
A primer on extramammary Paget’s disease for the urologist
Introduction
Extramammary Paget’s disease (EMPD) is a rare yet lethal cutaneous malignancy with an overall survival rate of 60.2% at 10 years postdiagnosis (1). This disease is distinct from Paget’s disease of the breast and predominantly affects the apocrine gland-bearing skin such as the perianal, genital and axillary regions of the body. Due to the rarity of this disease, controversies exist within the literature regarding its true prevalence, its association with concurrent internal malignancies and the diagnostic evaluation, therapeutic approaches and follow-up management. Currently, treatment recommendations for EMPD are based off single institution series and small case reports only, with many different treatment options, both in clinical and experimental use, yielding variable outcomes and results (2-4). We aim to provide an up-to-date review of the current knowledge of EMPD. In addition to discussing the clinical presentation and prognostic outcomes of this disease, we also focus and elaborate on the diagnostic approaches and treatment alternatives available to physicians when faced with patients presenting with this malignancy.
Epidemiology & pathophysiology
The incidence of EMPD has been reported to be as low as 0.12 per 100,000 people and represents 21% of primary scrotal cancers and 2% of primary vulvar cancers, respectively (5-7). With regards to gender and racial preponderance, Asian men seem to have a fourfold increased risk of being diagnosed with EMPD when compared to their Caucasian counterparts (1,8). Previous studies have also reported a female predominance in Caucasian populations (M:F ratio of 1:2–1:7) with the opposite being true among the Asian population (M:F ratio of 2:1–4:1) (5). EMPD represents 6.5% of all cutaneous Paget’s disease and it predominantly affects patients between ages 50 to 80 years, with a peak age of 66 years old (9-11). The most common sites affected by EMPD is the vulva (65%), followed by the perianal region (20%) and subsequently the penoscrotal and groin areas (14%) (12).
Two prognostically distinct pathogenesis of EMPD have been described (13). In the primary or intraepidermal form, carcinoma develops in situ from the apocrine gland ducts and is less commonly associated with an underlying malignancy; however, it is still capable of invading the dermal layers and metastasizing over time (14). In contrast, the secondary form arises from an epidermotropic spread of malignant cells from a primary tumor within a contiguous epithelium, such as the genitourinary or gastrointestinal tract, or from a dermal adnexal gland (15,16).
Clinical presentation
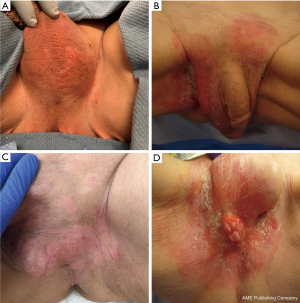
EMPD most commonly presents as a well delineated or poorly defined erythematous and scaly plaque that may encrust, ulcerate or develop pigmentation (Figure 1) (17). Due to its similar clinical presentation to many benign conditions, the diagnosis of EMPD is often delayed for years, with only 17% of patients being correctly diagnosed at first presentation (18). The most common presenting symptoms include pruritus (up to 60–72%), rash or erythema, which usually raises little suspicion for a malignant etiology (19,20). The initial differential diagnoses are commonly contact dermatitis, seborrheic eczema and fungal infections (12,18). Thus, conservative management such as topical emollients or corticosteroids, antifungal creams or other oral therapies are often prescribed first for these benign conditions. Patients typically experience a delay in definitive diagnosis for an average of 21 to 43 months after multiple rounds of failed treatment and persistent symptoms (10,11). Therefore, providers should biopsy any recalcitrant lesions that fail to respond to expectant treatments or acquire a specialist referral to decrease the risk of delayed diagnosis.
On histology, lesions usually reveal an epidermal infiltration of Paget cells, which appear as large, round cells with an abundant, pale-pink cytoplasm, surrounding a hypochromatic nuclei, occasionally with a prominent nucleolus (21). Pathognomonic cells contain intracytoplasmic sialomucin, which is capable of staining periodic acid-Schiff (PAS), mucicarmine, colloidal iron and alcian blue stains, which can aid in the diagnosis of EMPD (9). Cells may also express cytokeratin (CK), which are easily identifiable on immunochemical staining. While CK7 has been reported to have good sensitivity for EMPD, ranging from 86–100%, CK20 appears to be more specific for this disease (22-24). The expression of hormonal receptors has also been examined. A lack of both the estrogen and progesterone receptors together with the presence of androgen receptors and overexpression of HER-2 protein is often suggestive of EMPD (25,26). Furthermore, the presence of tumor suppressor protein p53 as well as the expression of tumor proliferation markers, such as Ki-67 and cyclin D1, has been linked to the secondary form of EMPD, which also predicts the invasiveness of EMPD lesions (27,28). While there are no grading systems for EMPD histology, the unified perception is that all EMPD lesions are considered high grade (29).
Course and prognosis
Patients with primary or intraepithelial EMPD have a favorable prognosis, with studies showing that the mortality rates of patients treated adequately for non-invasive disease do not differ significantly from that of the general matched population (30). Primary disease, though seemingly treatable, has the potential to disseminate and become invasive (31). Overall mortality rates for the secondary form of EMPD have been reported to be 26–66%, either from metastatic EMPD or from their associated internal malignancies (19). Moreover, mortality rates were found to be significantly higher in patients with an underlying adnexal carcinoma when compared to those who did not (46% vs. 18%, P<0.05) (12).
