
Urethral atrophy is now a rare cause for artificial urinary sphincter revision surgery in the contemporary 3.5 cm cuff era
Introduction
For nearly 50 years, the artificial urinary sphincter (AUS) has been widely accepted as the gold standard treatment for male stress urinary incontinence (SUI) treatment. While the long-term efficacy and patient satisfaction have been well demonstrated, many AUS patients eventually require reoperation for recurrent or persistent incontinence (1-8). Traditionally, urethral subcuff atrophy has been established as the leading reason for AUS revision (2,3,9,10).
Since the introduction of the 3.5 cm AUS cuff to the U.S. market in 2010, precise cuff sizing has been suggested to reduce revisions due to spongiosal atrophy (11). The availability of the smaller cuff theoretically prevents ongoing leakage due to improper cuff sizing in men with small urethras. We investigated our high volume experience with AUS revision and replacement procedures to identify the most common causes of recurrent incontinence over the past decade.
Methods
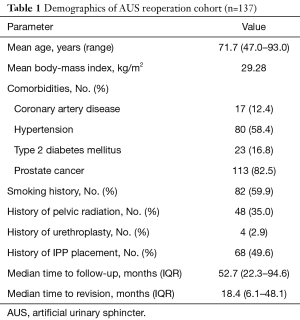
We retrospectively reviewed our tertiary referral center database of male AUS procedures performed by a single surgeon from 2007–2019. Men undergoing AUS revision or replacement surgeries were included; those without documented follow-up or with prior AUS cuff erosions were excluded. The need for AUS revision/replacement and device failure was identified in clinic. AUS cuff sizes and reasons for reoperation were recorded based on intraoperative findings and cystoscopy. Patient demographics, comorbidities, and relevant clinic visits were reviewed (Table 1).
Full table
Cases were stratified by cuff size of the initial AUS system (3.5 vs. ≥4.0 cm). Reasons for reoperation were determined by operative note review by year of revision amongst four three-year periods from 2008–2019. This analysis was intended to examine the changing trends across time in reasons for AUS failure spanning the years before and after the introduction of the 3.5 cm cuff in 2010. Urethral atrophy was recorded when there was no known fluid loss of the system and placement of a smaller cuff size resolved the inadequate cuff coaptation. No tandem cuff procedures were performed during this cohort.
Mechanical device failure was established by intraoperative device interrogation. In cases of suspected system leak, device components were systematically injected with saline and/or aspirated to assess component integrity. In cases of suspected PRB herniation, herniated balloons were palpable in the groin area and otherwise intact without evidence of fluid loss. Intraoperative cystoscopic improvement of sphincter coaptation was confirmed during application of manual pressure to the intact herniated balloon.
Results
Reasons for reoperation
From a series of 714 AUS procedures at our center from 2007–2019, 177 revision or replacement procedures were identified, with 137 meeting inclusion criteria [mean age 71.7 years, median follow-up 52.7 months (IQR 22.3–94.6 months)] (Table 1). Mechanical failure was the leading reason for reoperation (95/137, 69.3%). Urethral atrophy was cited as the reason for revision in only 8.0% (11/137) of cases.
PRB failure was the single most common reason for revision (47/95, 49.5%), followed by cuff failure due to cuff leak or defect (23/95, 24.2%). Of the revisions/replacements attributed to PRB failure, 48.9% (23/47) were due to PRB leak or fluid loss, and 25.5% (12/47) were due to PRB herniation. Failure of the AUS pump or tubing was the reason for reoperation in 8.0% (11/137) of cases. The reason for device failure or recurrent incontinence was unknown in 6.6% (9/137) of cases.
