility, visibility, and efficiency) further reduce the operating time and patient risk. The lower fiber degradation rate and resistance under extreme working deflections also make it safer, in particular for flexible scopes (13,19,41,65,95,112,114,116,117,157).
Patient and instrument safety at laser delivery point
The higher water absorption coefficient makes the TFL an even safer instrument than the Ho:YAG laser, the latter being considered safe for both the patient and instruments when properly used. Non-contact TFL ablation studies reported ablation stall out at working distances beyond 1.0 mm. The short TFL working distance and longer ureter perforation times may provide a greater safety margin from accidental perforation of tissues or damage to instruments (e.g., a nitinol stone basket) during laser irradiation, especially in comparison with the 4–5 mm maximum working distance previously reported for the Ho:YAG laser (127,158). Concerning the endoscopic safety distance for scopes, keeping the laser fiber tip visualized in 1/4 of the field of view of the endoscopic video screen (corresponding to a scope-to-fiber-tip distance of approximately 3 mm) appears to be safe for the treatment of both soft and hard stones or tissues, as demonstrated for the Ho:YAG laser (118,159). As for possible intrarenal pressure concerns, laser settings per se have not been demonstrated to cause any measurable change in intrarenal pressure (160).
Temperature increase during lithotripsy
Recently, the risk of local temperature rise in the kidney or ureter during laser emission has gained broad attention, particularly with the use of high-power lasers (99,100,161,162). One study using different irrigation fluid temperatures with a TFL did not find any significant differences in temperature increase in comparison with a Ho:YAG laser when using the same pulse frequency and energy settings (163). Thus, the same strategies that apply to the Ho:YAG laser also apply to the TFL, i.e., always using irrigation or cooled irrigation fluid and intermittent laser activation to mitigate thermal effects and avoid unnecessary damage to the surrounding tissues (19,99,100,164). However, when using intermittent laser activation with high-power settings, the temperature increase can still be dangerously high when irrigation is low (165). Even with commonly used irrigation pressures and laser settings slightly above 10 W, some authors have documented potentially dangerous intra-ureteral temperatures within seconds (166). As increasing temperatures also decrease the absorption coefficient of water for thulium radiation (86,87), and consequently reduce the TFL’s efficiency, this could be another argument for the use of irrigation fluid cooled to at least 20 °C to ensure maximum efficiency and safety.
Radiation and electrical hazards
One of the main concerns with laser radiation is eye safety (19). With its 1,940 nm wavelength, the TFL is far less dangerous than other lasers, as lasers emitting wavelengths longer than 1,400 nm are often referred to as being “eye-safe” because the light in this wavelength range is strongly absorbed in the eye’s cornea and lens, and cannot therefore reach the substantially more sensitive retina (167). This has already been studied and verified for the Ho:YAG laser (168,169). Considering that the absorption length at the TFL wavelength for the cornea is very small (well below 0.1 mm) (167) and that the TFL has a much shorter optical penetration depth (see Theory section), the TFL is expected to be even more “retina-safe” than the Ho:YAG laser. With regard to electrical safety, all electrical appliances and medical equipment must go through regulated quality control and so should be safe to use in this respect (170). Unlike Ho:YAG lasers, the TFL does not use high-voltage power supplies or three-phase high-amperage power outlets that could give rise to additional safety issues (153), and the TFL is therefore, in theory, a safer machine with respect to electrical-related hazards.
Noise level
One of the most overlooked safety hazards in Medicine (171,172) is the noise level. Most experienced readers are likely to agree that a working high-power Ho:YAG laser in the OR is quite noisy, and can make communication in the OR more difficult. Existing studies on the noise levels of laser lithotripsy are rare, but it has been shown that high-power Ho:YAG lasers produce sound levels up to 70 dB, which are higher than those of other technologies and exceed the widely stated 55 dB limit for work requiring concentration, or the normal communication protective level (at 60 dB). Such noise levels can also negatively affect surgeons by increasing with statistical significance their systolic blood pressure (173,174). The simple fact that one of the most recent Ho:YAG lasers includes a “silent mode” is a further indication of this noise-hazard problem (49). Fortunately, due to its internal components and construction, the TFL is significantly quieter than Ho:YAG flash lamp-pumped lasers (94,153), providing a calmer and less noisy working environment.
Limitations
This review has a key limitation common to all studies on very new technologies, namely, the low number of studies available upon which to base the conclusions. Despite this drawback, the authors have tried to remain as objective as possible, providing and summarizing the best evidence-based information available, and allowing the reader to make their own judgment. With consideration of the adherence of this review to PRISMA guidelines and the limited number of studies about the TFL, including in vitro laboratory studies and small clinical series, it did not make sense to perform an individual study-level or outcome-level assessment of the risk of bias (20), which could be considered another limitation.
Conclusions
The Ho:YAG laser has stood the test of time; it has been extensively studied and has become the gold-standard for laser lithotripsy since its first appearance over 30 years ago. Nevertheless, the apparent advantages of the TFL over the Ho:YAG laser (several times higher efficiency, wider and more comprehensive parameter combinations, significantly reduced retropulsion, ease of use, versatility, scope miniaturization possibilities, system compactness, foreseeable component durability, and increased safety profile) are simply too extensive to be ignored. The TFL appears to be a real alternative to the Ho:YAG laser, with the potential to become a true game-changer in laser lithotripsy. Due to its novelty, further studies are needed to broaden our understanding of the TFL, and comprehend the full implications and benefits of this new technology, as well its limitations.