Several factors such as dermal invasion, elevated CEA levels and lymph node metastasis portend a greater risk of adverse prognosis, with the former being the most significant. Hatta found that the 5-year survival rate for patients with deep invasion beneath the reticular dermis was significantly lower than patients with no invasion or with microinvasion to the papillary dermis (32). Histological assessment is therefore needed for appropriate risk stratification (33-35). Serum CEA levels have also been associated with predicting systemic metastasis in EMPD patients with a sensitivity of 70% and specificity of 94% (36). CEA may be used as an indicator to monitor treatment effects and outcomes for EMPD (37,38). Regional lymph node metastasis has also been shown to significantly affect the prognosis of EMPD. Unfortunately, limited studies have evaluated the utility of lymphadenectomy as a treatment strategy for EMPD (32,34). Further assessment of lymphadenectomy should be performed before it can be accepted as a standard treatment. Studies have also examined the efficacy of sentinel lymph node biopsy (SLNB) and reported a significant association between SLN positivity and increased dermal invasion rates, lower overall 5-year survival rates, and higher lymphovascular metastatic rates (39-44). Due to the limited existing data regarding lymphadenectomy, chemotherapy, immunotherapy and radiation therapy in EMPD disease, patients with metastatic disease should be evaluated via a multidisciplinary approach to develop a treatment plan that may maximize outcomes for individual patients.
Association with internal malignancy
In contrast to Paget’s disease of the breast, whereby 100% of patients have an underlying ductal breast carcinoma, the rates of EMPD with an associated malignancy is reported to be 21–29% (12,45). Controversy exists within the literature regarding this association with concurrent internal malignancies. Chanda demonstrated that topographic locations of EMPD appear to be closely related to the anatomic sites of the underlying malignancy. For example, neoplasms of the male genitourinary tract (e.g., prostate) were associated with penoscrotal EMPD, neoplasms of the gastrointestinal tract (e.g., rectal) were associated with perianal EMPD, and neoplasms of the female genitourinary tract (e.g., squamous cell and adenocarcinoma of the cervix, Bartholin glands) were associated with vulvar EMPD (12). Therefore, directed screening tests for underlying malignancies based on clinical presentations have been recommended.
Investigations and diagnostic evaluation
A thorough history and physical examination should be performed with special attention to the location, distribution, size, color and morphology of the EMPD lesion. Palpation for enlarged lymph nodes and hepatosplenomegaly should be performed with a breast, pelvic and digital rectal exam as indicated. A biopsy of the lesion should be performed if not done so already. Pathology should be reviewed for factors such as dermal invasion which portends a higher risk of adverse prognosis (18,32,45).
A comprehensive laboratory workup, including serum carcinoembryonic antigen (CEA) levels should be obtained. Cross-sectional imaging of the abdomen and pelvis may be performed to evaluate for nodal or metastatic disease. Screening for associated internal malignancies, such as cervical, colorectal, bladder and prostate may be done as clinically indicated with Pap smear, colonoscopy, cystoscopy and serum prostate-specific antigen, respectively. If patients have lapsed the recommended screening intervals, respective specialty referral may be warranted (46).
Treatment and management
A recent online questionnaire queried an international cohort and identified that EMPD is treated by a variety of providers using an array of treatment modalities. These phenomena pose a challenge to developing guidelines for diagnosis, management and follow-up (18). Currently, a few invasive and non-invasive treatment options exist for EMPD with limited head-to-head comparisons. In fact, there is only one existing clinical trial evaluating the safety and efficacy of topical imiquimod cream for non-invasive vulvar Paget’s disease (NCT02385188) (47). Other non-invasive treatments include radiation and chemotherapy, laser therapy or photodynamic therapy while the more invasive approaches include Mohs micrographic surgery (MMS) and wide local excision (WLE). Table 1 summarizes the response and recurrence rates for non-invasive treatment options while Figure 2 depicts the advantages and disadvantages of the main treatment modalities for EMPD.

Full table

Non-surgical treatment
Laser therapy
Laser treatment for EMPD has garnered much interest as a conservative approach that may preserve anatomy and sexual function. Several reports have demonstrated the effectiveness of both the CO2 and Nd:YAG lasers to treat EMPD with the advantage of shorter operative and hospitalization times; however, the lack of histologic evidence for analysis, post-operative pain and anesthesia requirements have prompted both physicians and patients to seek alternative treatments (48-50). Several reports have also noted high recurrence rates even up to 67–100% with this treatment modality (51,52). This is likely due to the multifocal and extensive nature of EMPD lesions and the overly superficial ablative techniques provided by laser therapy which may not adequately treat microinvasive or invasive disease (53).
Photodynamic therapy (PDT)
PDT has been used to treat several neoplasms such as non-melanoma skin cancer, esophageal carcinoma and even non-small cell lung carcinoma (54,55). It relies on the interaction between oxygen and a photosensitizer, either topical 5-aminolevulinic acid (ALA) or systemic porfimer sodium (PS), to generate reactive oxygen species that selectively destroy neoplastic tissue (56). While topical ALA offers excellent cosmetic outcomes, its ability to treat invasive and multifocal extensions of the disease remains questionable (57,58). While PS may alleviate this concern, systemic administration of a photosensitizer may generate a more severe local reaction which requires longer healing times. The safety and efficacy of PDT have yet to be clearly elucidated and therefore should be limited to patients who are unable to undergo surgery or with lesions in difficult anatomic locations (59).
