
Long-term device survival and quality of life outcomes following artificial urinary sphincter placement
Introduction
Artificial urinary sphincter (AUS) is a treatment option for incontinence after prostate treatment, and the preferred surgery for patients with severe symptoms (1). Since its introduction more than 40 years ago (2), the AUS has been implanted more than 150,000 times worldwide (3) and studies have shown rising rates of male incontinence procedures in the recent era (4). The AUS device has undergone little significant change since 1987 (narrow-back cuff and kink-free tubing) (5) with few significant technique changes since that time as well (transcorporal cuff, etc.). This affords increasing opportunity for reporting on long-term results. While the growing body of literature on AUS long-term outcomes reports predominantly on device survival and continence rates, there is still a paucity of data on patient-reported quality of life outcomes especially in large cohorts (6,7). We previously reported our experience on device survival in 1,082 patients with median 4-year follow-up (6). Since then, we have surveyed our surviving population of AUS patients concerning their continence rates and quality of life. We present herein an update on the largest reported series of AUS patients with device survival and additional key continence and quality of life outcomes (8).
Methods
After institutional review board approval was obtained, we queried our institutional AUS database and identified all patients who underwent primary AUS placement at our institution from 1983 to 2016. AUS revision surgeries were excluded from this analysis. Patients who underwent AUS implantation for neurogenic bladder or pelvic fracture, were less than 18 years of age, or declined research consent were also excluded.
Baseline clinical and demographic features of the cohort were evaluated. The etiology of incontinence was classified as radical prostatectomy (RP; includes both open and robotic approaches), RP with radiation (RP + RT), benign prostate surgery (including transurethral resection and photoselective vaporization of prostate) (TURP), and cryotherapy (Cryo). Of note, the majority of cryotherapy cases were performed as salvage therapy after recurrence following primary prostatectomy and/or radiation therapy. Due to low numbers, patients with a history of radiation therapy alone (prostate in situ) were excluded.
Regarding surgical technique, we perform AUS placement through a perineal incision. We divide the bulbospongiosus muscle and circumferentially dissect the proximal bulbar urethra. Urethral measurement is performed to determine appropriate cuff size. We perform abdominal placement of the reservoir through a separate incision and fill the reservoir with 22 mL of iso-osmotic contrast to aid in future device failure evaluation, if necessary.
All patients are seen back at 6 weeks for a postoperative visit with device activation and teaching. Otherwise, patient office visits are performed on an as-needed basis. Individual charts were reviewed to determine need for secondary surgery as well as the cause (explantation for infection/erosion or revision for device malfunction, urethral atrophy, or pump/tubing complication). Via our AUS registry, we also periodically contacted our patients via phone and mail to update patient and device outcomes. In recent mailings, we have requested that our patients report quality of life metrics including pad usage and validated survey questions including the Patient Global Impression of Improvement (PGI-I) (8,9).
Baseline patient characteristics were analyzed with descriptive statistics: continuous variables were described with medians and interquartile ranges (IQRs) while categorical variables were summarized with frequencies and percentages. Kaplan-Meier analysis was used to determine time to all-cause secondary surgery, with a breakdown by cause of secondary surgery (infection/erosion, mechanical failure, urethral atrophy, pump malposition/other) tabulated. Univariate and multivariable analyses were performed to test association of baseline variables with the need for secondary surgery. Quality of life outcomes were grouped in time ranges because time intervals between AUS placement and survey responses were variable between patients. All analyses were performed using the SAS software package (SAS Institute, Inc. Cary, NC) using 2-sided statistical tests with a P value of <0.05 considered statistically significant.
Results
During the study time frame, 1,154 men who underwent primary AUS were eligible and included in the analysis. Baseline clinical and demographic features of the cohort are shown in Table 1. RP was the most common etiology of incontinence, with 61% of men having had RP alone and 27% with an additional history of radiation therapy. Coronary artery disease (27%), peripheral vascular disease (5%), and diabetes (16%) were not particularly prevalent; however, only 45% were never-smokers. Twenty-nine percent had a history of bladder neck contracture or vesicourethral anastomotic stenosis and 17% had been previously treated for incontinence via urethral sling or bulking agent injection. Concerning operative technique, all patients received a 4 cm or larger cuff, as the 83 (7%) patients measuring 3.5 cm or less received a transcorporal cuff placement.
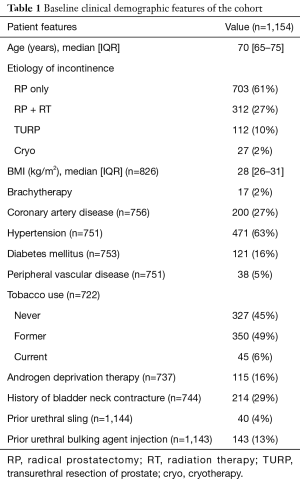
Full table
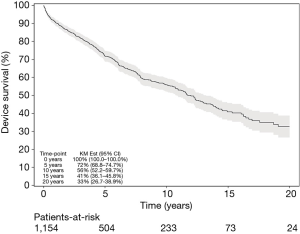
Median follow-up for the cohort was 5.4 years (IQR, 1.6–10.5 years). The overall secondary surgery rate (explantation or revision at any timepoint) was 35% (n=404). The rate of secondary surgery at 5, 10, 15, and 20 years was 72%, 56%, 41%, and 33%, respectively (Figure 1). As shown in Table 2, 11% of revisions were early for pump malposition, kinked tubing, or similar issue. Infection/erosion prompted 25% of secondary surgeries at a median of 1.3 years after initial AUS placement, whereas urethral atrophy (26%) and device malfunction (39%) accounted for the majority of revisions at 4.4 and 4.5 years, respectively.
