
A new strategy for the treatment of sorafenib-refractory metastatic renal cell carcinoma in China: combination with intermittent chemotherapy
Introduction
Renal cell carcinoma (RCC) is a kind of malignancy with genetic and metabolic disorder. There are estimated 330,000 new RCC cases and over 100,000 deaths each year worldwide, with a rising incidence as high as 3% yearly (1). Moreover, in China, 78,000 new cases of RCC were detected each year with an increasing incidence, of which 19,500 cases were advanced RCC. Additionally, 20–30% RCC were found metastatic at diagnosis, and 20% patients had recurrence or metastasis during follow-up. Although the treatment of metastatic RCC (mRCC) had achieved advances in the last few years, it remained one of the most deadly cancers due to its poor prognosis, and the 5-year survival rate is lower than 10% (2-4), which makes it a major problem all around the world.
Nowadays, comprehensive therapy is recommended for mRCC instead of surgical treatment alone. In the last decade, with the emergence of targeted therapy, the transition from cytotoxic therapy to highly selective molecules has completely changed and expanded the selection of RCC patients. At present, most patients with advanced RCC prefer to targeted therapy using antiangiogenic agents and tyrosine kinase inhibitors (TKIs). The FDA has approved 7 agents for the advanced RCC treatment, including sorafenib (5). Sorafenib is a category 2A treatment option for those who have relapsed or medically unresectable stage IV predominantly RCC by the National Comprehensive Cancer Network (NCCN) kidney Cancer Panel.
Nevertheless, due to little data about optimal therapy for sorafenib-refractory cases, there are challenges in the treatment planning for patients with progressive disease (PD). In a RECORD 1 trial, the comparison between everolimus and placebo was implemented for mRCC treatment in sunitinib-refractory or sorafenib-refractory patients. The median PFS was 4.0 vs. 1.9 months (6). Axitinib and sorafenib as the second-line therapy were compared in a multicenter randomized phase III (AXIS) after 1 prior systemic therapy. The overall median PFS for axitinib and sorafenib was 6.7 and 4.7 months, respectively (7). Besides, pazopanib, bevacizumab and temsirolimus were also recommended by NCCN kidney Cancer Panel as the optional treatment after tyrosine kinase failure (8-10). However, most of the drugs have not yet entered the Chinese market and most mRCC patients suffered from heavy economic burden. In consequence, a new strategy for sorafenib-refractory mRCC patients was urgently needed in China.
Historically, the role of the traditional cytotoxic agents was limited in treating mRCC. Moreover, in phase-2 studies, some programs like thermotherapy regimes have already been carried out, and the little response rates are lower than 10% (11). Interestingly, 17% of patients had the response of combining continuous infusion 5-FU and gemcitabine (12). Based on the inconvenience of prolonged infusion 5-FU, combination of capecitabine and gemcitabine have been explored. Fifty-five previous treated RCC patients had the response rate of 15%. In addition, the median duration of response was improved to 7.1 months (13).
Therefore, we hereby decided to explore the role of combination chemotherapy in sorafenib-refractory mRCC patients. To our knowledge, no study had been reported on Gemcitabine and S-1 combination chemotherapy after tyrosine kinase failure in mRCC. In this study, clinical safety and efficacy of Gemcitabine and S-1 combination chemotherapy were retrospectively analyzed to evaluate the potential benefit for advanced mRCC.
Methods
Patients
From January 2010 to April 2014, baseline characteristics and survival outcomes of 19 sorafenib-refractory mRCC patients with combination chemotherapy in the affiliated Cancer Hospital of Jiangsu Province of Nanjing Medical University were retrospectively collected and analyzed. Certainly, study outcomes will not affect the further management of the patients and the Ethics Committee of Nanjing medical University, Jiangsu Cancer Hospital has approved the study.
After receiving treatment with sorafenib, all patients had progressive mRCC at least 6 months. The eligible criteria for combination chemotherapy are as follows: (I) Measurable lesions based on the response evaluation criteria in solid tumor (RECIST) criteria, ver.1.0; (II) Karnofsky performance status (KPS) ≥80; (III) adequate bone marrow function, including platelet count ≥100×109/L, leucocyte count ≥4×109/L and hemoglobin ≥100 g/L; (IV) adequate hepatic function, including alanine aminotransferase and aspartate aminotransferase ≤2× upper normal limit, and bilirubin ≤2.0 mg/dL; (V) adequate renal function, serum creatinine ≤2× upper normal limit; (VI) patients will not accept VEGFR-TKI therapy, cytokines and immunotherapy during or after the chemotherapy until death.