Acknowledgments
The authors wish to thank and acknowledge the help of Dr. Arminda Sustelo, head of the Library Department of Hospital Prof. Doutor Fernando Fonseca, Amadora, Portugal, and Dr. Helena Donato, head of the Library Departments of Centro Hospitalar e Universitário de Coimbra, Portugal for providing some of the difficult to access articles; Mr. Alexander Vybornov from IPG Medical Corporation for providing the official but difficult to access Urolase SP+ brochure; and Drs. Gregory Altshuler and Ilya Yaroslavsky from IPG Medical Corporation, Marlboro, USA, and Dr. Nathaniel Fried from the University of North Carolina at Charlotte, USA for critically reviewing the text of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Hofstetter A. Laser--science fiction or a new dimension in medicine? Urologe A 1985;24:310-2. [PubMed]
- Li AA. Physical methods in the treatment of patients with kidney and ureteral calculi. Vopr Kurortol Fizioter Lech Fiz Kult 1985.65-8. [PubMed]
- Schmidt-Kloiber H, Reichel E, Schoffmann H. Laserinduced shock-wave lithotripsy (LISL). Biomed Tech (Berl) 1985;30:173-81. [Crossref] [PubMed]
- Coptcoat MJ, Ison KT, Watson G, Wickham JE. Lasertripsy for ureteric stones in 120 cases: lessons learned. Br J Urol 1988;61:487-9. [Crossref] [PubMed]
- Hofmann R, Hartung R. Use of pulsed Nd:YAG laser in the ureter. Urol Clin North Am 1988;15:369-75. [PubMed]
- Ritchey M, Patterson DE, Kelalis PP, Segura JW. A case of pediatric ureteroscopic lasertripsy. J Urol 1988;139:1272-4. [Crossref] [PubMed]
- Adkins WC, Dulabon DA, Chorazy ZJ, et al. Consider Ho:YAG for low-cost, effective laser lithotripsy. Clin Laser Mon 1994;12:139-41. [PubMed]
- Anidjar M, Cussenot O, Ravery V, et al. Le rôle du laser en urologie. Prog Urol 1995;5:175-92. [PubMed]
- Denstedt JD, Razvi HA, Sales JL, et al. Preliminary experience with holmium: YAG laser lithotripsy. J Endourol 1995;9:255-8. [Crossref] [PubMed]
- Matsuoka K, Iida S, Nakanami M, et al. Holmium: yttrium-aluminum-garnet laser for endoscopic lithotripsy. Urology 1995;45:947-52. [Crossref] [PubMed]
- Erhard MJ, Bagley DH. Urologic applications of the holmium laser: preliminary experience. J Endourol 1995;9:383-6. [Crossref] [PubMed]
- IPG Photonics. UROLASE SP+ Brochure; 2018.
- Blackmon RL, Irby PB, Fried NM. Thulium fiber laser lithotripsy using tapered fibers. Lasers Surg Med 2010;42:45-50. [Crossref] [PubMed]
- Hardy LA, Wilson CR, Irby PB, et al. Thulium fiber laser lithotripsy in an in vitro ureter model. J Biomed Opt 2014;19:128001. [Crossref] [PubMed]
- Fried NM, Irby PB. Advances in laser technology and fibre-optic delivery systems in lithotripsy. Nat Rev Urol 2018;15:563-73. [Crossref] [PubMed]
- Palepu A, Kendall C, Moher D. Open Medicine endorses PROSPERO. Open Med 2011;5:e65-6. [PubMed]
- Chien PFW, Khan KS, Siassakos D. Registration of systematic reviews: PROSPERO. BJOG 2012;119:903-5. [Crossref] [PubMed]
- Davies S. The importance of PROSPERO to the National Institute for Health Research. Syst Rev 2012;1:5. [Crossref] [PubMed]
- Kronenberg P, Somani B. Advances in Lasers for the Treatment of Stones-a Systematic Review. Curr Urol Rep 2018;19:45. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- EAU15 - 30th Annual Congress of the European Association of Urology Abstracts. Eur Urol Suppl 2015;14:e1-eV77.
- EAU16 - 31st Annual Congress of the European Association of Urology Abstracts. Eur Urol Suppl 2016;15:e1-eV79.
- Abstracts EAU. 17 - 32nd Annual EAU Congress. Eur Urol Suppl 2017;16:e1-2160.
- Abstracts EAU. 18 - 33rd Annual EAU Congress. Eur Urol Suppl 2018;17:e1-2003.
- 2015 Annual Meeting Program Abstracts. J Urol 2015;193:e1-1118.
- 2016 Annual Meeting Program Abstracts. J Urol 2016;195:e1-1192.
- 2017 Annual Meeting Program Abstracts. J Urol 2017;197:1-1376.
