
Current management and management controversies in early- and intermediate-stage of nonseminoma germ cell tumors
Introduction
Testicular cancer (GCT) is the most common solid organ cancer diagnosed in adult men under the age of 34. An estimated 9,000 new cases were diagnosed in 2018 in the United States, and the disease’s prevalence is 5.7 per 100,000 men (based on 2011–2015). Overall survival rates are high, with 5-year survival reaching 95%; however, approximately 400 men die of GCT each year (1,2). Approximately half of GCT cases are classified as pure seminoma, and half are nonseminomatous germ cell tumors (NSGCT). The latter consists of embryonal carcinoma (11% pure and 80% mixed NSGCT cases), choriocarcinoma (0.6% pure and 33% mixed NSGCT cases), yolk sac tumors (0.6% pure and 12% mixed NSGCT), and teratoma (3.5% pure and 60% mixed NSGCT) (3). Two-thirds (68%) of patients present with stage I disease, while 19% of diagnosed cases are stage II and 12% are stage III (2).
Management of testicular care is primarily driven by clinical stage and whether the tumor is a pure seminoma or a non-seminoma. These factors hold significant prognostic value, and are directly related to recommended treatment options. Though multimodal treatment approaches combining surgery, radiation therapy, and chemotherapy are responsible for high cure rates, retroperitoneal lymph node dissection (RPLND) is therapeutic in several well-defined settings, such as in stage I NSGCT characterized by a high-risk of micrometastatic lymph node involvement and in low-volume stage II disease characterized by non-bulky retroperitoneal lymph node involvement (stage IIa/b). In such cases, the role of RPLND includes pathologic staging, prognostic risk stratification and cancer control. In cases of non-seminomatous disease, alternatives to RPLND for high-risk stage I disease and low-risk stage II disease primarily consists of adjuvant cisplatin-based chemotherapy. NSGCT is highly sensitive to cisplatin, which when combined with surgery, contributes to cancer specific survival exceeding 95% (4). Because nuances exist between different treatment strategies, a patient with stage I NSGCT may need to decide between observation, primary RPLND or adjuvant chemotherapy while those with low-volume stage II disease may need to choose between RPLND and chemotherapy. In this review, we will discuss management options for early- and intermediate-stage nonseminomatous GCT, which we define as clinical stage (CS) I-IIb disease.
Clinical staging for GCT
GCT is staged using the Tumor-Node-Metastasis-Serum Tumor Markers (TNM-S) classification set by the American Joint Committee on Cancer (AJCC) (Tables 1,2). Pathologic T stage is established after orchiectomy while clinical N and M status are determined by cross sectional imaging. Staging for GCT also utilizes serum tumor markers (after radical orchiectomy) as part of TNM-S classification for GCT.
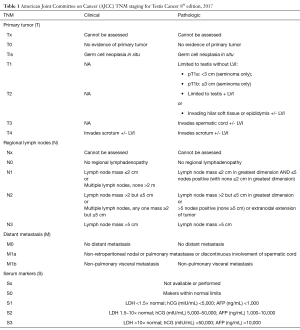
Full table
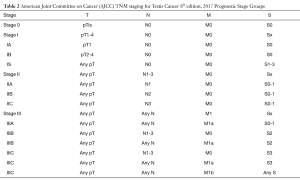
Full table
Notable changes to the 8th edition of the AJCC Staging Classification System include the new terminology of germ cell neoplasia in situ for pTis, subclassification of pT1 disease based on size criteria for seminoma, upstaging to pT2 based on epididymal or hilar soft tissue invasion, and M1 classification in cases characterized by discontinuous involvement of the spermatic cord (5).
Different post-orchiectomy management approaches are largely driven by the risk of retroperitoneal disease. Despite advances in imaging technology over the last several decades, clinical staging is not definitive.
Various studies, for example have reported a consistent 25–35% rate of clinical understaging of the retroperitoneum. In addition, consensus agreement regarding an absolute criteria indicating normal or abnormal retroperitoneal lymph node on CT scan does not exist. Hudolin et al. reviewed findings of staging RPLND on CS1 NSGCT patients and found 31.8% patients with lymph node metastasis with an average size of 1.05 cm, indicating that a >1 cm threshold for abnormal lymph nodes would miss 60% of metastatic cases (6). Some have advocated using a combination of size and location to determine if an enlarged lymph node is significant. Leibovitch et al. used a size cutoff of 4 mm in the primary landing zone and 10 mm outside this region and found a sensitivity of 91% and a specificity of 58% for pathologic stage II disease (7).