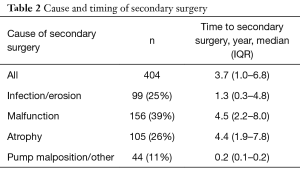
Full table
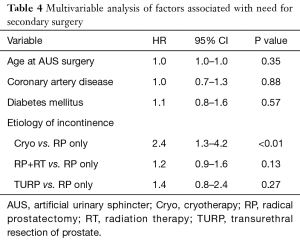
On univariate analysis of patient factors associated with need for any secondary surgery (Table 3), only history of cryosurgery (HR 2.7; 95% CI, 1.6–4.6; P<0.01) and radiation therapy (HR 1.4; 95% CI, 1.1–1.7; P=0.01) demonstrated significant association. Notably, coronary artery disease, peripheral vascular disease, diabetes, history of smoking, androgen deprivation therapy, and prior incontinence treatment did not demonstrate association with secondary surgery in our study. On multivariable analysis (Table 4) only history of cryosurgery retained significance (HR 2.4; 95% CI, 1.3–4.2; P<0.01).
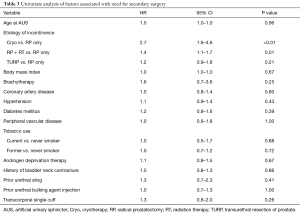
Full table
Full table
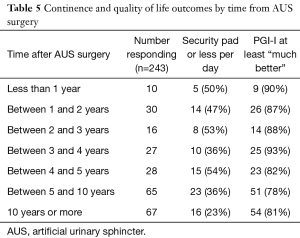
Concerning continence and quality of life outcomes (Table 5), 32% of patients that did not need a secondary surgery (243/750) had survey responses available. Of these, survey data demonstrated that 50% of patients responding less than 1 year from AUS placement used a security pad or less per day. Ninety percent of these patients reported a PGI-I of at least “much better”. Use of a security pad or less was reported by fewer patients who responded at greater time intervals from initial placement, down to 36% for 5–10 year responders and 23% for >10-year responders. However, these patients continued to report sustained subjective improvement, with 78% and 81% reporting PGI-I at least “much better”, respectively.
Full table
Discussion
Our study reports the outcomes of a large cohort [1,154] of primary AUS placements over extended follow-up (median 5.4 years, maximum 31.1 years). While the percentage of devices free from explantation/revision decreases with time (down to 56% at 10 years and 33% at 20 years) and pad usage increases with time, up to 80% of patients continue to report a PGI-I that is at least “much better” with greater than 10-year follow-up.
Our device durability rates without the need for secondary surgery are consistent with those reported in the literature (10), including in smaller studies focused on long-term follow-up (7,11-14).
Concerning patient-reported continence and quality of life outcomes, the literature contains a variety of definitions for successful outcomes (8,10). We chose to focus on the need for a security pad per day or less and the PGI-I because previous work has demonstrated that these variables hold the strongest association to patient-reported AUS success (8). The need for a security pad or less per day declined with greater time from primary AUS placement such that only 36% of patients 5–10 years from placement and 23% >10 years out reported continence by this definition. Comparison of continence rates across studies is difficult due to the varied definitions, but a systematic review summarized that the AUS appears to offer about 50% dry rates at mid-term follow-up (10).
Interestingly, our longest follow-up patients continue to report high responses on the PGI-I, despite fewer meeting the successful continence definition of needing a security pad or less daily. While prior studies have shown that patient satisfaction correlates inversely with the number of pads needed per day (14), our findings suggest that many of the patients who require more than a security pad per day are still experiencing such a significant improvement from their severe preoperative incontinence to consider the AUS successful. While our study addressed quality of life outcomes only in patients with a primary implant in place, other studies have reported that patient satisfaction is not affected by the need for secondary surgery (14-16). This fact suggests that even though the absolute revision rate for AUS is high (>70% by 20 years), even patients requiring another surgery can experience ongoing significant improvement, reflective of overall success.
Interestingly, a history of cryotherapy demonstrated a strong association with the need for secondary surgery which we had previously noted in a subset of our patients (17). While cryotherapy, in some instances, is performed as a primary therapy, many patients receive cryotherapy as salvage treatment after prostatectomy and/or radiation. Therefore, it is unclear if the cryotherapy modality itself is at the root of the association or if it serves as a surrogate for complex or repeated cancer treatments.
