
Tamsulosin dispensation patterns in the United States: a real-world, longitudinal, population claims database analysis
Introduction
The histological prevalence of benign prostatic hyperplasia (BPH) increases steadily with age, affecting 10% of men aged 30–40 years and 80–90% of men aged 70–80 years (1). Many, but not all men with histologically confirmed BPH develop lower urinary tract symptoms (LUTS) (1). Over the last 30 years, alpha-adrenergic sympathomimetic blocking agents have been the first-line treatment of bothersome, moderate-to-severe LUTS attributable to BPH (male LUTS) (2,3). However, for much of that time, initiation of alpha-blockers was an arduous process, often requiring dose adjustments, considerations regarding timing of administration, and frequent blood pressure monitoring to avoid orthostatic hypotension (4-6). The introduction in 1997 of tamsulosin (7)—a third-generation alpha1 adrenoceptor antagonist that targets the alpha1A-receptors expressed on the prostate stromal smooth muscle cells (8)—represented a breakthrough in the medical treatment of male LUTS because of its comparable efficacy and improved side effect profile over prior non-selective alpha-blockers (9,10). Tamsulosin facilitated the treatment of male LUTS by urologists and subsequently, was readily available for dispensation by many medical specialties (8). Despite the emergence of several newer uroselective alpha-blockers, tamsulosin is the most popular of these agents (11). It is also the preferred alpha1A antagonist available in the Veterans Affairs medical system (12,13), as well as commercial insurance plans (14).
In the United States (US), urologists have traditionally been the gatekeepers for the medical management of male LUTS, providing higher dispensation of BPH medications than other medical specialties (15). Although tamsulosin was approved by the US Food and Drug Administration (FDA) for the treatment of the signs and symptoms of BPH (7), with the changing healthcare landscape and evaluation of alpha-blockers for the treatment of urolithiasis (16), prostatitis (17), neurogenic bladder (18), and even female voiding dysfunction (19), it may now be dispensed to men and women for conditions other than male LUTS.
To identify potential changes to prescribing patterns and the extent of tamsulosin use for conditions beyond its original indication, we evaluated tamsulosin dispensing patterns in the US, using a large, multi-payer claims database. Specifically, we aimed to identify and characterize individuals who were dispensed tamsulosin, particularly for the first time, and men who received a BPH diagnosis. Additionally, we sought to characterize the health care professional (HCP) specialties which prescribed tamsulosin, as well as pre-existing comorbid conditions and newly diagnosed conditions of interest in men initiating tamsulosin for the first time.
Methods
Data source
Claims data were retrieved from the IMS PharMetrics Plus™ database (20)—a large, multi-payer Health Insurance Portability and Accountability Act-compliant database containing adjudicated medical and pharmacy claims from over 55 US health plans. The interested reader can find additional details about the database, study design and assessments in a Supplementary file.
Study design and assessments
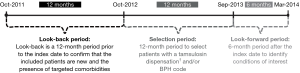
This retrospective, exploratory analysis comprised 2 main time periods (Figure 1): a 12-month selection period (October 1, 2012 to September 30, 2013) for identifying men or women who were dispensed tamsulosin or men who received a BPH diagnosis [documented International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) 600.xx code] and an 18-month analysis period [12 months before (look-back period) and 6 months after (look-forward period) tamsulosin initiation or first documentation of BPH diagnosis (index dates)]. These diagnostic codes were utilized as they are most specific to BPH-related diagnoses. Additionally, the rationale for investigating tamsulosin, 1 of 3 uroselective agents available in the US, is that it has emerged as the most popular of these agents.
Patients
Patients dispensed tamsulosin or diagnosed with BPH during the selection period were assigned to 1 or both the cohorts. The first cohort (tamsulosin users) comprised men or women who were dispensed tamsulosin during the selection period and were further categorized as continuing or new tamsulosin users. The second cohort comprised men who received a BPH diagnosis during the selection period and were further categorized based on existing or newly diagnosed BPH.