- 2018 Annual Meeting Program Abstracts. J Urol 2018;199:1-1250. [PubMed]
- Scientific Program of 33rd World Congress of Endourology & SWL Program Book. J Endourol 2015;29 Suppl 1:1-A457. [Crossref] [PubMed]
- Scientific program of 34th world congress of endourology & SWL program book and abstracts. J Endourol 2016;30:1-A464. [Crossref] [PubMed]
- Scientific program of 35TH world congress of endourology program book and abstracts. J Endourol 2017;31:1-A474. [Crossref] [PubMed]
- Scientific program of 36th world congress of endourology program book and abstracts. J Endourol 2018;32:1-A573. [Crossref] [PubMed]
- SIU 2015 Abstracts. World J Urol 2015;33 Suppl 1:1-256. [Crossref] [PubMed]
- Abstract SIU. 2016 Buenos Aires, Argentina book. World J Urol 2016;34:1-248. [Crossref] [PubMed]
- Abstracts from the 37th Congress of the Société Internationale d'Urologie, Centro de Congressos de Lisboa, October 19-22, 2017. World J Urol 2017;35:1-360. [Crossref] [PubMed]
- Abstracts from 38th Congress of the Société Internationale d’Urologie Seoul Dragon City, October 4-7, 2018. World J Urol 2018;36:1-380. [Crossref] [PubMed]
- Floratos DL, de la Rosette JJ. Lasers in urology. BJU Int 1999;84:204-11. [Crossref] [PubMed]
- Vicente Rodríguez JJ, Fernández González I, Hernández Fernández C, et al. Láser en Urología. Actas Urológicas Españolas 2006;30:879-95. [Crossref] [PubMed]
- Shigemura K, Yamamichi F, Kitagawa K, et al. Does Surgeon Experience Affect Operative Time, Adverse Events and Continence Outcomes in Holmium Laser Enucleation of the Prostate? A Review of More Than 1,000 Cases. J Urol 2017;198:663-70. [Crossref] [PubMed]
- Shigemura K, Fujisawa M. Current status of holmium laser enucleation of the prostate. Int J Urol 2018;25:206-11. [Crossref] [PubMed]
- Kronenberg P, Traxer O. Update on lasers in urology 2014: current assessment on holmium:yttrium-aluminum-garnet (Ho:YAG) laser lithotripter settings and laser fibers. World J Urol 2015;33:463-9. [Crossref] [PubMed]
- Leveillee RJ, Lobik L. Intracorporeal lithotripsy: which modality is best? Curr Opin Urol 2003;13:249-53. [Crossref] [PubMed]
- Bader MJ, Gratzke C, Hecht V, et al. Impact of collateral damage to endourologic tools during laser lithotripsy--in vitro comparison of three different clinical laser systems. J Endourol 2011;25:667-72. [Crossref] [PubMed]
- Dornier. The Dornier Medilas H20 Brochure: The Holmium Laser for Lithotripsy. DMT007-06/04; 2004. Available online: http://www.lcrhea.ro/data/MedilasH20Brochure.pdf
- Zattoni F. Low-power holmium:Yag laser applications in endourology. Tuttlingen: Endo-Press; 2010.
- Lumenis. Lumenis PULSE 120H: Operator's Manual. UM-10012510 Rev. F; 2017. Available online: https://prd-medweb-cdn.s3.amazonaws.com/documents/periopservices/files/UM-10012510_F%20Lumenis%20Pulse%20120H%20Op%20Manual_English.pdf
- Quanta System. Cyber Ho 100 Brochure. BRO-CYBERHO100-rev001-ROTW; 2018. Available online: https://www.quantasystem.com/laser/cyber-ho/
- Olympus. Olympus EMPOWER H Laser Portfolio Brochure. S00169EN 09/18 OEKG; 2018. Available online: https://www.olympus.co.uk/medical/rmt/media/Content/Content-MSD/Documents/Brochures/Olympus_EMPOWER-Laser_Brochure_EN_43000.pdf
- Dornier. Dornier Medilas® H 140 Brochure. DMT357-022018-REV A EN; 2018. Available online: https://dornier.com/wp-content/uploads/2018/03/Medilas-H-140-English-Product-Brochure.pdf
- Jena Surgical. MultiPulse HoPLUS Brochure. J51-2100-000/EN/Rev.02; 2018. Available online: http://www.jenasurgical.com/wordpress/wp-content/uploads/2018/10/JS_MultiPulse-HoPLUS_EN_REV_02_WEB.pdf
- Matlaga BR, Chew B, Eisner B, et al. Ureteroscopic Laser Lithotripsy: A Review of Dusting vs Fragmentation with Extraction. J Endourol 2018;32:1-6. [Crossref] [PubMed]
- Aldoukhi AH, Roberts WW, Hall TL, et al. Holmium Laser Lithotripsy in the New Stone Age: Dust or Bust? Front Surg 2017;4:57. [Crossref] [PubMed]