Treatment regimen and response evaluation
Patients with sorafenib-refractory mRCC received the treatment combining Gemcitabine (1,000 mg/m2, day 1 and day 8 of every cycle of 21 days) and S-1 (40 mg/m2, twice daily for 14 days, followed by a rest period of 7 days). Patients were continually given sorafenib 400 mg twice every day in a cycle of 28 days. All patients underwent at least 2 cycles of chemotherapy. The plan was not interrupted unless the uncontrollable toxicity grade 3/4 due to sorafenib. The treatment would be suspended when meeting the followings situations: (I) disease progression; (II) intolerable toxicity; (III) after six cycles of treatment. Curative effects were assessed with contrast-enhanced computed tomography (CT) scans two cycles of chemotherapy following RECIST criteria, ver.1.0. The National Cancer Institute Common Terminology Criteria for Adverse Events, ver.3.0, was used to evaluate the toxicity monitoring in every cycle.
Statistical analysis
Time to progression (TTP) was measured. Overall survival (OS) was calculated as the time from the first day of chemotherapy to death or the last follow-up date. Surviving patients were all censored at the final follow-up date. The disease control rate indicated SD and partial response (PR) after at least 24 weeks. Kaplan-Meier method was employed to determine OS and TTP. Statistical relationship among the prognostic factors, TTP and OS was analyzed by Cox regression models. SPSS, ver.16.0, was used to perform statistical analyses and a P<0.05 was statistically significant.
Results
Patient characteristics
Basic characteristics of included patients were shown in Table 1. Six women and 13 men were involved in this study. The mean age was 53 years, ranged from 24 to 69 years. Fifteen patients had previous surgery before sorafenib chemotherapy, 12 patients (63.2%) had lung metastasis 6 patients (31.6%) had lymph nodes metastasis.
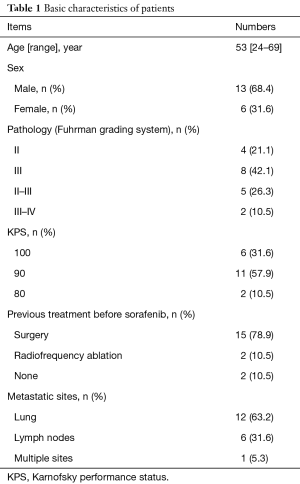
Full table
Evaluation of curative effect
Nineteen patients received 86 cycles of chemotherapy in total, with an average of 4 cycles per patient. Five patients (26.3%) had SD, 6 patients (31.6%) had PD, and 8 patients (42.1%) had PR. The disease control rate (PR + SD) was 68.4%. The median TTP was 6.3 months (range, 2.0–32.7 months), and the median OS was 19.7 months (range, 5.7–45.0 months).
In the survival analysis, disease control (PR + SD) group showed an obviously longer TTP (median TTP: 9.5 vs. 2.0 months, P<0.001) and OS (median OS: 21.0 vs. 8.3 months, P<0.001) when compared with PD group (Figure 1). TTP and OS were related to disease control in univariate analysis [TTP: hazard ratio (HR) =41.667, P=0.001; OS: HR =12.743, P=0.001] while no statistically significances were found between gender, age and TTP or OS (Table 2). The same results were also detected in multivariate analysis (TTP: HR =33.608, P=0.002; OS: HR =10.905, P=0.001).
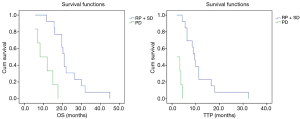
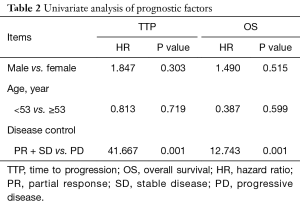
Full table
Toxicity profiles
Treatment related toxicities were assessed after the treatment cycle. No treatment related death was observed. All patients reported side-effects at different degrees after two combination chemotherapy cycles, while only 3 patients suffered grade 3/4 toxicities (15.8%). Neutropenia and thrombocytopenia were the most common and severe hematologic toxicity, but grade 3/4 only occupied a small part of all side-effects. Nausea (78.9%) was the most common non-hematologic toxicities (Table 3). All toxicities were manageable. What’s more, the combination chemotherapy did not increase the previous side-effects of sorafenib.
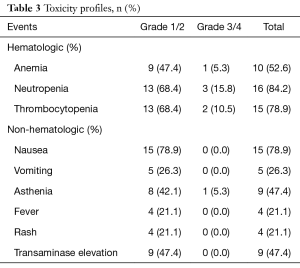
Full table
Discussion
Nowadays, molecular targeted therapy is the standard treatment for advanced RCC, including anti VEGF antibodies and TKIs, which have a wide use in treatment. Sorafenib tosylate a small molecule inhibiting multiple isoforms of intracellular serine/threonine kinase, RAF, and some receptor tyrosine kinases, such as VEGFR-1, -2, and -3, RET, c-KIT, FLT-3 and PDGFR-β (14,15). It has been reported that sorafenib could extend the median PFS and median OS in RCC patients (16). When RCC progresses after treatment of sorafenib, the most common alternative is replacing one kind of TKI to another kind or mTOR drugs (17). However, the mechanism of these alternative drugs is almost the same, and the curative effect is limited. Moreover, the side-effects are superimposed without report of obvious extension of TTP or OS.