Additional information on methods is included in the Supplementary file.
Since alpha-blockers may affect blood pressure and can increase the possibility of orthostatic hypotensive events, especially after the first dose (21,22), the proportion of male new tamsulosin users who received a hypotension diagnosis within 1 month of tamsulosin initiation was determined. Occurrence of other targeted comorbidities in these men was also evaluated.
Results
Tamsulosin users
A total of 133,977 patients [119,136 (88.9%) men; 14,861 (11.1%) women] were dispensed tamsulosin during the selection period and were continuously enrolled in a health plan during the analysis period. Approximately half [72,583 (54.2%)] of the patients were new tamsulosin users: 59,197 (81.6%) men and 13,386 (18.4%) women (Figure 2 and Table 1). Interestingly, urologists were not the primary dispensers of tamsulosin among new users. Tamsulosin was initiated in men most often by primary care physicians [PCPs; 18,709/59,197 (31.6%)], followed by urologists [10,667/59,197 (18.0%)] and emergency medicine physicians [6,803/59,197 (11.5%); Table 1], whereas among female new users, tamsulosin was most often initiated by emergency medicine physicians [2,890/13,386 (21.6%)].
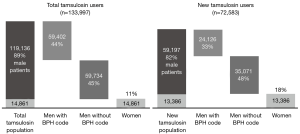
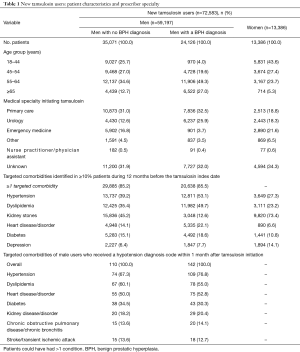
Full table
Almost half of male tamsulosin users, overall [59,734/133,997 (44.6%)] and newly initiated [35,071/72,583 (48.3%)], did not have a BPH diagnosis during the 18-month analysis period. Of 40,262 male new tamsulosin users without a BPH diagnosis in the 12-month look-back period, only 5,191 (12.9%) received a BPH diagnosis in the 6-month look-forward period, mostly during month 1 (5.5%).
Tamsulosin use varied depending on whether or not the men received a BPH diagnosis. New male tamsulosin users without a BPH diagnosis and new female tamsulosin users had fewer dispensations (1.5 average dispensations) than men with a BPH diagnosis (3 average dispensations). Tamsulosin users with a BPH code had day supply indicative of chronic use [8,832/24,125 (36.6%) with 151- to 180-day supply], while among those without a BPH code, more than half [22,290/35,071 (63.6%)] had ≤30-day supply and 5,239 (14.9%) had 151- to 180-day supply, indicative of episodic use.
Overall, 50,523 (85.3%) male new tamsulosin users had ≥1 targeted comorbidity in the 12-month look-back period (Table 1). Overall, 0.4% (252/59,197) of male new tamsulosin users received a hypotension diagnosis code within 1 month of tamsulosin dispensation; many of them also had cardiovascular comorbidities (Table 1). Because tamsulosin dispensation to women was not expected, the percentage of female new tamsulosin users who received a hypotension diagnosis was not evaluated.
Men diagnosed with BPH
Overall, 199,468 men were diagnosed with BPH during the selection period, of which, 143,444 (71.9%) were newly diagnosed (Table 2). Most men diagnosed with BPH were middle-aged or older (≥45 years), with approximately half in the 55- to 64-year-old group [overall, 97,508 (48.9%); newly diagnosed, 71,103 (49.6%)]. A diagnosis of BPH was made by urologists in nearly half of men (overall, 50.6%; newly diagnosed, 49.1%), followed by PCPs in approximately one-third of men (overall, 34.3%; newly diagnosed, 35.5%).