Radiotherapy
Use of radiation therapy in the treatment of EMPD has been reported in several case reports (60-64). The majority of these patients presented with primary lesions on functionally delicate areas or were non-surgical candidates. Acute and chronic radiation toxicity is the major adverse effect. Initial investigations have described many different treatment techniques with a diverse range of radiation beam types, energy and dosages, all of which have yielded varying results and outcomes. Further studies evaluating the safety and efficacy of radiotherapy should be conducted before conclusions can be drawn.
Topical chemotherapy
Topical chemotherapeutic agents have been utilized for EMPD. While these agents have reported response rates as high as 57–100% in localized disease, the side effects related to these agents including severe pain and dermatitis, moist desquamation and allergic reactions have mostly caused them to fall out of favor (65-67). 5-fluorouracil (5-FU), mostly used as an adjunct to surgery, has been reported to be useful in clearing residual lesions which may not be excisable during surgery (67). It may also have a role in highlighting subclinical areas of EMPD, allowing better visibility during surgery or be useful postoperatively to detect recurrence of disease (68). Most investigators, however, recommend against the use of 5-FU as a monotherapy as it has only been shown to clear clinical but not pathological disease. Both studies by Haberman and Kawatsu described the use of 5-FU in genital EMPD and found that although the primary lesions cleared clinically, biopsy specimens still demonstrated persistent disease (69,70). Topical bleomycin has also been considered for the treatment of EMPD. Watring et al. reported varying responses and outcomes in a series of seven patients treated for recurrent vulvar EMPD. Of these, four patients experienced complete therapeutic response with bleomycin, one of which required retreatment 30 months later and subsequently showed no evidence of recurrence (65).
Topical 5% imiquimod cream, more commonly known for treating genital warts, has been increasingly prescribed for off-label use in localized EMPD. EMPD response rates have been reported to be as high at 52–80% with a 19% recurrence rate (71). Imiquimod is an immune response modifier that enhances both innate and acquired immunity via the stimulation of cytokines, such as interferon-α and tumor necrosis factor-α. These cytokines in turn augment the anti-tumoral immune system to increase neoplastic cell death and destruction. Although the safety and efficacy of imiquimod for EMPD have yet to be completely exemplified, its side effect profile is better tolerated than much of the earlier chemotherapeutic agents, with mild dermatitis being the most commonly reported symptom (2,72-74). Depigmentation is another adverse effect that has been previously described and may obscure visible margins on gross examination at the time of excision or post-operative follow-up (75,76). Further studies thoroughly evaluating imiquimod are required before it may be considered as an adjunct to surgery, a potential alternative in non-surgical candidates or as part of a therapeutic combination with other non-invasive treatment modalities (77).
Surgical treatment
Surgical excision with negative margins is the mainstay treatment for EMPD. Lesions may skip, be multifocal, or develop asymmetrically, which makes achieving negative surgical margins challenging (20). Furthermore, margins cannot be clearly identified with visual inspection alone as malignant cells are capable of extending microscopically beyond the clinically evident lesion. Achieving negative margins is paramount for long-term survival as it has shown to prevent further invasion, metastasis and recurrence (Table 2) (17,78-81). Choi et al. identified that marginal status and lymphovascular invasion were the most valid prognosticating factors for EMPD recurrence, while Long et al. demonstrated that patients with positive margins had a four-fold increased risk of suffering local recurrence when compared to those with negative margins (82,83).

Full table
Several techniques have been adopted to help achieve negative margins, including the use of preoperative mapping biopsies, wide margins, intraoperative frozen sections and immunohistochemical and fluorescein staining (24,84,85,87-90). Yang et al. reported a 92% rate of obtaining negative margins with intraoperative frozen sections versus 26% without frozen sections. Misas et al. reported a 97.4% positive predictive value and 99.9% negative predictive value when assessing disease extent with fluorescein when compared to direct visualization (84,88). Despite these efforts, obtaining negative margins remains challenging (91). A study in 2005 found that 10 out of 19 patients (53%) were found to have positive margins despite undergoing intraoperative frozen sections during WLE (11). This may occur when only limited amounts of pathology frozen sections are assessed intraoperatively and complicated by the presence of multicentric, multifocal disease (83,86,92). Given the importance of obtaining negative margin status, we recommended surgical treatment to be performed at higher volume centers with the resources available to effectively perform adjunctive approaches.
The decision of surgical approach has been a long-standing debate among physicians. While MMS involves examining all the histological margins of the tumor during surgery, intraoperative frozen sections during WLE may sample a more limited area. Conceptually, MMS may seem like the preferred option over WLE for the treatment of EMPD. As such, certain investigations have shown that MMS may be superior to WLE in achieving negative margins (8,90,93). Unfortunately, this disease develops multifocal and skip lesions, which may account for some of the treatment failures with MMS. Effective use of MMS requires specialized training and may not be appropriate for larger lesions or lesions located in sensitive genital locations.