- Santiago JE, Hollander AB, Soni SD, et al. To Dust or Not To Dust: A Systematic Review of Ureteroscopic Laser Lithotripsy Techniques. Curr Urol Rep 2017;18:32. [Crossref] [PubMed]
- Wu Z, Gao P, Feng C, et al. MP-04.10 Flexible Ureteroscopic Stone Dusting with Holmium: YAG Laser for Treatment of Renal Stones. World J Urol 2016;34:19. [PubMed]
- Electro Medical Systems. Swiss Laserclast Brochure. FA -398; 2012. Available online: http://www.ems-company.com/media/PDF%20NEW/FA-398_EN_Ed_08-2012_LaserClast%20Brochure_single.pdf
- Rocamed. MH01 Laser Lithotripter Brochure. V.Uk.2; 2013. Available online: http://www.rocamed-urology.com/images/stories/doc/br.uk.v2.laser.pdf
- Kang HW, Lee H, Teichman JMH, et al. Dependence of calculus retropulsion on pulse duration during Ho: YAG laser lithotripsy. Lasers Surg Med 2006;38:762-72. [Crossref] [PubMed]
- Wollin DA, Ackerman A, Yang C, et al. Variable Pulse Duration From a New Holmium: YAG Laser: The Effect on Stone Comminution, Fiber Tip Degradation, and Retropulsion in a Dusting Model. Urology 2017;103:47-51. [Crossref] [PubMed]
- Bell JR, Penniston KL, Nakada SY. In Vitro Comparison of Holmium Lasers: Evidence for Shorter Fragmentation Time and Decreased Retropulsion Using a Modern Variable-pulse Laser. Urology 2017;107:37-42. [Crossref] [PubMed]
- Sroka R, Pongratz T, Scheib G, et al. Impact of pulse duration on Ho: YAG laser lithotripsy: treatment aspects on the single-pulse level. World J Urol 2015;33:479-85. [Crossref] [PubMed]
- CoolTouch - Syneron Candela. StoneLight 30 brochure: Laser Therapy; 2016. Available online: http://www.lcrhea.ro/data/MedilasH20Brochure.pdf
- Kronenberg P, Traxer O. PI-05 ultra-short, short, medium and long-pulse laser lithotripsy performance. J Urol 2016;195:e410. [Crossref]
- Bell JR, Penniston KL, Nakada SY. In Vitro Comparison of Stone Fragmentation When Using Various Settings with Modern Variable Pulse Holmium Lasers. J Endourol 2017;31:1067-72. [Crossref] [PubMed]
- Eisel M, Ströbl S, Pongratz T, et al. In vitro investigations of propulsion during laser lithotripsy using video tracking. Lasers Surg Med 2018;50:333-9. [Crossref] [PubMed]
- Fried NM. Recent advances in infrared laser lithotripsy Invited. Biomed Opt Express 2018;9:4552-68. [Crossref] [PubMed]
- Vizziello D, Acquati P, Clementi M, et al. MP27-17 Virtual Basket technology - Impact on high frequency lithotripsy in a urological simulator. J Endourol 2018;32:A277.
- Kronenberg P, Traxer O. MP22-13 burst laser lithotripsy - a novel lithotripsy mode. J Urol 2016;195:e258. [Crossref]
- Dornier. Dornier Medilas® H 140 specification sheet. DMT340-062018-REV B EN; 2018. Available online:
- Lumenis. VersaPulse PowerSuite Brochure. PB-1106750 Rev. B; 2013. Available online: http://www.surgical.lumenis.com/pdf/PB-1106750_rB_VPPS_P20v_br_LowRes%20(1).pdf
- Richard Wolf. Mega Pulse 70+ Brochure. D 711.XI.17.en.2; 2017.
- White MD, Moran ME, Calvano CJ, et al. Evaluation of retropulsion caused by holmium:YAG laser with various power settings and fibers. J Endourol 1998;12:183-6. [Crossref] [PubMed]
- Cansino Alcaide JR, Hidalgo Togores L, Cabrera Castillo PM, et al. Tratamiento de la litiasis con láser en condiciones especiales. Arch Esp Urol 2008;61:1111-4. [Crossref] [PubMed]
- Scott NJ, Cilip CM, Fried NM. Thulium fiber laser ablation of urinary Stones through small-core Optical fibers. IEEE J Sel Top Quantum Electron 2009;15:435-40. [Crossref]
- Blackmon RL, Irby PB, Fried NM. Comparison of holmium:YAG and thulium fiber laser lithotripsy: ablation thresholds, ablation rates, and retropulsion effects. J Biomed Opt 2011;16:071403. [Crossref] [PubMed]
- Sarp ASK, Gulsoy M. Determining the optimal dose of 1940-nm thulium fiber laser for assisting the endodontic treatment. Lasers Med Sci 2017;32:1507-16. [Crossref] [PubMed]
- Hardy LA, Gonzalez DA, Irby PB, et al. Fragmentation and dusting of large kidney stones using compact, air-cooled, high peak power, 1940-nm, Thulium fiber laser. Proc SPIE 10468 Therapeutics and Diagnostics in Urology 2018;2018:104680O.