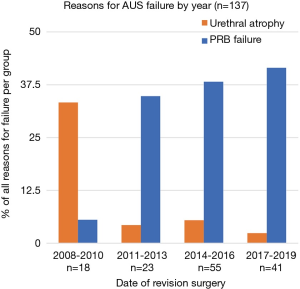
For the cases with available data on date of primary AUS placement (92%), median time to AUS revision or replacement surgery from the date of immediate prior AUS placement was 18.4 months (IQR 6.1–48.1 months). The mean time to revision was longest for reoperation due to urethral atrophy (42.0 months) compared to PRB failure (27.0 months), cuff failure (37.7 months), and pump failure (15.7 months). Of revisions performed from 2008–2010 in our cohort, 33.3% were cited as due to urethral atrophy, while the incidence of atrophy decreased to 4.3%, 5.45%, and 2.4% amongst revisions performed in 2011–2013, 2014–2016, and 2017–2019, respectively. Revisions due to PRB failure made up 5.56% of AUS revisions performed from 2008–2010, and increased to account for 34.8%, 38.2%, and 41.5% of revisions performed in 2011–2013, 2014–2016, and 2017–2019, respectively (Figure 1).
3.5 vs. ≥4.0 cm cuff systems
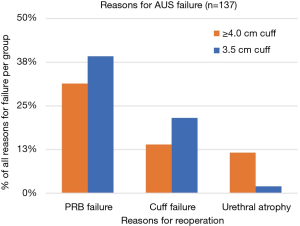
Of the 137 revision surgeries, 51 (37.2%) involved previously placed 3.5 cm cuff AUS systems [median follow-up 54.2 months (IQR 28.4–90.4 months)], and 86 (62.8%) were on previously placed ≥4.0 cm cuff AUS systems [median follow-up 52.7 months (IQR 13.6–95.7)]. No tandem cuffs were placed at the time of AUS revision/replacement. Fewer reoperations occurred due to urethral atrophy in the 3.5 cm cuff group (1/51, 2.0%) compared to the ≥4.0 cm cuff group (10/86, 11.6%, P=0.05) (Figure 2). PRB failure was the leading reason for reoperation for both the 3.5 cm cuff group (20/51, 39.2%) and the ≥4.0 cm cuff group (27/86, 31.4%). There were no other significant differences between the two groups regarding the number of AUS failures attributed to a certain cause (Table 2).

Full table
Discussion
Urethral atrophy revisited
Although AUS placement still represents the gold standard for male SUI, it is a delicate system prone to revision or complete replacement for recurrent SUI. Urethral atrophy has traditionally been cited as the most common cause of device failure, accounting for more than half of AUS reoperation cases in multiple series over the past three decades (3,9,10). Long-term compression of the urethra by the AUS cuff has been proposed to cause ischemia of the corpus spongiosum, thus leading to inadequate coaptation within the cuff (3). Decreased urethral circumference has been associated with prostate cancer treatments including radiation and radical prostatectomy (12). Recent investigators propose that the atrophic urethra may further be compressed by a fibrotic psuedocapsule sheath developing on the inner surface of the cuff (13,14).
High atrophy rates of 52.9–74%, recorded from older studies dating from 1995–2005, were published long before availability of 3.5 cm cuffs (3,10). We suspect that large cuffs on small urethras likely led to many AUS revision/replacements to be misclassified as urethral atrophy in those earlier reports. After the introduction and regular use of the 3.5 cm cuff, the incidence of AUS revision for urethral atrophy decreased sharply (Figure 2). Atrophy comprised nearly a third of the revisions performed from 2008–2010, but later dropped to less than ten percent of revisions in later years.
This overall temporal trend away from atrophy as cause for revision is further emphasized when comparing cuff sizes. Far fewer revisions were attributed to urethral atrophy among 3.5 cm cuff patients compared to ≥4.0 cm cuff cases, thus suggesting that appropriate primary use of the 3.5 cm cuff in men with smaller urethras prevents the need for further revisions due to atrophy. We utilize the 3.5 cm cuff primarily for men with urethral circumference <3.5 cm. Although transcorporal cuff placement has been proposed for men with small urethras, we reserve the transcorporal option for salvage cases in men with prior urethral reconstruction or cuff erosion (15).