Although composite rates of achieving negative margins with MMS (97%) was higher than WLE (65%), other studies have also demonstrated comparable outcomes in obtaining adequate cancer control with both methods (6,11,17,83,94). Lesion size may play a role in selecting the surgical approach. A review of 38 cases of EMPD treated with MMS found that 76% of lesions were ≤10 cm while 97% of cases had lesion sizes ≤15 cm (93,95,96). Conversely, 95% of lesions treated with WLE were found to be ≥10 cm with 70% of lesions treated by Chung et al. being ≥15 cm, with the largest lesion measuring 30 cm in diameter (6,97). Several studies have demonstrated the ability of WLE to be performed safely and adequately and to yield equally satisfactory and durable outcomes to MMS with recurrence rates ranging from 21–60% in WLE and 0–26% in MMS (Table 3) (78,80,98-103). This suggests that while MMS may be effective in obtaining negative margins, it may be less amendable to treating larger EMPD lesions, in which case WLE may be the more favorable option.
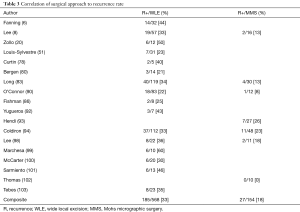
Full table
Morbidity and complication rates differ between these two surgical approaches. MMS allows for maximal tissue sparing and decreased complication rates when compared to WLE which is associated with higher risk of neurovascular injuries, lymphedema and skin defects (94). However, MMS is limited to smaller lesions, is performed under local anesthesia and may require closure of the defect at a separate encounter by another surgeon (93). Conversely, WLE may be performed for larger lesions or lesions in sensitive areas. Patients undergoing WLE may also benefit from simultaneous primary closure or admission for inpatient wound care prior to delayed closure as final pathology is being reviewed (97). Both MMS and WLE have their respective advantages and an individualized, shared-decision making approach is recommended.
EMPD defects may require reconstruction with the use of complex primary closures, skin grafts and flaps (11). Skin grafts are a versatile reconstructive method with reported use as high as 44–80% in the management of EMPD. Advancement flaps, rotational flaps and myocutaneous flaps of the gluteal and thigh muscles may also be used for coverage and may require the assistance of a plastic surgeon (104-108). In general, penile shaft lesions may best be covered with skin grafts to preserve cosmesis and reduce the risk of contracture or chordee formation. Suprapubic, scrotal, inguinal, and perineal lesions may be amenable to complex primary closure by aggressive mobilization of neighboring tissues, flaps, or skin grafts. Due to the skills needed to both excise and reconstruct the genitalia, a reconstructive urologist may be best suited to manage localized genital EMPD.
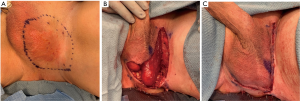
At our institution, WLE is the preferred method for excision of genital EMPD lesions (Figure 3) (97). Pre-operative mapping biopsies and intra-operative frozen sections are used in conjunction with a 2-cm margin to help overcome the insidious nature of EMPD. Murata et al. found that the distance between the resected edge of the EMPD lesion to the last lesional cell on histopathology measured 1.02 cm on average (109). This, coupled with surgeon preference, justified our rationale for obtaining 2-cm margins when managing EMPD patients. Our protocol is to delay complex wound closure (skin grafting or flaps) until negative margins have been confirmed on final pathology. Patients requiring complex wound closure are admitted for inpatient wound care with wet-to-dry dressings or xenografts while permanent specimens undergo expeditious pathology review. If positive margins are identified, further excision of the corresponding region is performed. Once negative margins are confirmed, complex wound closure with or without split thickness skin grafting or local flaps is performed during the same admission (97).
Although there are no strict guidelines regarding the need and frequency of post-operative management, continued surveillance and follow-up is absolutely warranted due to the high incidence of recurrence with EMPD. We follow patients every 3 months in the first year, 6 months in the second and annually thereafter. A routine physical examination is required during each clinic visit, while cross sectional imaging of the abdomen and pelvis can be performed with either CT or MRI to rule out systemic metastasis. Mapping biopsies may be performed according to clinical suspicion for disease recurrence while serum CEA levels are obtained to monitor treatment response.