- Chiron PH, Doizi S, Coninck VD, et al. PT066: Impact of SuperPulse Thulium Fiber Laser settings and curve diameter on optical fiber fracture during intracorporeal lithotripsy. Eur Urol Suppl 2019;18:e1756. [Crossref]
- Martov AG, Andronov AS, Dutov SV, et al. V36 Thulium SuperPulse Fiber Laser (TSPFL) for micro-PCNL. Eur Urol Suppl 2019;18:e2251. [Crossref]
- Traxer O, Rapoport L, Tsarichenko D, et al. V03-02 First clinical study on superpulse thulium fiber laser for lithotripsy. J Urol 2018;199:e321-2. [Crossref]
- Ergakov D, Martov AG, Guseynov M. 991 - The comparative clinical study of Ho: YAG and SuperPulse Tm fiber laser lithotripters. Eur Urol Suppl 2018;17:e1391. [Crossref]
- Glybochko P, Altshuler G, Vinarov A, et al. 226 - Comparison between the possibilities of holmium and thulium laser in lithotripsy in vitro. Eur Urol Suppl 2017;16:e391-2. [Crossref]
- Zamyatina V, Gapontsev V, Enikeev D, et al. Super pulse thulium fiber laser for lithotripsy. Lasers Surg Med 2016;48:10.
- Yaroslawsky I, Vinnichenko V, McNeill T, et al. Optimization of a novel Tm fiber laser lithotripter in terms of stone ablation efficiency and retropulsion reduction. Proc SPIE 10468 Therapeutics and Diagnostics in Urology 2018;2018:104680H.
- Dymov A, Rapoport L, Enikeev D, et al. 383: Prospective clinical study on superpulse thulium fiber laser: Initial analysis of optimal laser settings. Eur Urol Suppl 2019;18:e500. [Crossref]
- Herrmann TR, Liatsikos EN, Nagele U, et al. Guidelines on Lasers and Technologies. Eur Urol 2012;61:783-95. [Crossref] [PubMed]
- Lange BI, Brendel T, Hüttmann G. Temperature dependence of light absorption in water at holmium and thulium laser wavelengths. Appl Opt 2002;41:5797-803. [Crossref] [PubMed]
- Theisen-Kunde D, Danicke V, Wendt M, et al. Temperature dependence of water absorption for wavelengths at 1920 nm and 1940 nm. 4th European Conference of the International Federation for Medical and Biological Engineering. IFMBE Proceedings, Berlin, Heidelberg: Springer, 2008.
- Schomacker KT, Domankevitz Y, Flotte TJ, et al. Co:MgF2 laser ablation of tissue: effect of wavelength on ablation threshold and thermal damage. Lasers Surg Med 1991;11:141-51. [Crossref] [PubMed]
- Hardy LA, Vinnichenko V, Fried NM. High power holmium:YAG versus thulium fiber laser treatment of kidney stones in dusting mode: ablation rate and fragment size studies. Lasers Surg Med 2019;51:522-30. [Crossref] [PubMed]
- Traxer O, Keller EX. Thulium fiber laser: the new player for kidney stone treatment? A comparison with Holmium:YAG laser. World J Urol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Paschotta R. Encyclopedia of laser physics and technology. 1st ed. Weinheim: Wiley-VCH; 2008.
- Jackson SD, Lauto A. Diode-pumped fiber lasers: a new clinical tool? Lasers Surg Med 2002;30:184-90. [Crossref] [PubMed]
- Fried NM. Thulium fiber laser lithotripsy: An in vitro analysis of stone fragmentation using a modulated 110-watt Thulium fiber laser at 1.94 microm. Lasers Surg Med 2005;37:53-8. [Crossref] [PubMed]
- Paschotta R. Encyclopedia of laser physics and technology: diode-pumped lasers. 1st ed. Weinheim: Wiley-VCH; 2008.
- Wilson CR, Hardy LA, Irby PB, et al. Microscopic analysis of laser-induced proximal fiber tip damage during holmium: YAG and thulium fiber laser lithotripsy. Opt Eng 2016;55:46102. [Crossref]
- Gross A, Becker B, Taratkin M, et al. MP24-10 Wavelength and pulse shape effects on stone fragmentation of laser lithotripters. J Urol 2018;199:e293-4. [Crossref]
- Ali S, Rapoport L, Tsarichenko D, et al. VS1-3 Clinical study on Superpulse Thulium Fiber Laser for Lithotripsy. J Endourol 2018;32:A496.
- Chiron PH, Berthe LL, Coninck VD, et al. MP5-4 Comparison of vapor bubbles induced by an Holmium:YAG Laser and a SuperPusled Thulium Fiber Laser. Toward high speed lithotripsy at KHz repetition rate. J Endourol 2018;32:A42.
- Aldoukhi AH, Ghani KR, Hall TL, et al. Thermal Response to High-Power Holmium Laser Lithotripsy. J Endourol 2017;31:1308-12. [Crossref] [PubMed]
- Butticè S, Sener TE, Proietti S, et al. Temperature Changes Inside the Kidney: What Happens During Holmium:Yttrium-Aluminium-Garnet Laser Usage? J Endourol 2016;30:574-9. [Crossref] [PubMed]
- Molina WR, Silva IN, Donalisio da Silva R, et al. Influence of saline on temperature profile of laser lithotripsy activation. J Endourol 2015;29:235-9. [Crossref] [PubMed]
- Johnson JP, Oz MC, Chuck RS, et al. Comparison of methods for transcatheter fragmentation of gallstones. Surg Endosc 1989;3:7-10. [Crossref] [PubMed]
- Johnson DE, Cromeens DM, Price RE. Use of the holmium:YAG laser in urology. Lasers Surg. Med 1992;12:353-63. [Crossref] [PubMed]
- Dymov A, Glybochko P, Alyaev Y, et al. V11-11 thulium lithotripsy: from experiment to clinical practice. J Urol 2017;197:e1285. [Crossref]
- Keller EX, Coninck VD, Chiron PH, et al. VS1-1 Thulium fiber laser for lithotripsy of large renal stones: initial experience. J Endourol 2018;32:A495.