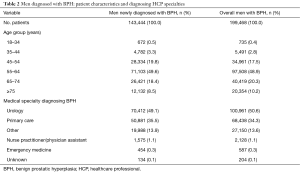
Full table
Among men with newly diagnosed BPH, 36,531 (25.5%) were dispensed tamsulosin during the 18-month analysis period. Tamsulosin was initiated before BPH was diagnosed in 22,336 (61.1%) newly diagnosed men [4,895 (13.4%) men before the BPH index date only; 17,441 (47.7%) before and after the BPH index date].
Overall, 98,371 (68.6%) men with newly diagnosed BPH had ≥1 targeted comorbidity in the 12 months before the BPH index date (Table 3), and 640 men received a hypotension diagnosis code (Table 4).

Full table

Full table
As mentioned above, men receiving tamsulosin may be at an increased risk of orthostatic hypotensive events. To further understand the potential for a first-dose effect, the proportion of men with a hypotension diagnosis code within 1 month of the BPH index date was determined, and occurrence of other targeted comorbidities was assessed. Relatively few [640/143,444 (0.4%)] men with newly diagnosed BPH received a diagnosis of hypotension within 1 month of the BPH index date. The most frequently recorded other targeted comorbidities among these men were hypertension (69.8%), dyslipidemia (55.8%), and heart disease/disorder (50.6%).
Discussion
In this retrospective, claims-based analysis, we identified men and women who were dispensed tamsulosin and men who were diagnosed with BPH over a 12-month period. We used the IMS PharMetrics Plus™ medical claims database, which captures a comprehensive clinical picture and provides a temporal snapshot of typical national healthcare delivery (20).
Although tamsulosin is approved for the treatment of male LUTS, it has been evaluated for the treatment of other conditions such as neurogenic bladder, ureteral stones, and even for voiding dysfunctions in women (18,19,23,24). Results of the current analysis show that 11.1% of all tamsulosin users and 18.4% of new tamsulosin users were women. Kidney stones were the most common (73.4%) targeted comorbidity identified in women during the 12 months before tamsulosin initiation, and emergency medicine physicians most often initiated tamsulosin use in women.
Interestingly, tamsulosin was dispensed commonly to men without a reported BPH diagnosis. For example, tamsulosin was dispensed before BPH was diagnosed in 61.1% of all men with newly diagnosed BPH. Further, almost half of the men dispensed tamsulosin—44.6% overall and 48.3% of new tamsulosin users—did not have a BPH diagnosis during the entire 18-month analysis period. Among male new tamsulosin users without a BPH diagnosis in the 12 months before tamsulosin initiation, most (87.1%) did not record a subsequent BPH diagnosis within 6 months of their initial tamsulosin dispensation. Several factors may contribute to these findings. A BPH diagnosis could have been documented >12 months before initiation of tamsulosin, a code for LUTS may have been entered instead of a code for BPH, the presence of comorbidities may have confounded diagnosis code entry in the submitted claim, and/or, as reported in previous studies (18,19,23,24), tamsulosin may have been dispensed for conditions other than BPH. In this analysis, 2 different treatment patterns were observed among new tamsulosin users. Consistent with the chronic nature of BPH, over one-third (36.6%) of men with a BPH diagnosis had a 151- to 180-day supply, suggestive of long-term use. Interestingly, 14.9% of men without a BPH diagnosis had a 151- to 180-day supply, suggesting that tamsulosin was used for a chronic condition—most likely BPH—and supports the finding that physicians might not be coding BPH even if it is present and they are treating it. The other two-thirds (63.6%) had a ≤30-day supply, suggestive of episodic use.
Traditionally, urologists were considered the primary caregivers for patients with LUTS attributable to BPH because managing these patients was primarily a surgical endeavor (25). However, PCPs became more involved in BPH diagnosis and management once efficacious pharmacological options became available in the 1990s (15,26,27). In a US survey conducted in 1998, half of the men with symptomatic BPH initially visited PCPs (25%) or internal medicine specialists (24%), although first-care management was provided by urologists in 37% cases (15). Results from a retrospective cohort study using medical claims from a large, non-profit, managed care organization showed that despite accounting for less than one-third of the office visits for management of BPH, patients were twice as likely to be treated with medical therapy by urologists than PCPs (28).