Conclusions
Surgical excision to achieve negative margins remains the mainstay of treatment to decrease local recurrence rates and maximize durable cure in noninvasive disease. Further studies are still necessary to examine the implications of dermal invasion and lymph node involvement in EMPD and to elucidate the most efficacious treatment modality with the least morbidity. EMPD patients should be referred to centers of excellence with the experience and resources of a multidisciplinary panel that can ensure homogeneity of care, eventually allowing for the development of treatment protocols and consensus guidelines.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget's disease in males: survival outcomes in 495 patients. Ann Surg Oncol 2015;22:1625-30. [Crossref] [PubMed]
- Cowan RA, Black DR, Hoang LN, et al. A pilot study of topical imiquimod therapy for the treatment of recurrent extramammary Paget's disease. Gynecol Oncol 2016;142:139-43. [Crossref] [PubMed]
- Wollina U, Goldman A, Bieneck A, et al. Surgical Treatment for Extramammary Paget's Disease. Curr Treat Options Oncol 2018;19:27. [Crossref] [PubMed]
- Yoshino K, Fujisawa Y, Kiyohara Y, et al. Usefulness of docetaxel as first-line chemotherapy for metastatic extramammary Paget's disease. J Dermatol 2016;43:633-7. [Crossref] [PubMed]
- Cheng PS, Lu CL, Cheng CL, et al. Significant male predisposition in extramammary Paget disease: a nationwide population-based study in Taiwan. Br J Dermatol 2014;171:191-3. [Crossref] [PubMed]
- Fanning J, Lambert HC, Hale TM, et al. Paget's disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget's disease, and recurrence after surgical excision. American journal of obstetrics and gynecology 1999;180:24-7. [Crossref] [PubMed]
- Wright JL, Morgan TM, Lin DW. Primary scrotal cancer: disease characteristics and increasing incidence. Urology 2008;72:1139-43. [Crossref] [PubMed]
- Lee SJ, Choe YS, Jung HD, et al. A multicenter study on extramammary Paget's disease in Korea. Int J Dermatol 2011;50:508-15. [Crossref] [PubMed]
- Kanitakis J. Mammary and extramammary Paget's disease. J Eur Acad Dermatol Venereol 2007;21:581-90. [PubMed]
- Kang Z, Zhang Q, Li X, et al. Clinical and pathological characteristics of extramammary Paget's disease: report of 246 Chinese male patients. Int J Clin Exp Pathol 2015;8:13233-40. [PubMed]
- Jung JH, Kwak C, Kim HH, et al. Extramammary Paget Disease of External Genitalia: Surgical Excision and Follow-up Experiences With 19 Patients. Korean J Urol 2013;54:834-9. [Crossref] [PubMed]
- Chanda JJ. Extramammary Paget's disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol 1985;13:1009-14. [Crossref] [PubMed]
- Wagner G, Sachse MM. Extramammary Paget disease - clinical appearance, pathogenesis, management. J Dtsch Dermatol Ges 2011;9:448-54. [PubMed]
- Goldblum JR, Hart WR. Vulvar Paget's disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol 1997;21:1178-87. [Crossref] [PubMed]
- Shepherd V, Davidson EJ, Davies-Humphreys J. Extramammary Paget's disease. BJOG 2005;112:273-9. [Crossref] [PubMed]
- Lloyd J, Flanagan AM. Mammary and extramammary Paget's disease. J Clin Pathol 2000;53:742-9. [Crossref] [PubMed]
- Hegarty PK, Suh J, Fisher MB, et al. Penoscrotal extramammary Paget's disease: the University of Texas M. D. Anderson Cancer Center contemporary experience. J Urol 2011;186:97-102. [Crossref] [PubMed]
- Chung PH, Kampp JT, Voelzke BB. Patients' Experiences With Extramammary Paget Disease: An Online Pilot Study Querying a Patient Support Group. Urology 2018;111:214-9. [Crossref] [PubMed]
- Parker LP, Parker JR, Bodurka-Bevers D, et al. Paget's disease of the vulva: pathology, pattern of involvement, and prognosis. Gynecol Oncol 2000;77:183-9. [Crossref] [PubMed]
- Zollo JD, Zeitouni NC. The Roswell Park Cancer Institute experience with extramammary Paget's disease. Br J Dermatol 2000;142:59-65. [Crossref] [PubMed]
- Park S, Grossfeld GD, McAninch JW, et al. Extramammary Paget's disease of the penis and scrotum: excision, reconstruction and evaluation of occult malignancy. J Urol 2001;166:2112-6; discussion 7. [Crossref] [PubMed]
- Battles OE, Page DL, Johnson JE. Cytokeratins, CEA, and mucin histochemistry in the diagnosis and characterization of extramammary Paget's disease. Am J Clin Pathol 1997;108:6-12. [Crossref] [PubMed]
- Lundquist K, Kohler S, Rouse RV. Intraepidermal cytokeratin 7 expression is not restricted to Paget cells but is also seen in Toker cells and Merkel cells. Am J Surg Pathol 1999;23:212-9. [Crossref] [PubMed]
- Smith KJ, Tuur S, Corvette D, et al. Cytokeratin 7 staining in mammary and extramammary Paget's disease. Mod Pathol 1997;10:1069-74. [PubMed]