- Martov AG, Ergakov DV, Guseynov M, et al. VS1-2 SuperPulse Thulium Fiber Laser for Ureteroscopic Lithotripsy: 1 Year Experience. J Endourol 2018;32:A495-6.
- Rapoport LM, Vinarov AZ, Sorokin NI, et al. Experimental verification of thulium lithotripsy. Urologiia 2018.74-80. [Crossref] [PubMed]
- Chiron PH, Berthe LL. MP5-20 SuperPulsedThulium Fiber Laser for endocorporeal lithotripsy: superior from the very first pulse? J Endourol 2018;32:A49-50.
- Molina WR, Knudsen BE, Chew BH. MP5-19 Comparison of Rapid-Pulse Tm Fiber LASER (RPFL) vs High Power 120W Holmium-YAG LASER (Ho:YAG): Stone ablation efficiency at the same average power settings. J Endourol 2018;32:A49.
- Coninck Vd, Keller E, Kovalenko A, et al. PT067: Dusting efficiency comparison between Moses technology of Ho: YAG laser and superpulse thulium fiber laser. Eur Urol Suppl 2019;18:e1757-8. [Crossref]
- Coninck Vd, Keller EX, Chiron PH, et al. MP5-6 Dusting Efficiency Comparison between Moses Technology of Ho:YAG Laser and SuperPulse Thulium Fiber Laser. J Endourol 2018;32:A42-3.
- Kronenberg P, Traxer O. V1718 Laser fibers, pulse energy and retropulsion-what we can see and what we can't. J Urol 2013;189:e707. [Crossref]
- Martov AG, Ergakov DV, Guseinov MA, et al. Initial experience in clinical application of thulium laser contact lithotripsy for transurethral treatment of urolithiasis. Urologiia 2018.112-20. [Crossref] [PubMed]
- Blackmon RL, Hutchens TC, Hardy LA, et al. Thulium fiber laser ablation of kidney stones using a 50 -µm -core silica optical fiber. Opt Eng 2015.54.
- Kronenberg P, Traxer O. E60. The truth about laser fiber diameters. Eur Urol Suppl 2013;12:50. [Crossref]
- Pasqui F, Dubosq F, Tchala K, et al. Impact on active scope deflection and irrigation flow of all endoscopic working tools during flexible ureteroscopy. Eur Urol 2004;45:58-64. [Crossref] [PubMed]
- Spore SS, Teichman JM, Corbin NS, et al. Holmium: YAG lithotripsy: optimal power settings. J Endourol 1999;13:559-66. [Crossref] [PubMed]
- Chiron PH, Berthe L, Keller EX, et al. MP5-11 The ‘‘Safety distance concept’’ applied to ureterorenoscopy with a SuperPulsed Thulium Fiber Laser: are all parameters really usable? J Endourol 2018;32:A44-5.
- Blackmon RL, Irby PB, Fried NM. Holmium:YAG (lambda = 2,120 nm) versus thulium fiber (lambda = 1,908 nm) laser lithotripsy. Lasers Surg Med 2010;42:232-6. [Crossref] [PubMed]
- Chan KF, Vassar GJ, Pfefer TJ, et al. Holmium:YAG laser lithotripsy: A dominant photothermal ablative mechanism with chemical decomposition of urinary calculi. Lasers Surg Med 1999;25:22-37. [Crossref] [PubMed]
- Hardy LA, Irby P, Fried N. Scanning electron microscopy of real and artificial kidney stones before and after Thulium fiber laser ablation in air and water. Proc. SPIE, Vol 10468; 2018.
- Vinnichenko V, Hardy L, Fried N. MP5-9 Comparison of high power Holmium:YAG and Thulium fiber lasers for dusting of calcium oxalate monohydrate stones. J Endourol 2018;32:A43-4.
- Ghani KR, Gagin G, Hollingsworth J, et al. V14-3 Developments in Ureteroscopic Stone Treatment (DUST): Tips and tricks for lithotripsy using multi-cavity high-power holmium lasers. J Endourol 2015;29 Suppl 1:A392-3.
- Tracey J, Gagin G, Morhardt D, et al. MP51-07 Flexible ureteroscopy and laser lithotripsy for renal stones using ‘pop-dusting’: comparison of outcomes between traditional dusting settings versus ultra-high frequency settings. J Urol 2016;195:e683. [Crossref]
- Netsch C, Becker B, Taratkin M, et al. MP2-13 Effects of Wavelength and Pulse Shape on Stone Fragmentation Rate of Laser Lithotripter Devices. J Endourol 2018;32:A16.
- Becker B, Gross AJ, Netsch C. Ho: YaG laser lithotripsy: recent innovations. Curr Opin Urol 2019;29:103-7. [Crossref] [PubMed]
- Hardy LA, Kennedy JD, Wilson CR, et al. Analysis of thulium fiber laser induced bubble dynamics for ablation of kidney stones. J Biophotonics 2017;10:1240-9. [Crossref] [PubMed]
- Isner J, Clarke R, Katzir A, et al. Transmission characteristics of individual wavelengths in blood do not predict ability to accomplish laser ablation in a blood field: Inferential evidence for the ‘Moses effect'. Circulation 1986;74:II-361.