The healthcare landscape seems to be changing, however, and PCPs are now actively prescribing alpha-blockers such as tamsulosin. Results of an observational study based on 2004–2005 BPH Registry data showed that significantly more men consulting PCPs than urologists (51.6% vs. 43.0%; P<0.001) used alpha-blockers (e.g., alfuzosin and tamsulosin) (29). In the present analysis, PCPs initiated tamsulosin in approximately 31.6% male new tamsulosin users, while urologists and emergency medicine physicians initiated tamsulosin in approximately 18.0% and 11.5% male new tamsulosin users, respectively. Interestingly, only 25.5% of men with newly diagnosed BPH were dispensed tamsulosin during the analysis period. Under-treatment of men with BPH is well recognized; in prior US and multinational surveys, approximately 10% of symptomatic men received relevant medications for BPH/LUTS (30,31). Nonetheless, the low rate of prescribing could reflect other factors, such as treatment with alternative drugs or procedures, the presence of mild symptoms not necessitating treatment, or patient preference for no treatment.
Comorbidities are frequently age related and many are correlated with BPH. Therefore, we evaluated targeted comorbidities present during the 12 months before tamsulosin initiation among male new tamsulosin users. Most (85.3%) male new tamsulosin users had ≥1 targeted comorbidity before starting tamsulosin—most frequently hypertension, dyslipidemia, kidney stones, heart disease/disorder, and diabetes. Although not assessed in this analysis, these men were likely taking medications for some or all of their comorbid conditions. Despite comorbidities and presumed polypharmacy, tamsulosin was initiated. Similarly, many (68.6%) men with newly diagnosed BPH had ≥1 targeted comorbidity during the 12 months before being diagnosed, and most conditions of interest were identified before the BPH diagnosis was made or tamsulosin treatment was initiated.
Alpha-blockers are generally safe (10); however, they are associated with the occurrence of orthostatic hypotension, especially following the first dose (21,22). Additionally, concomitant use of pharmacological agents for comorbid conditions, especially agents with known hypotensive effects, can potentially increase the risk of hypotension. In clinical trials, reports of hypotensive adverse events with tamsulosin use were usually mild and transient (32,33). Signs and symptoms of orthostasis were detected more frequently in patients treated with tamsulosin than placebo; however, incidence rates were low for symptomatic postural hypotension (0.2–0.4% vs. 0.0%), syncope (0.2–0.4% vs. 0.6%), and vertigo (0.6–1.0% vs. 0.6%); incidence of dizziness was 15–17% vs. 10% (7). In a meta-analysis of alpha-blockers, rates of orthostatic hypotension associated with tamsulosin and alfuzosin use were comparable with that of placebo (1% each) (9). In the present analysis, 0.4% of male new tamsulosin users were diagnosed with hypotension within 1 month of starting tamsulosin. Of note, cardiovascular comorbidities were more common in these men than in the overall population.
In a previous retrospective cohort study that also employed the IMS PharMetrics Plus™ database, a temporal association between tamsulosin use and severe hypotension requiring hospitalization during the first 2 months after starting or restarting tamsulosin was reported among men aged 40–85 years (21). However, half [165,292/324,255 (51.0%)] of the men had ≥19 prescriptions dispensed in the 6-month period before starting tamsulosin; dispensed medications included concomitant antihypertensive agents (ranging from 20.8% for calcium channel blockers to 43.7% for angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors). Although the authors included an assessment of the Charlson comorbidity score, they did not report on the prevalence of hypertension and other common significant age-related comorbidities often seen in men presumed to have BPH. A full understanding of the association between hypotension and tamsulosin should consider the effect of comorbid conditions and their pharmacologic management.