Gemcitabine and Fluorouracil are chemotherapeutic drugs for a variety of cancers. The active form of gemcitabine, which occurs via phosphorylation upon cellular uptake and yields gemcitabine diphosphate and gemcitabine triphosphate, has the potential to inhibit DNA synthesis processes. The latter type is a fierce competitor of deoxycytidine triphosphate and is capable of obstructing DNA chain elongation, causing DNA fragmentation and death of cells. Some self-potentiating pharmacological activities of these derivatives that can enhance the above mechanism most likely accounts for gemcitabine metabolites superiority over deoxycytidine to maintain high intracellular concentrations (18). The mechanism of action is yet unknown, and although gemcitabine reportedly has the potency of cytotoxic drugs in RCC (19), the efficiency rate, at 10–15%, is minimal.
S-1 is an oral fluoropyrimidine and a novel 5-FU analog containing tegafur, a metabolically activated prodrug of 5-FU. 5-Chloro-2, 4-dihydroxypyridine, could potently inhibit the degradation by dihydropyrimidine dehydrogenase, thereby improving the pharmacological action of 5-FU (20). The incidence of gastrointestinal (GI) toxicities could be reduced by potassium in mucosal cells of GI tract after oral administration because the activation of 5-Fu in the GI tract was suppressed. Compared with 5-FU, S-1 has the following advantages, maintaining high blood drug concentration, increasing anti-tumor activity, significantly reducing drug toxicity and more convenient administration (21).
Though security and validity have been verified by the initial trial of gemcitabine and fluorouracil independently, there were limited reports on combination of gemcitabine and fluorouracil chemotherapy plus sorafenib for early treatment of patients with mRCC (22). Additionally, to the best of our knowledge, no researches have been performed before about combination therapy after failure of first-line chemotherapy in mRCC. In our innovative research, the results indicated that the sensitivity of tumor cells to chemotherapeutic drugs can be increased after treatment with TKIs. Whether sequential combination chemotherapy could prolong the total survival time in sorafenib-refractory mRCC requires further confirmation. However, limited studies could present satisfied results in combination chemotherapy of gemcitabine and capecitabine in treatment which had not been recommended by NCCN Clinical Practice Guidelines in oncology. As reported, the median OS for mRCC treated with gemcitabine and capecitabine was 14.5 months, and the median PFS was 5.6 months (23), which was obviously worse than our results.
Our results showed a satisfied response rate (68.4%) in sorafenib-refractory mRCC. The median OS for mRCC patients was 19.7 months, and the median TTP was 6.3 months, which were obvious prolonged than previous studies. Disease control (PR + SD) group showed an obviously longer TTP and OS when compared with PD group. Besides, TTP and OS were significantly related to disease control in univariate and multivariate analysis (all P<0.05). The results indicated that combination chemotherapy might partly restore chemotherapy sensitivity of sorafenib in sorafenib-refractory mRCC patients. However, potential mechanism requires to be further studied. The frequent side-effects were hematologic: anemia (52.6%), neutropenia (84.2%) and thrombocytopenia (78.9%). Nausea (78.9%), asthenia (47.4%) and transaminase elevation (47.4%) were the most common non-hematologic toxicities. Grade 3/4 toxicities were limited and all the toxicities were manageable. Moreover, combination chemotherapy did not increase the previous side-effects of sorafenib.
Most clinical trials for advanced mRCC investigated new targeted agents after the sorafenib era. The NCCN guideline recommends another TKI or mTOR replacement as the treatment options after sorafenib failure. However, combination with intermittent chemotherapy as an alternative treatment might be appropriate for patients with high KPS grade and without chemotherapy contraindications. In consequence, combination chemotherapy was suggested to serve as an alternative treatment option after sorafenib failure for advanced mRCC patients. Despite the promising results of our study, some limitations should be taken into consideration. Firstly, it was a retrospective study. Secondly, the sample size was small which made it difficult to compare the curative effect between combination chemotherapy and sorafenib alone. Last but not least, no intensive study was carried out on how this chemotherapy combination affects the estimated glomerular filtration rate (eGFR). Studies had shown that eGFR might have an important effect on survival because it could be a major driver of other cause mortality in mRCC patients (24,25). Prospective, large sample size studies are needed to further verify our results in the future.
Conclusions
In total, combination chemotherapy could be an optional treatment for advanced mRCC patients after sorafenib refractory.