- Diaz de Leon E, Carcangiu ML, Prieto VG, et al. Extramammary Paget disease is characterized by the consistent lack of estrogen and progesterone receptors but frequently expresses androgen receptor. Am J Clin Pathol 2000;113:572-5. [Crossref] [PubMed]
- Tanskanen M, Jahkola T, Asko-Seljavaara S, et al. HER2 oncogene amplification in extramammary Paget's disease. Histopathology 2003;42:575-9. [Crossref] [PubMed]
- Aoyagi S, Akiyama M, Shimizu H. High expression of Ki-67 and cyclin D1 in invasive extramammary Paget's disease. J Dermatol Sci 2008;50:177-84. [Crossref] [PubMed]
- Zhang C, Zhang P, Sung CJ, et al. Overexpression of p53 is correlated with stromal invasion in extramammary Paget's disease of the vulva. Hum Pathol 2003;34:880-5. [Crossref] [PubMed]
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: Retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci 2016;83:234-9. [Crossref] [PubMed]
- Pierie JP, Choudry U, Muzikansky A, et al. Prognosis and management of extramammary Paget's disease and the association with secondary malignancies. J Am Coll Surg 2003;196:45-50. [Crossref] [PubMed]
- Feuer GA, Shevchuk M, Calanog A. Vulvar Paget's disease: the need to exclude an invasive lesion. Gynecol Oncol 1990;38:81-9. [Crossref] [PubMed]
- Hatta N. Prognostic Factors of Extramammary Paget's Disease. Curr Treat Options Oncol 2018;19:47. [Crossref] [PubMed]
- Ito Y, Igawa S, Ohishi Y, et al. Prognostic indicators in 35 patients with extramammary Paget's disease. Dermatol Surg 2012;38:1938-44. [Crossref] [PubMed]
- Tsutsumida A, Yamamoto Y, Minakawa H, et al. Indications for lymph node dissection in the treatment of extramammary Paget's disease. Dermatol Surg 2003;29:21-4. [PubMed]
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol 2015;40:473-8. [Crossref] [PubMed]
- Hatta N, Yamada M, Hirano T, et al. Extramammary Paget's disease: treatment, prognostic factors and outcome in 76 patients. Br J Dermatol 2008;158:313-8. [PubMed]
- Oji M, Furue M, Tamaki K. Serum carcinoembryonic antigen level in Paget's disease. Br J Dermatol 1984;110:211-3. [Crossref] [PubMed]
- Zhu Y, Ye DW, Yao XD, et al. Clinicopathological characteristics, management and outcome of metastatic penoscrotal extramammary Paget's disease. Br J Dermatol 2009;161:577-82. [Crossref] [PubMed]
- Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992;127:392-9. [Crossref] [PubMed]
- Cochran AJ, Essner R, Rose DM, et al. Principles of sentinel lymph node identification: background and clinical implications. Langenbecks Arch Surg 2000;385:252-60. [Crossref] [PubMed]
- Hatta N, Morita R, Yamada M, et al. Sentinel lymph node biopsy in patients with extramammary Paget's disease. Dermatol Surg 2004;30:1329-34. [PubMed]
- Ogata D, Kiyohara Y, Yoshikawa S, et al. Usefulness of sentinel lymph node biopsy for prognostic prediction in extramammary Paget's disease. Eur J Dermatol 2016;26:254-9. [Crossref] [PubMed]
- Fujisawa Y, Yoshino K, Kiyohara Y, et al. The role of sentinel lymph node biopsy in the management of invasive extramammary Paget's disease: Multi-center, retrospective study of 151 patients. J Dermatol Sci 2015;79:38-42. [Crossref] [PubMed]
- Nakamura Y, Fujisawa Y, Ishikawa M, et al. Usefulness of sentinel lymph node biopsy for extramammary Paget disease. Br J Dermatol 2012;167:954-6. [Crossref] [PubMed]
- Lai YL, Yang WG, Tsay PK, et al. Penoscrotal extramammary Paget's disease: a review of 33 cases in a 20-year experience. Plast Reconstr Surg 2003;112:1017-23. [Crossref] [PubMed]
- Lam C, Funaro D. Extramammary Paget's disease: Summary of current knowledge. Dermatol Clin 2010;28:807-26. [Crossref] [PubMed]
- van der Linden M, Meeuwis K, van Hees C, et al. The Paget Trial: A Multicenter, Observational Cohort Intervention Study for the Clinical Efficacy, Safety, and Immunological Response of Topical 5% Imiquimod Cream for Vulvar Paget Disease. JMIR Res Protoc 2017;6:e178. [Crossref] [PubMed]
- Valentine BH, Arena B, Green E. Laser ablation of recurrent Paget's disease of vulva and perineum. J Gynecol Surg 1992;8:21-4. [Crossref] [PubMed]
- Weese D, Murphy J, Zimmern PE. Nd: YAG laser treatment of extramammary Paget's disease of the penis and scrotum. J Urol (Paris) 1993;99:269-71. [PubMed]
- Ewing TL. Paget's disease of the vulva treated by combined surgery and laser. Gynecol Oncol 1991;43:137-40. [Crossref] [PubMed]
- Louis-Sylvestre C, Haddad B, Paniel BJ. Paget's disease of the vulva: results of different conservative treatments. Eur J Obstet Gynecol Reprod Biol 2001;99:253-5. [Crossref] [PubMed]
- Choi JB, Yoon ES, Yoon DK, et al. Failure of carbon dioxide laser treatment in three patients with penoscrotal extramammary Paget's disease. BJU Int 2001;88:297-8. [Crossref] [PubMed]
- Becker-Wegerich PM, Fritsch C, Schulte KW, et al. Carbon dioxide laser treatment of extramammary Paget's disease guided by photodynamic diagnosis. Br J Dermatol 1998;138:169-72. [Crossref] [PubMed]