Strengths of the study include a large sample size, lengthy follow-up duration, and use of validated symptom questionnaires. Furthermore, for device survival we report the need for any secondary surgery to limit ambiguity between specific causes such as that between infection and erosion. We chose continence and quality of life outcomes based on study-proven associations with patient satisfaction as well (8).
The retrospective nature of our study introduces significant limitations that should also be acknowledged. For instance, the severity of preoperative incontinence could not be assessed, and this may provide insight in to the discrepancy between pad use on long-term follow-up and continued improvement on PGI-I. Furthermore, since our study addressed only patients with a primary implant and patients who were treated at a tertiary referral center in a high-volume AUS practice, the results may not be generalizable to all practices. The impact of changes in surgical technique, including more common use of a transcorporal approach in the most contemporary cases, may also impact our findings. Unfortunately, given limited numbers and follow-up in this subset of patients, specific statistical analysis was not feasible. Further studies on the use of this technique are needed.
In conclusion, primary AUS devices have acceptable long-term durability and function, and despite decreasing continence rates with time, patients continue to report high rates of overall symptom improvement.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was completed without any sources of extra-institutional funding. Mayo Clinic Institutional Review Board approval was received prior to study commencement (IRB 18-011023).
References
- Sandhu JS, Breyer B, Comiter C, et al. Incontinence after Prostate Treatment: AUA/SUFU Guideline. J Urol 2019;202:369-78. [Crossref] [PubMed]
- Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J Urol 1974;112:75-80. [Crossref] [PubMed]
- Lucas MG, Bosch RJL, Burkhard FC, et al. EAU guidelines on assessment and nonsurgical management of urinary incontinence. Eur Urol 2012;62:1130-42. [Crossref] [PubMed]
- Liu JS, Hofer MD, Milose J, et al. Male Sling and Artificial Urethral Sphincter for Male Stress Urinary Incontinence Among Certifying American Urologists. Urology 2016;87:95-9. [Crossref] [PubMed]
- Light JK, Reynolds JC. Impact of the new cuff design on reliability of the AS800 artificial urinary sphincter. J Urol 1992;147:609-11. [Crossref] [PubMed]
- Linder BJ, Rivera ME, Ziegelmann MJ, et al. Long-term Outcomes Following Artificial Urinary Sphincter Placement: An Analysis of 1082 Cases at Mayo Clinic. Urology 2015;86:602-7. [Crossref] [PubMed]
- Léon P, Chartier-Kastler E, Rouprêt M, et al. Long-term functional outcomes after artificial urinary sphincter implantation in men with stress urinary incontinence. BJU Int 2015;115:951-7. [Crossref] [PubMed]
- Linder BJ, Rangel LJ, Elliott DS. Evaluating Success Rates After Artificial Urinary Sphincter Placement: A Comparison of Clinical Definitions. Urology 2018;113:220-4. [Crossref] [PubMed]
- Suskind AM, Dunn RL, Morgan DM, et al. The Michigan Incontinence Symptom Index (M-ISI): a clinical measure for type, severity, and bother related to urinary incontinence. Neurourol Urodyn 2014;33:1128-34. [Crossref] [PubMed]
- Van der Aa F, Drake MJ, Kasyan GR, et al. The Artificial Urinary Sphincter After a Quarter of a Century: A Critical Systematic Review of Its Use in Male Non-neurogenic Incontinence. Eur Urol 2013;63:681-9. [Crossref] [PubMed]
- Abello A, Das AK. Long-term (>5 years) outcomes of patients implanted with artificial urinary sphincter: A single-center experience. Urol Ann 2019;11:15-9. [Crossref] [PubMed]
- Kim SP, Sarmast Z, Daignault S, et al. Long-Term Durability and Functional Outcomes Among Patients With Artificial Urinary Sphincters: A 10-Year Retrospective Review From the University of Michigan. J Urol 2008;179:1912-6. [Crossref] [PubMed]
- Montague DK, Angermeier KW, Paolone DR. Long-term Continence and Patient Satisfaction After Artificial Sphincter Implantation for Urinary Incontinence After Prostatectomy. J Urol 2001;166:547-9. [Crossref] [PubMed]
- Gousse AE, Madjar S, Lambert MM, et al. Artificial urinary sphincter for post-radical prostatectomy urinary incontinence: long-term subjective results. J Urol 2001;166:1755-8. [Crossref] [PubMed]
- Viers BR, Linder BJ, Rivera ME, et al. Long-Term Quality of Life and Functional Outcomes among Primary and Secondary Artificial Urinary Sphincter Implantations in Men with Stress Urinary Incontinence. J Urol 2016;196:838-43. [Crossref] [PubMed]
- Litwiller SE, Kim KB, Fone PD, et al. Post-Prostatectomy incontinence and the Artificial Urinary Sphincter: A Long-Term Study of Patient Satisfaction and Criteria for Success. J Urol 1996;156:1975-80. [Crossref] [PubMed]
- Miller AR, Linder BJ, Rangel LJ. The impact of incontinence etiology on artificial urinary sphincter outcomes. Investig Clin Urol 2017;58:241-6. [Crossref] [PubMed]