Management options for clinical stage I NSGCT
Determining the need for adjuvant treatments should prompt careful consideration of both the presence of high risk features as well as patient preference for or against certain adjuvant therapies given that up to 70% of clinical stage I NSGCT patients are cured by radical orchiectomy alone (8,9). Acceptable management options for CS 1 NSGCT include active surveillance (AS), RPLND, or adjuvant chemotherapy. Current National Comprehensive Cancer Network (NCCN) guidelines (Version 1.2019) recommend a risk-adapted approach that corresponds to clinical stage and histologic features found on orchiectomy pathology. For example, the absence or presence of lymphovascular invasion (LVI) stratifies stage I cases into clinical stage IA (pT1N0M0S0) and clinical stage IB disease (pT2N0M0S0), respectively (10). LVI is associated with a 40–55% relapse rate compared to 10–14% relapse risk in cases without LVI (11). Other histologic features have been investigated as predictors of relapse such as predominance of embryonal carcinoma, but the value of these features in the absence of LVI is debated (12).
While observation and AS may be appropriate for CS IA disease, adjuvant therapy (AT) is typically recommended for stage IB cases. The NCCN recommends AS (preferred), adjuvant chemotherapy with 1 cycle of bleomycin, etoposide, and cisplatin (BEP), or nerve sparing RPLND for stage IA patients. For stage IB patients, AS, 1 cycle BEP, and adjuvant RPLND are all options. The European Association of Urology (EAU) offers more definitive recommendations with surveillance being recommended as the standard option for CS IA patients. A single cycle (1) of BEP is recommended only if there are conditions against surveillance and nerve sparing RPLND only if there conditions both against surveillance AND chemotherapy. For stage IB patients the EAU recommends BEP ×1 cycle as the standard with nerve sparing RPLND or surveillance only if there are circumstances that preclude chemotherapy. The American Association of Urology (AUA) does not currently have guidelines for the management of GCT, but are currently in development. Table 3 highlights the current recommendations of both the NCCN (10), and the EAU (13).

Full table
AS
AS is an attractive option for cases of CS I without high-risk features (e.g., LVI) as the vast majority of these cases are cured with radical orchiectomy and observation avoids the morbidity associated with more invasive treatments. One series reported patients undergoing AS had an overall relapse rate of 19%, with a 5-year cancer specific survival at 99.4% (14). NCCN guidelines recommend AS as the preferred option in stage IA NSGCT, with AS being an option in stage IB NSGCT. Concerns that employing AS broadly for all patients with CS I disease subjects a significant number of patients who experience recurrence to full dose induction chemotherapy have been previously expressed and underpin the rationale for primary RPLND. However, in comparing overall number of relapses and treatment burden per 100 patients, AS results in the fewest number of total chemotherapy cycles (75–90 cycles of chemotherapy) and post chemotherapy surgeries (5–10 surgeries) compared to adjuvant BEP (110–210 cycles of chemotherapy with 3 postchemotherapy surgeries) or primary RPLND (100 surgeries and 45 cycles of chemotherapy) (15).
The use of AS has increased over time; one study reported a temporal shift in surveillance rates, with an increase in the number of patients managed with surveillance from 65% in 2004–2005 to 74% in 2012–2013 (OR 1.50; 95% CI, 1.14–1.98; P=0.004) (16). However, in a survey of panelists at the third European consensus conference on diagnosis and treatment of germ-cell cancer, the preferred strategy for stage I NSGCT varied among participants (surveillance in all patients: 28.0%; surveillance in low-risk, adjuvant 2 cycles BEP in high-risk: 30.0%; surveillance in low-risk, 1 cycle adjuvant BEP in high-risk: 36.0%; surveillance in low-risk, nerve-sparing RPLND in high-risk; nerve sparing RPLND in all patients: 6.0%) (17).
Adherence to follow-up is a key concern among patients undergoing surveillance. Yu and colleagues reported that nearly 40% of stage I cases reported through the NCDB database (including both seminoma and nonseminoma) received inadequate follow-up and testing. Despite a high rate of noncompliance to the recommended surveillance schedule, the recurrence rate was 14.3% (18).
Adjuvant chemotherapy
The evidence for adjuvant BEP for NSGCT was based on a phase II United Kingdom Medical Research Council study in which 2 cycles of adjuvant BEP resulted in a relapse-free rate at 98% at 2 years (with a median follow-up of 4 years) (19). However, because of increasing concerns regarding late term toxicities associated with chemotherapy for GCT patients, more recent studies have examined the effectiveness of a single cycle of adjuvant BEP. Long- and late-term consequences of chemotherapy include an increased risk of cardiovascular disease or secondary malignancy (1.9 fold) (20), nephrotoxicity, ototoxicity, and peripheral neuropathy (20,21). A landmark phase III RCT from Germany compared primary RPLND with 1 cycle BEP, and reported a 1.04% recurrence rate in the chemotherapy arm (median follow-up of 4.7 years) (22). The SWENOTECA group found a 3.2% risk of relapse in high-risk patients with LVI compared to 1.6% in patients without LVI at 5 years follow-up, and an overall cancer specific survival of 100% (23). A randomized controlled trial comparing 1 versus 2 cycles of adjuvant chemotherapy was abandoned, but mature results from the SWENOTECA study (median follow-up of 7.9 years) showed a total of 12 relapses in 517 patients (4). Current NCCN guidelines recommend 1 cycle of BEP if used in an adjuvant setting (10).