Acknowledgments
Funding: This work was supported by grants from the Six Talents Peaks Program of Jiangsu Province (No. 2016-WSW-021), National Natural Science Foundation of China (No. 81702520), Medical Research Project of Jiangsu Provincial Health and Family Planning Commission (No. H2018052), Research Project of Jiangsu Cancer Hospital (No. ZN201602), and the Young Talents Program of Jiangsu Cancer Hospital (No. 2017YQL-04).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee of Nanjing medical University, Jiangsu Cancer Hospital has approved the study (2017-053). All involved patients provided written informed consent. The study outcomes will not affect the future management of the patients.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations), National Cancer Institute. Bethesda, MD. Accessed 5 January 2013. Available online: https://seer.cancer.gov/csr/1975_2009_pops09/
- Woodward E, Jagdev S, McParland L, et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 2011;48:160-6. [Crossref] [PubMed]
- Lin PP, Mirza AN, Lewis VO, et al. Patient survival after surgery for osseous metastases from renal cell carcinoma. J Bone Joint Surg Am 2007;89:1794-801. [Crossref] [PubMed]
- Ng CS, Wood CG, Silverman PM, et al. Renal cell carcinoma: diagnosis, staging, and surveillance. AJR Am J Roentgenol 2008;191:1220-32. [Crossref] [PubMed]
- Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib vs. interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:1280-9. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449-56. [Crossref] [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib vs. sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9. [Crossref] [PubMed]
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061-8. [Crossref] [PubMed]
- Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427-34. [Crossref] [PubMed]
- Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 2004;22:909-18. [Crossref] [PubMed]
- Aronoff GR, Brier ME. Prescribing drugs in renal disease. In: Brenner BM, Rector FC. editors. Brenner & Rector’s The Kidney. Philadelphia: WB Saunders, 2004;2849-70.
- Kobak S. Efficacy and safety of adalimumab in a patient with ankylosing spondylitis on peritoneal dialysis. Rheumatol Int 2012;32:1785-7. [Crossref] [PubMed]
- Van Veldhuizen PJ, Hussey M, Lara PN Jr, et al. A phase ii study of gemcitabine and capecitabine in patients with advanced renal cell cancer: Southwest Oncology Group Study S0312. Am J Clin Oncol 2009;32:453-9. [Crossref] [PubMed]
- Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 2005;23:965-72. [Crossref] [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [Crossref] [PubMed]
- Wang HK, Zhang HL, Zhu Y, et al. A Phase II trial of dosage escalation of sorafenib in Asian patients with metastatic renal cell carcinoma. Future Oncol 2014;10:1941-51. [Crossref] [PubMed]
- Dudek AZ, Zolnierek J, Dham A, et al. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. Cancer 2009;115:61-7. [Crossref] [PubMed]
- von der Maase H. Gemcitabine and cisplatin in locally advanced and/or metastatic bladder cancer. Eur J Cancer 2000;36 Suppl 2:13-6. [Crossref] [PubMed]
- George CM, Stadler WM. The role of systemic chemotherapy in the treatment of kidney cancer. Cancer Treat Res 2003;116:173-82. [Crossref] [PubMed]
- Jiang W, Lu Z, He Y, et al. Dihydropyrimidine dehydrogenase activity in hepatocellular carcinoma: implication in 5-fluorouracil-based chemotherapy. Clin Cancer Res 1997;3:395-9. [PubMed]
- Furuse J, Okusaka T, Kaneko S, et al. Phase I/II study of the pharmacokinetics, safety and efficacy of S-1 in patients with advanced hepatocellular carcinoma. Cancer Sci 2010;101:2606-11. [Crossref] [PubMed]
- Tagawa ST, Milowsky MI, Jeske S, et al. A phase I trial of sorafenib plus gemcitabine and capecitabine for patients with advanced renal cell carcinoma: New York Cancer Consortium Trial NCI 6981. Am J Clin Oncol 2011;34:443-8. [Crossref] [PubMed]
- Stadler WM, Halabi S, Rini B, et al. A phase II study of gemcitabine and capecitabine in metastatic renal cancer: a report of Cancer and Leukemia Group B protocol 90008. Cancer 2006;107:1273-9. [Crossref] [PubMed]
- Martini A, Cumarasamy S, Hemal AK, et al. Renal cell carcinoma: the oncological outcome is not the only endpoint. Transl Androl Urol 2019;8:S93-5. [Crossref] [PubMed]
- Lane BR, Demirjian S, Derweesh IH, et al. Survival and Functional Stability in Chronic Kidney Disease Due to Surgical Removal of Nephrons: Importance of the New Baseline Glomerular Filtration Rate. Eur Urol 2015;68:996-1003. [Crossref] [PubMed]