We recognize that use of the 3.5 cm cuff remains controversial, as evidenced by a recent series of over 1000 AUS cases from Mayo Clinic in which the 3.5 cm cuff was never used (2). USC investigators recently reported an increased susceptibility to failure among 3.5 cm cuffs in their experience, while Kretschmer et al. reported decreased revisions among 3.5 cm cuff cases (16,17). Nevertheless, our extended experience has been that the safety and long-term success of the 3.5 cm cuff are nearly identical to larger cuffs (18). A unique strength of the current study is the large volume of cases with 3.5 cm cuffs placed at the time of primary AUS implantation in our cohort [40/51 (78.4%) revisions of 3.5 cm cuff systems], which likely provides a more accurate representation of 3.5 cm cuff performance compared to series with few cases of failure after primary 3.5 cm cuff placement.
Primary 3.5 cm cuff placement appears to offer some advantages in men with small urethras. When the smallest cuff size resides in the most proximal location on the urethra, the need for subsequent perineal dissection during reoperation is often eliminated; for example, if no fluid loss is confirmed by ultrasound, CT scan, palpation, or other clinical means, attention can be focused on the PRB. Mechanical weakness, herniation, and pressure-related phenomena of the PRB are more often detected as the cause of device failure in this setting because improper cuff sizing has been eliminated as a potential cause of failure.
PRB failure
PRB failure has now become the most common specific cause for AUS reoperation in our experience. Our finding that roughly one-third of revisions were attributed to PRB issues is virtually identical to a recent large series reporting 36.5% of failures attributed to PRB failure (19). Other recent studies cite similar rates of mechanical device failure as the most common reason for revision, reporting 38.8–62% of AUS failures in their respective cohorts (2,13,14,19,20). Intraoperative interrogation of balloon integrity has revealed that material fatigue is common—roughly two-thirds of PRBs showed pressures less than the manufacturer range (13,14). Patients should be counseled on the delicate nature of the AUS system, especially the PRB, and the frequent need for reoperation. We have recently seen many cases in which PRB replacement alone has yielded dramatic improvement of continence. Larger series are necessary to confirm best practices for prevention, diagnosis, and management options in cases of PRB failure.
Limitations
This study is limited by its single-surgeon retrospective nature and associated inherent biases. In addition, the remote location of many of our patients from our regional tertiary referral center often compromises our ability to ensure detailed follow-up. Many patients travel from considerable distances to seek treatment at our center—32% (128/406) of a cohort of our AUS patients traveled >100 miles to our center. Some patients choose to follow-up with their local urologists, only presenting at our clinic if complications arise.
Urethral atrophy has traditionally been diagnosed imprecisely, through visualization of poor urethral coaptation via cystoscopy (4). Only in 2010 was the device assembly kit modified to include a measuring tape that extended down to 3.5 cm. We found that when poor coaptation was noted, it was often due to fluid loss within a single device component and coaptation was often restored upon replacement of that component. Greater awareness of the importance of device interrogation during reoperation cases over the duration of the study may have contributed to the increasing recognition of the exact site of mechanical device failures. While follow-up was similar between the 3.5 and ≥4.0 cm cuff groups (median 54.2 and 52.7 months, respectively), the more recent implementation of 3.5 cm cuff usage inherently lending itself to lesser follow-up may create an element of follow-up bias in detecting urethral atrophy.
Finally, Linder et al. proposed three years as a cutpoint for whole rather than partial replacement. We have not seen delayed failures after isolated PRB replacements, thus suggesting that the three year cutpoint supported by Linder et al. is arbitrary. Further study is warranted to better establish guidelines for this question.