- Wu H, Minamide T, Yano T. Role of photodynamic therapy in the treatment of esophageal cancer. Dig Endosc 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Shafirstein G, Battoo A, Harris K, et al. Photodynamic Therapy of Non-Small Cell Lung Cancer. Narrative Review and Future Directions. Ann Am Thorac Soc 2016;13:265-75. [PubMed]
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic Therapy: A Clinical Consensus Guide. Dermatol Surg 2016;42:804-27. [Crossref] [PubMed]
- Shieh S, Dee AS, Cheney RT, et al. Photodynamic therapy for the treatment of extramammary Paget's disease. Br J Dermatol 2002;146:1000-5. [Crossref] [PubMed]
- Henta T, Itoh Y, Kobayashi M, et al. Photodynamic therapy for inoperable vulval Paget's disease using delta-aminolaevulinic acid: successful management of a large skin lesion. Br J Dermatol 1999;141:347-9. [Crossref] [PubMed]
- Mikasa K, Watanabe D, Kondo C, et al. 5-Aminolevulinic acid-based photodynamic therapy for the treatment of two patients with extramammary Paget's disease. J Dermatol 2005;32:97-101. [Crossref] [PubMed]
- Moreno-Arias GA, Conill C, Castells-Mas A, et al. Radiotherapy for genital extramammary Paget's disease in situ. Dermatol Surg 2001;27:587-90. [PubMed]
- Guerrieri M, Back MF. Extramammary Paget's disease: role of radiation therapy. Australas Radiol 2002;46:204-8. [Crossref] [PubMed]
- Brierley JD, Stockdale AD. Radiotherapy: an effective treatment for extramammary Paget's disease. Clin Oncol (R Coll Radiol) 1991;3:3-5. [Crossref] [PubMed]
- Tackenberg S, Gehrig A, Dummer R, et al. External beam radiotherapy of extramammary Paget disease. Cutis 2015;95:109-12. [PubMed]
- Yanagi T, Kato N, Yamane N, et al. Radiotherapy for extramammary Paget's disease: histopathological findings after radiotherapy. Clin Exp Dermatol 2007;32:506-8. [Crossref] [PubMed]
- Watring WG, Roberts JA, Lagasse LD, et al. Treatment of recurrent Paget's disease of the vulva with topical bleomycin. Cancer 1978;41:10-1. [Crossref] [PubMed]
- Bewley AP, Bracka A, Staughton RC, et al. Extramammary Paget's disease of the scrotum: treatment with topical 5-fluorouracil and plastic surgery. Br J Dermatol 1994;131:445-6. [Crossref] [PubMed]
- Del Castillo LF, Garcia C, Schoendorff C, et al. Spontaneous apparent clinical resolution with histologic persistence of a case of extramammary Paget's disease: response to topical 5-fluorouracil. Cutis 2000;65:331-3. [PubMed]
- Eliezri YD, Silvers DN, Horan DB. Role of preoperative topical 5-fluorouracil in preparation for Mohs micrographic surgery of extramammary Paget's disease. J Am Acad Dermatol 1987;17:497-505. [Crossref] [PubMed]
- Haberman HF, Goodall J, Llewellyn M. Extramammary Paget's disease. Can Med Assoc J 1978;118:161-2. [PubMed]
- Kawatsu T, Miki Y. Triple extramammary Paget's disease. Arch Dermatol 1971;104:316-9. [Crossref] [PubMed]
- Machida H, Moeini A, Roman LD, et al. Effects of imiquimod on vulvar Paget's disease: a systematic review of literature. Gynecol Oncol 2015;139:165-71. [Crossref] [PubMed]
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget's disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol 2013;33:479-83. [Crossref] [PubMed]
- Liau MM, Yang SS, Tan KB, et al. Topical imiquimod in the treatment of extramammary Paget's disease: A 10 year retrospective analysis in an Asian tertiary centre. Dermatol Ther 2016;29:459-62. [Crossref] [PubMed]
- Dogan A, Hilal Z, Krentel H, et al. Paget's Disease of the Vulva Treated with Imiquimod: Case Report and Systematic Review of the Literature. Gynecol Obstet Invest 2017;82:1-7. [Crossref] [PubMed]
- Jacob SE, Blyumin M. Vitiligo-like hypopigmentation with poliosis following treatment of superficial basal cell carcinoma with imiquimod. Dermatol Surg 2008;34:844-5. [PubMed]
- Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol 2016;55:831-44. [Crossref] [PubMed]
- Ye JN, Rhew DC, Yip F, et al. Extramammary Paget's disease resistant to surgery and imiquimod monotherapy but responsive to imiquimod combination topical chemotherapy with 5-fluorouracil and retinoic acid: a case report. Cutis 2006;77:245-50. [PubMed]
- Curtin JP, Rubin SC, Jones WB, et al. Paget's disease of the vulva. Gynecol Oncol 1990;39:374-7. [Crossref] [PubMed]
- Baehrendtz H, Einhorn N, Pettersson F, et al. Paget's disease of the vulva: the Radiumhemmet series 1975-1990. Int J Gynecol Cancer 1994;4:1-6. [Crossref] [PubMed]
- Bergen S, DiSaia PJ, Liao SY, et al. Conservative management of extramammary Paget's disease of the vulva. Gynecol Oncol 1989;33:151-6. [Crossref] [PubMed]
- Black D, Tornos C, Soslow RA, et al. The outcomes of patients with positive margins after excision for intraepithelial Paget's disease of the vulva. Gynecol Oncol 2007;104:547-50. [Crossref] [PubMed]