While informative in many respects, this retrospective analysis has some limitations, mainly associated with the database used and the study design. The IMS PharMetrics Plus™ database primarily represents privately insured patients aged ≤65 years; therefore, individuals >65 years are underrepresented in this analysis. Additionally, the data are intended for reimbursement and the quality of the claims is dependent on the accuracy of reporting and coding (34). Therefore, the number of patients with a BPH diagnosis and those with comorbidities may not have been captured accurately in the database. The association between the drug dispensed and the diagnosis or reason for its prescription is not provided. An understanding of the dynamic circumstances that affect the assignment of diagnostic codes is needed when interpreting findings, as large variations in coding and reporting, such as change in codes and addition of new codes, can occur among clinicians and over time. Although dispensation and day supply data were captured and analyzed, these parameters may be influenced by the services provided (i.e., insurance company) and may not represent actual tamsulosin use.
Conclusions
In this retrospective analysis of a large cohort of privately insured patients, we found that although tamsulosin is approved for the signs and symptoms of BPH (7), treatment initiation with tamsulosin was observed among men and women, which suggests that HCPs seem comfortable prescribing tamsulosin for conditions other than BPH. Among men dispensed tamsulosin, many did not have a BPH diagnosis before receiving tamsulosin. Most men dispensed tamsulosin had ≥1 pre-existing comorbidity and were presumably receiving corresponding concomitant medications, suggesting that physicians were comfortable prescribing tamsulosin in the presence of comorbidity and polypharmacy. Furthermore, PCP and emergency medicine physicians primarily prescribed tamsulosin to men and women, respectively; however, urologists primarily diagnosed BPH. Importantly, the incidence of reported hypotension within 1 month of starting tamsulosin treatment was low, which supports the findings from randomized controlled trials that suggest that the first-dose orthostatic hypotensive adverse effect of tamsulosin is usually transient and mild in nature. In conclusion, the results of this exploratory analysis have important implications for further retrospective research into the use of this pharmaceutical agent.
Supplementary
Methods
Data source
At the time of the study, the IMS PharMetrics Plus™ database included paid claims data from ~70 million unique patients and over 48 million unique patients with medical and pharmacy insurance coverage (20). The database comprises detailed, de-identified patient, medical, pharmaceutical insurance claim form information, including inpatient and outpatient diagnoses and procedures [International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM)]; retail and mail order prescription records; and cost data. The age distribution of patients included in the IMS PharMetrics Plus™ database closely resembles that of the 2011 United States (US) census up to age 65. Individuals >65 years are underrepresented in this database (6.2% of the population versus 13.3% in the 2011 US census) because it primarily represents privately insured patients ≤65 years; many individuals >65 years are not enrolled in a commercial health plan. However, this database is a suitable source of data because benign prostatic hyperplasia (BPH) commonly becomes symptomatic between 40 and 65 years of age, and male lower urinary tract symptoms (LUTS) diagnosis and/or consequent treatment with tamsulosin often begins in the <65-year-old age group (1).
Study design and assessments
During the 12-month look-back period, new tamsulosin users and men newly diagnosed with BPH were identified. In addition, the incidence of targeted comorbidities, defined as commonly reported medical conditions in aging men and urologic conditions that may be observed with the presence of male LUTS (e.g., diabetes mellitus, urinary tract infection, prostatitis, prostate cancer, bladder cancer, urinary calculi, and urinary retention), was determined. During the 6-month look-forward period, tamsulosin use (chronic or episodic) based on dispensation and day supply data was determined. The timing of tamsulosin dispensation and day supply was used to describe duration of tamsulosin use. Furthermore, the incidence of newly diagnosed conditions of interest among new tamsulosin users or men newly diagnosed with BPH was determined. If a condition of interest was documented within the 6-month look-forward period and not in the 12-month look-back period, this condition was considered newly diagnosed.