- Isner J, Steg G, Clarke R. Current status of cardiovascular laser therapy, 1987. IEEE Journal of Quantum Electronics 1987;23:1756-71. [Crossref]
- Isner JM, Lucas AR, Fields CD. Laser therapy in the treatment of cardiovascular disease. Br J Hosp Med 1988;40:172-8. [PubMed]
- Blackmon RL, Case JR, Trammell SR, et al. Fiber-optic manipulation of urinary stone phantoms using holmium:YAG and thulium fiber lasers. J Biomed Opt 2013;18:28001. [Crossref] [PubMed]
- Wilson C, Kennedy JD, Irby P, et al. Miniature ureteroscope distal tip designs for potential use in thulium fiber laser lithotripsy. J Biomed Opt 2018;23:1-9. [Crossref] [PubMed]
- Hardy LA, Wilson CR, Irby PB, et al. Rapid Thulium Fiber Laser Lithotripsy at Pulse Rates up to 500 Hz Using a Stone Basket. IEEE J Sel Top Quantum Electron 2014;20:138-41. [Crossref]
- Wilson CR, Hardy LA, Kennedy JD, et al. Miniature ball-tip optical fibers for use in thulium fiber laser ablation of kidney stones. J Biomed Opt 2016;21:18003. [Crossref] [PubMed]
- Hutchens TC, Blackmon RL, Irby PB, et al. Hollow steel tips for reducing distal fiber burn-back during thulium fiber laser lithotripsy. J Biomed Opt 2013;18:078001. [Crossref] [PubMed]
- Hutchens TC, Blackmon RL, Irby PB, et al. Detachable fiber optic tips for use in thulium fiber laser lithotripsy. J Biomed Opt 2013;18:038001. [Crossref] [PubMed]
- Hutchens TC, Gonzalez DA, Irby PB, et al. Fiber optic muzzle brake tip for reducing fiber burnback and stone retropulsion during thulium fiber laser lithotripsy. J Biomed Opt 2017;22:18001. [Crossref] [PubMed]
- Hall LA, Gonzalez DA, Fried NM. Thulium fiber laser ablation of kidney stones using an automated, vibrating fiber. J Biomed Opt 2019;24:1-10. [Crossref] [PubMed]
- Wilson CR, Hutchens TC, Hardy LA, et al. A Miniaturized, 1.9F Integrated Optical Fiber and Stone Basket for Use in Thulium Fiber Laser Lithotripsy. J Endourol 2015;29:1110-4. [Crossref] [PubMed]
- Rouse L, Cleveland R, Turney BW. MP29-6 Renal Pelvic Pressure during Ureteroscopy: Sheath versus Ureteroscope alone. J Endourol 2018;32:A294.
- Doizi S, Uzan A, Keller EX, et al. MP2-5 Comparison of intrarenal pelvic pressure levels during flexible ureteroscopy, mini-percutaneous nephrolithotomy and standard percutaneous nephrolithotomy in a kidney model. J Endourol 2018;32:A12-3.
- Enikeev D, Glybochko P, Rapoport L, et al. A Randomized Trial Comparing The Learning Curve of 3 Endoscopic Enucleation Techniques (HoLEP, ThuFLEP, and MEP) for BPH Using Mentoring Approach-Initial Results. Urology 2018;121:51-7. [Crossref] [PubMed]
- Enikeev D, Okhunov Z, Rapoport L, et al. Novel Thulium Fiber Laser for Enucleation of Prostate: A Retrospective Comparison with Open Simple Prostatectomy. J Endourol 2019;33:16-21. [Crossref] [PubMed]
- Enikeev D, Glybochko P, Alyaev Y, et al. V65 - Thulium fiber laser enucleation of the prostate in management of giant BPH (>200 cc). Eur Urol Suppl 2018;17:e1978. [Crossref]
- Enikeev D, Glybochko P, Alyaev Y, et al. MP62-10 Monopolar versus laser (thuflep, holep) endoscopic enucleation of the prostate: a single-center experience. J Urol 2018;199:e835-6. [Crossref]
- Enikeev D, Glybochko P, Rapoport L, et al. Impact of endoscopic enucleation of the prostate with thulium fiber laser on the erectile function. BMC Urol 2018;18:87. [Crossref] [PubMed]
- Rapoport L, Glybochko P, Enikeev D, et al. PE69 - Peri-operative Outcomes of Robotic-assisted simple prostatectomy versus Thulium Fiber Laser Enucleation of the Prostate. Eur Urol Suppl 2018;17:e2354. [Crossref]
- Taratkin M, Enikeev D, Rapoport L, et al. PT182 - Initial results of a prospective randomized trial on the learning curve of three endoscopic enucleation techniques (HoLEP, ThuFLEP and MEP) for BPH. Eur Urol Suppl 2019;18:e1914. [Crossref]
- Glybochko P, Alyaev Y, Rapoport L, et al. V55 PDD-guided thulium fiber laser en-bloc enucleation of bladder tumor. Eur Urol Suppl 2018;17:e1967. [Crossref]
- Fried NM. Therapeutic applications of lasers in urology: an update. Expert Rev Med Devices 2006;3:81-94. [Crossref] [PubMed]
- Dołowy Ł, Krajewski W, Dembowski J, et al. The role of lasers in modern urology. Cent European J Urol 2015;68:175-82. [Crossref] [PubMed]
- Villa L, Haddad M, Capitanio U, et al. Which Patients with Upper Tract Urothelial Carcinoma Can be Safely Treated with Flexible Ureteroscopy with Holmium:YAG Laser Photoablation? Long-Term Results from a High Volume Institution. J Urol 2018;199:66-73. [Crossref] [PubMed]
- Paschotta R. Encyclopedia of laser physics and technology: lamp-pumped lasers. 1st ed. Weinheim: Wiley-VCH; 2008.