Conclusions
Our updated twelve-year experience with AUS revision surgery confirms that urethral atrophy is now rarely a cause for persistent or recurrent urinary leakage. PRB failure is now the leading cause of AUS reoperation.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. AF Morey receives honoraria for being a guest lecturer/meeting participant for Boston Scientific and Coloplast Corp. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of The University of Texas Southwestern Medical Center at Dallas (ID# 102012-019). The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J Urol 1974;112:75-80. [Crossref] [PubMed]
- Linder BJ, Rivera ME, Ziegelmann MJ, et al. Long-term Outcomes Following Artificial Urinary Sphincter Placement: An Analysis of 1082 Cases at Mayo Clinic. Urology 2015;86:602-7. [Crossref] [PubMed]
- Martins FE, Boyd SD. Artificial urinary sphincter in patients following major pelvic surgery and/or radiotherapy: are they less favorable candidates? J Urol 1995;153:1188-93. [Crossref] [PubMed]
- Lai HH, Hsu EI, Teh BS, et al. 13 Years of Experience With Artificial Urinary Sphincter Implantation at Baylor College of Medicine. J Urol 2007;177:1021-5. [Crossref] [PubMed]
- Cohen AJ, Kuchta K, Park S, et al. Patterns and timing of artificial urinary sphincter failure. World J Urol 2018;36:939-45. [Crossref] [PubMed]
- Te Dorsthorst MJ, van der Doelen MJ, Farag F, et al. Survival of the artificial urinary sphincter in a changing patient profile. World J Urol 2019;37:899-906. [Crossref] [PubMed]
- Radomski SB, Ruzhynsky V, Wallis C, et al. Complications and Interventions in Patients with an Artificial Urinary Sphincter: Long-Term Results. J Urol 2018;200:1093-8. [Crossref] [PubMed]
- Van der Aa F, Drake MJ, Kasyan GR, et al. The Artificial Urinary Sphincter After a Quarter of a Century: A Critical Systematic Review of Its Use in Male Non-neurogenic Incontinence. Eur Urol 2013;63:681-9. [Crossref] [PubMed]
- Anusionwu II, Wright EJ. Indications for Revision of Artificial Urinary Sphincter and Modifiable Risk Factors for Device-Related Morbidity. Neurourol Urodyn 2013;32:63-5. [Crossref] [PubMed]
- Raj GV, Peterson AC, Toh KL, et al. Outcomes Following Revisions and Secondary Implantation of the Artificial Urinary Sphincter. J Urol 2005;173:1242-5. [Crossref] [PubMed]
- Simhan J, Morey AF, Zhao LC, et al. Decreasing Need for Artificial Urinary Sphincter Revision Surgery by Precise Cuff Sizing in Men with Spongiosal Atrophy. J Urol 2014;192:798-803. [Crossref] [PubMed]
- Viers BR, Mathur S, Hofer MD, et al. Clinical Risk Factors Associated With Urethral Atrophy. Urology 2017;103:230-3. [Crossref] [PubMed]
- Bugeja S, Ivaz SL, Frost A, et al. Urethral atrophy after implantation of an artificial urinary sphincter: fact or fiction? BJU Int 2016;117:669-76. [Crossref] [PubMed]
- Pearlman AM, Rasper AM, Terlecki RP. Proof of concept: Exposing the myth of urethral atrophy after artificial urinary sphincter via assessment of circumferential recovery after capsulotomy and intraoperative pressure profiling of the pressure regulating balloon. Investig Clin Urol 2018;59:275-9. [Crossref] [PubMed]
- Wingate JT, Erickson BA, Murphy G, et al. Multicenter Analysis of Patient Reported Outcomes Following Artificial Urinary Sphincter Placement for Male Stress Urinary Incontinence. J Urol 2018;199:785-90. [Crossref] [PubMed]
- Loh-Doyle JC, Harmtan N, Nazemi A, et al. Mechanical failure rates of artificial urinary sphincter components: Is the 3.5-cm urethral cuff at higher risk? Neurourol Urodyn 2019;38:187-92. [Crossref] [PubMed]
- Kretschmer A, Buchner A, Grabbert M, et al. Risk factors for artificial urinary sphincter failure. World J Urol 2016;34:595-602. [Crossref] [PubMed]
- McKibben MJ, Shakir N, Fuchs JS, et al. Erosion rates of 3.5-cm artificial urinary sphincter cuffs are similar to larger cuffs. BJU Int 2019;123:335-41. [Crossref] [PubMed]
- Srivastava A, Joice GA, Patel HD, et al. Causes of Artificial Urinary Sphincter Failure and Strategies for Surgical Revision: Implications of Device Component Survival. Eur Urol Focus 2019;5:887-93. [Crossref] [PubMed]
- Moses RA, Keihani S, Craig JR, et al. Efficacy of Pressure Regulating Balloon Exchange in Men With Post Artificial Urinary Sphincter Persistent or Recurrent Stress Urinary Incontinence. Urology 2019;123:252-7. [Crossref] [PubMed]