Patients
The 2 cohorts were not mutually exclusive; men may have received a BPH diagnosis and initiated tamsulosin during the selection period. Patients who met the selection criteria and were continuously enrolled in a health plan for the entire analysis (≥12 months pre-index to 6 months post-index) were included in the analysis. Relevant information pertaining to patient demography, medical history, details of tamsulosin dispensation, documentation of BPH diagnosis codes, and specialty of prescribing physician was extracted from the database.
Data analysis
Data extracted from the IMS PharMetrics Plus™ database were summarized using descriptive statistics. This analysis was of purely exploratory nature; hence, no formal hypothesis testing was conducted.
Acknowledgments
This study was sponsored by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI), Ridgefield, CT, USA. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors received no direct compensation related to the development of the manuscript. Writing, editorial support, and formatting assistance was provided by Suchita Nath-Sain, PhD, Asim Priyendu, PhD, and Maribeth Bogush, MCI, PhD, of Cactus Communications, which was contracted and funded by BIPI. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Footnote
Conflicts of Interest: BR Kava serves as a consultant for Merit Pharmaceuticals and Endo Pharmaceuticals outside the submitted work. M Gittelman is a consultant for Boehringer Ingelheim. JM Wruck is an employee of Boehringer Ingelheim. AE Verbeek was an employee of Boehringer Ingelheim at the time the study was conducted and is currently an employee of Sanofi.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol 2005;7:S3-14. [PubMed]
- Deters LA. Benign prostatic hyperplasia (BPH) treatment & management. Medscape. Updated 2016. Available online: http://emedicine.medscape.com/article/437359-treatment#d1, accessed February 28, 2018.
- Binder SP. Alpha-blockers top choice for first-line LUTS treatment. Urology Times 2014. Available online: https://www.urologytimes.com/modern-medicine-feature-articles/alpha-blockers-top-choice-first-line-luts-treatment, accessed July 17, 2019.
- Lepor H. The evolution of alpha-blockers for the treatment of benign prostatic hyperplasia. Rev Urol 2006;8:S3-9. [PubMed]
- HytrinTM Product Information 2010. Available online: https://gp2u.com.au/static/pdf/H/HYTRIN-PI.pdf, accessed March 6, 2018.
- Cardura Highlights of Prescribing Information 2016. Available online: https://www.pfizermedicalinformation.com/en-us/patient/cardura, accessed February 28, 2018.
- FLOMAX (tamsulosin hydrochloride, USP) Capsules. Highlights of prescribing information. Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals, Inc; 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020579s026lbl.pdf, accessed July 17, 2019.
- American Urological Association Guideline: Management of Benign Prostatic Hyperplasia (BPH) 2010 American Urological Association Education and Research, Inc. Available online: https://www.auanet.org/guidelines/benign-prostatic-hyperplasia-(bph)-guideline/benign-prostatic-hyperplasia-(2010-reviewed-and-validity-confirmed-2014), accessed February 28, 2018.
- Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol 1999;36:1-13. [Crossref] [PubMed]
- Yuan J, Liu Y, Yang Z, et al. The efficacy and safety of alpha-1 blockers for benign prostatic hyperplasia: an overview of 15 systematic reviews. Curr Med Res Opin 2013;29:279-87. [Crossref] [PubMed]
- Filson CP, Wei JT, Hollingsworth JM. Trends in medical management of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology 2013;82:1386-92. [Crossref] [PubMed]
- Burk M, Furmaga E, Dong D, et al. Multicenter drug use evaluation of tamsulosin and availability of guidance criteria for nonformulary use in the veterans affairs health system. J Manag Care Pharm 2004;10:423-32. [Crossref] [PubMed]
- Britt RB, Hashem MG, Bryan WE 3rd, et al. Economic outcomes associated with a pharmacist-adjudicated formulary consult service in a veterans affairs medical center. J Manag Care Spec Pharm 2016;22:1051-61. [Crossref] [PubMed]
- Moon HW, Yang JH, Choi JB, et al. Prescription pattern of alpha-blockers for management of lower urinary tract symptoms/benign prostatic hyperplasia. Sci Rep 2018;8:13223. [Crossref] [PubMed]
- Bruskewitz R. Management of symptomatic BPH in the US: who is treated and how? Eur Urol. 1999;36:7-13. [Crossref] [PubMed]
- Pourmand A, Nadendla R, Mazer-Amirshahi M, et al. Tamsulosin for urolithiasis: a review of the recent literature and current controversies. Am J Emerg Med 2016;34:2217-21. [Crossref] [PubMed]
- Nickel JC, O'Leary MP, Lepor H, et al. Silodosin for men with chronic prostatitis/chronic pelvic pain syndrome: results of a phase II multicenter, double-blind, placebo controlled study. J Urol 2011;186:125-31. [Crossref] [PubMed]
- Cameron AP. Medical management of neurogenic bladder with oral therapy. Transl Androl Urol 2016;5:51-62. [PubMed]
- Meyer LE, Brown JN. Tamsulosin for voiding dysfunction in women. Int Urol Nephrol 2012;44:1649-56. [Crossref] [PubMed]
- IMS real-world data adjudicated claims: USA [IMS PharMetrics Plus]. Available online: http://www.bridgetodata.org/node/824, accessed February 28, 2018.