- Rassweiler J, Rassweiler MC, Kenngott H, et al. The past, present and future of minimally invasive therapy in urology: a review and speculative outlook. Minim Invasive Ther Allied Technol 2013;22:200-9. [Crossref] [PubMed]
- Stern KL, Monga M. The Moses holmium system - time is money. Can J Urol 2018;25:9313-6. [PubMed]
- Dubrovin V, Tabakov A, Egoshin A. UP. 330 Thulium Laser Lithotripsy for Dusting of Ureteral Stones, Initial Experience. World J Urol 2018;36:314.
- Kronenberg P, Traxer O. The truth about laser fiber diameters. Urology 2014;84:1301-7. [Crossref] [PubMed]
- Wilson CR, Hardy LA, Irby PB, et al. Collateral damage to the ureter and Nitinol stone baskets during thulium fiber laser lithotripsy. Lasers Surg Med 2015;47:403-10. [Crossref] [PubMed]
- Talso M, Emiliani E, Haddad M, et al. Laser Fiber and Flexible Ureterorenoscopy: The Safety Distance Concept. J Endourol 2016;30:1269-74. [Crossref] [PubMed]
- Shrestha A. MP29-8 Intra-renal pressure profile during flexible ureteroscopy. J Endourol 2018;32:A295.
- Wollin DA, Carlos EC, Tom WR, et al. Effect of Laser Settings and Irrigation Rates on Ureteral Temperature During Holmium Laser Lithotripsy, an In Vitro Model. J Endourol 2018;32:59-63. [Crossref] [PubMed]
- Kallidonis P, Amanatides L, Panagopoulos V, et al. Does the Heat Generation by the Thulium:Yttrium Aluminum Garnet Laser in the Irrigation Fluid Allow Its Use on the Upper Urinary Tract? An Experimental Study. J Endourol 2016;30:422-7. [Crossref] [PubMed]
- Dragos LB, Somani B, Keller E, et al. 386: Super-pulse thulium fiber versus high power holmium lasers. What about temperature? Eur Urol Suppl 2019;18:e505-8. [Crossref]
- Gross AJ, Netsch C. Editorial Comment on: Thermal Response to High-Power Holmium Laser Lithotripsy by Aldoukhi et al. J Endourol 2017;31:1313. [Crossref] [PubMed]
- Aldoukhi AH, Hall TL, Ghani KR, et al. MP24-11 ‘‘Operator Duty-Cycle’’ effect of heat generation during holmium laser lithotripsy. J Endourol 2018;32:A236-7.
- Terry RS, Winship BB, Wollin D, et al. MP29-12 The Rise and Fall of Dangerous Temperature Changes During Ureteroscopic Laser Lithotripsy. J Endourol 2018;32:A296-7.
- Paschotta R. Encyclopedia of laser physics and technology: Eye-safe lasers. 1st ed. Weinheim: Wiley-VCH; 2008.
- Doizi S, Villa L, Cloutier J, et al. Doit-on porter des lunettes protectrices lors de l’utilisation du laser Holmium:YAG lors des procédures endourologiques ? Étude chez un modèle animal. Prog Urol 2015;25:751. [Crossref] [PubMed]
- Villa L, Cloutier J, Compérat E, et al. Do We Really Need to Wear Proper Eye Protection When Using Holmium:YAG Laser During Endourologic Procedures? Results from an Ex Vivo Animal Model on Pig Eyes. J Endourol 2016;30:332-7. [Crossref] [PubMed]
- Food and Drug Administration. Medical devices; current good manufacturing practice (CGMP) final rule; quality system regulation. Fed Regist 1996;61:52602-62. [PubMed]
- Nicolau A, Casal D, Lopes P, et al. O Ruído nas Unidades de Cidados Intensivos Neonatais de Lisboa e Vale do Tejo 2005;36:15-21.
- Hodge B, Thompson JF. Noise pollution in the operating theatre. Lancet 1990;335:891-4. [Crossref] [PubMed]
- Gu M, Liu C, Chen YB, et al. Comparison of Vela and holmium laser enucleation of the prostate: a retrospective clinical trial with a 12-month follow-up. Int Urol Nephrol 2018;50:819-23. [Crossref] [PubMed]
- Xu H, Chen YB, Gu M, et al. Evaluation of noise hazard during the holmium laser enucleation of prostate. BMC Urol 2017;17:71. [Crossref] [PubMed]