- Choi YD, Cho NH, Park YS, et al. Lymphovascular and marginal invasion as useful prognostic indicators and the role of c-erbB-2 in patients with male extramammary Paget's disease: a study of 31 patients. J Urol 2005;174:561-5. [Crossref] [PubMed]
- Long B, Schmitt AR, Weaver AL, et al. A matter of margins: Surgical and pathologic risk factors for recurrence in extramammary Paget's disease. Gynecol Oncol 2017;147:358-63. [Crossref] [PubMed]
- Yang WJ, Kim DS, Im YJ, et al. Extramammary Paget's disease of penis and scrotum. Urology 2005;65:972-5. [Crossref] [PubMed]
- Wang Z, Lu M, Dong GQ, et al. Penile and scrotal Paget's disease: 130 Chinese patients with long-term follow-up. BJU Int 2008;102:485-8. [Crossref] [PubMed]
- Fishman DA, Chambers SK, Schwartz PE, et al. Extramammary Paget's disease of the vulva. Gynecol Oncol 1995;56:266-70. [Crossref] [PubMed]
- Stacy D, Burrell MO, Franklin EW. Extramammary Paget's disease of the vulva and anus: use of intraoperative frozen-section margins. Am J Obstet Gynecol 1986;155:519-23. [Crossref] [PubMed]
- Misas JE, Cold CJ, Hall FW. Vulvar Paget disease: fluorescein-aided visualization of margins. Obstet Gynecol 1991;77:156-9. [PubMed]
- Appert DL, Otley CC, Phillips PK, et al. Role of multiple scouting biopsies before Mohs micrographic surgery for extramammary Paget's disease. Dermatol Surg 2005;31:1417-22. [PubMed]
- O'Connor WJ, Lim KK, Zalla MJ, et al. Comparison of mohs micrographic surgery and wide excision for extramammary Paget's disease. Dermatol Surg 2003;29:723-7. [Crossref] [PubMed]
- Gunn RA, Gallager HS. Vulvar Paget's disease: a topographic study. Cancer 1980;46:590-4. [Crossref] [PubMed]
- Yugueros P, Keeney GL, Bite U. Paget's disease of the groin: report of seven cases. Plast Reconstr Surg 1997;100:336-9. [Crossref] [PubMed]
- Hendi A, Brodland DG, Zitelli JA. Extramammary Paget's disease: surgical treatment with Mohs micrographic surgery. J Am Acad Dermatol 2004;51:767-73. [Crossref] [PubMed]
- Coldiron BM, Goldsmith BA, Robinson JK. Surgical treatment of extramammary Paget's disease. A report of six cases and a reexamination of Mohs micrographic surgery compared with conventional surgical excision. Cancer 1991;67:933-8. [Crossref] [PubMed]
- Mohs FE, Blanchard L. Microscopically controlled surgery for extramammary Paget's disease. Arch Dermatol 1979;115:706-8. [Crossref] [PubMed]
- Wagner RF, Cottel WI. Treatment of extensive extramammary Paget disease of male genitalia with Mohs micrographic surgery. Urology 1988;31:415-8. [Crossref] [PubMed]
- Chung PH, Leong JY, Voelzke BB. Surgical Experience With Genital and Perineal Extramammary Paget's Disease. Urology 2019;128:90-5. [Crossref] [PubMed]
- Lee KY, Roh MR, Chung WG, et al. Comparison of Mohs micrographic surgery and wide excision for extramammary Paget's Disease: Korean experience. Dermatol Surg 2009;35:34-40. [PubMed]
- Marchesa P, Fazio VW, Oliart S, et al. Long-term outcome of patients with perianal Paget's disease. Ann Surg Oncol 1997;4:475-80. [Crossref] [PubMed]
- McCarter MD, Quan SH, Busam K, et al. Long-term outcome of perianal Paget's disease. Dis Colon Rectum 2003;46:612-6. [Crossref] [PubMed]
- Sarmiento JM, Wolff BG, Burgart LJ, et al. Paget's disease of the perianal region--an aggressive disease? Dis Colon Rectum 1997;40:1187-94. [Crossref] [PubMed]
- Thomas CJ, Wood GC, Marks VJ. Mohs micrographic surgery in the treatment of rare aggressive cutaneous tumors: the Geisinger experience. Dermatol Surg 2007;33:333-9. [PubMed]
- Tebes S, Cardosi R, Hoffman M. Paget's disease of the vulva. Am J Obstet Gynecol 2002;187:281-3; discussion 3-4. [Crossref] [PubMed]
- Rajendran S, Koh CE, Solomon MJ. Extramammary Paget's disease of the perianal region: a 20-year experience. ANZ J Surg 2017;87:132-7. [Crossref] [PubMed]
- Parashurama R, Nama V, Hutson R. Paget's Disease of the Vulva: A Review of 20 Years' Experience. Int J Gynecol Cancer 2017;27:791-3. [Crossref]
- Shen K, Luo H, Hu J, et al. Perianal Paget disease treated with wide excision and thigh skin flap reconstruction: a case report and review of literature. Medicine (Baltimore) 2018;97:e11638. [Crossref] [PubMed]
- Kyriazanos ID, Stamos NP, Miliadis L, et al. Extra-mammary Paget's disease of the perianal region: a review of the literature emphasizing the operative management technique. Surg Oncol 2011;20:e61-71. [Crossref] [PubMed]
- Singh J, Row D, Schaub T, et al. A Novel, Multidisciplinary Approach in the Treatment of Perianal Paget Disease. Dermatol Surg 2018;44:1653-5. [Crossref] [PubMed]
- Murata Y, Kumano K. Extramammary Paget's disease of the genitalia with clinically clear margins can be adequately resected with 1 cm margin. Eur J Dermatol 2005;15:168-70. [PubMed]