- Bird ST, Delaney JAC, Brophy JM, et al. Tamsulosin treatment for benign prostatic hyperplasia and risk of severe hypotension in men aged 40-85 years in the United States: risk window analyses using between and within patient methodology. BMJ 2013;347:f6320. [Crossref] [PubMed]
- Oelke M, Gericke A, Michel MC. Cardiovascular and ocular safety of α1-adrenoceptor antagonists in the treatment of male lower urinary tract symptoms. Expert Opin Drug Saf 2014;13:1187-97. [Crossref] [PubMed]
- Lee KS, Han DH, Lee YS, et al. Efficacy and safety of tamsulosin for the treatment of non-neurogenic voiding dysfunction in females: a 8-week prospective study. J Korean Med Sci 2010;25:117-22. [Crossref] [PubMed]
- Malo C, Audette-Côté JS, Emond M, et al. Tamsulosin for treatment of unilateral distal ureterolithiasis: a systematic review and meta-analysis. CJEM 2014;16:229-42. [Crossref] [PubMed]
- Hollingsworth JM, Wei JT. Economic impact of surgical intervention in the treatment of benign prostatic hyperplasia. Rev Urol 2006;8:S9-15. [PubMed]
- Fawzy A, Fontenot C, Guthrie R, et al. Practice patterns among primary care physicians in benign prostatic hyperplasia and prostate cancer. Fam Med 1997;29:321-5. [PubMed]
- Collins MM, Barry MJ, Bin L, et al. Diagnosis and treatment of benign prostatic hyperplasia. Practice patterns of primary care physicians. J Gen Intern Med 1997;12:224-9. [PubMed]
- Hollingsworth JM, Hollenbeck BK, Daignault S, et al. Differences in initial benign prostatic hyperplasia management between primary care physicians and urologists. J Urol 2009;182:2410-4. [Crossref] [PubMed]
- Wei JT, Miner MM, Steers WD, et al. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol 2011;186:971-6. [Crossref] [PubMed]
- Hall SA, Link CL, Hu JC, et al. Drug treatment of urological symptoms: estimating the magnitude of unmet need in a community-based sample. BJU Int 2009;104:1680-8. [Crossref] [PubMed]
- Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol 2003;44:637-49. [Crossref] [PubMed]
- Lepor H. Long-term evaluation of tamsulosin in benign prostatic hyperplasia: placebo-controlled, double-blind extension of phase III trial. Tamsulosin Investigator Group. Urology 1998;51:901-6. [Crossref] [PubMed]
- Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol 1998;160:1701-6. [Crossref] [PubMed]
- Joyce GF, Escarce JJ, Solomon MD, et al. Employer drug benefit plans and spending on prescription drugs. JAMA 2002;288:1733-9. [Crossref] [PubMed]