RPLND
Whereas surveillance is preferred modality for low-risk stage I NSGCT, both RPLND and adjuvant chemotherapy are preferred options for patients with high-risk pathologic features. Though utilized less often, advantages for RPLND include accurate pathologic staging minimizing risk of recurrence of chemoresistant NSGCT, and complete resection of teratoma. In the largest randomized controlled trial comparing RPLND and 1 course BEP, Albers et al. showed that there were 2 recurrences in the BEP arm and 15 recurrences in the RPLND arm (P=0.0011) with a HR of 7.937 (95% CI, 1.808–34.78) (22). An important criticism of this trial was that patients underwent a modified template RPLND so complete control of the retroperitoneum was not achieved in all cases. Prior studies have reported presence of extra-template disease in 3–23% of all cases managed with bilateral RPLND (24). The recurrence rate of the Albers study was also higher than that reported from high-volume US centers, which have been reported as less than 2% in some RPLND series (9,25). Another study demonstrated that only 15% of patients require chemotherapy after nerve-sparing bilateral RPLND (26), and de Wit et al. argue that chemotherapy risks are lowest following primary RPLND and should be part of counseling patients (27). Major complication rates after RPLND performed at high-volume centers are low at 2–3% (28,29). Another factor favoring RPLND is that adjuvant chemotherapy does not treat teratoma, which has been found in up to 15% of patients with occult metastases (25,30). Therefore, RPLND is a good option for carefully selected patients with early stage disease, particularly patients who are not candidates for or wish to avoid chemotherapy.
Management of CS IS
Stage IS NSGCT manifest with persistent elevation of tumor markers after radical orchiectomy without evidence of radiographic disease. Mild elevation of tumor markers after orchiectomy can be product of nonmalignant causes. Hypogonadism, marijuana use, and hepatobiliary disease should be ruled out before proposing any form of AT. If beta-hCG is elevated and stable, then testing with a different assay is recommended. Patients should not be treated based on isolated elevations of LDH as other non-cancerous conditions may cause a non-specific elevation of LDH.
Historically, RPLND was recommended in patients who had elevated tumor markers but no obvious evidence of metastatic disease on staging imaging, but subsequent reports observed high relapse rates after primary RPLND. For example, Davis et al., published a small single-institution retrospective series including 15 patients with stage IS NSGCT. All 11 patients who underwent upfront RPLND relapsed during follow-up. Of the 4 patients who received upfront chemotherapy, only 1 relapse occurred and required surgery (31).
Saxman et al., suggested that patients with persistently elevated tumors markers should be treated initially with chemotherapy (32). Another small retrospective study compared cisplatin-based (16 patients) vs. carboplatin-based (4 patients) upfront chemotherapy. Tumor markers returned to normal in all 20 patients, though 3 patients experienced retroperitoneal relapses and 1 died due to progression of disease (33). In 2008, Dash and colleagues examined 24 patients with stage IS NSGCT, of which 17 received upfront chemotherapy and three then were treated with elective RPLND. Of the patients who received chemotherapy, 3 (of 14) had a retroperitoneal relapse. All 7 patients who were treated with RPLND relapsed. These data indicate the lack of effectiveness of RPLND alone in stage IS NSGCT (34).
The NCCN recommends that stage IS NSGCT patients with elevated AFP and/or beta-hCG in the S1 range should be treated with primary chemotherapy for good risk disease. Either 3 cycles of BEP or 4 cycles of EP are preferable treatments because the majority of these patients have disseminated disease (35,36).
Management of CS IIA NSGCT
Management for stage IIA NSGCT depends on the status of tumor markers. If markers persist, the NCCN recommends treatment with induction chemotherapy. With negative markers, patients have the option of treatment with upfront nerve-sparing RPLND or primary induction chemotherapy (10).
As noted for role of RPLND for stage I NSGCT, potential advantages include accurate pathologic staging and minimizing risk of recurrence of chemoresistant GCT and teratoma, which can be found in up to 20% of patients (37). While teratoma is histologically benign, its biologic potential is unpredictable and can undergo growth and malignant transformation (38,39). In addition, between 12–35% of patients undergoing primary RPLND for clinical stage IIA are found to have pathologically negative lymph nodes (pN0) (25,30,40,41), and such patients would be spared any additional chemotherapy. The risk of relapse after RPLND is >50% for patients with pN2 or pN3 disease (42,43), with this risk reduced to less than 1% after 2 cycles of adjuvant chemotherapy (26,44). If found to have pN1 or pN2 disease after primary RPLND, the NCCN guidelines recommends observation (which is preferred for pN1 disease) or 2 cycles of adjuvant chemotherapy (which is preferred for pN2 disease), and full induction chemotherapy for pN3 patients (10).
There are no randomized controlled trials comparing upfront RPLND to induction chemotherapy in the setting of CS II disease. A series by Stephenson et al. looked at patients treated CS II disease treated with primary RPLND [136] or induction chemotherapy followed by post-chemotherapy RPLND [116]. Primary chemotherapy followed by PC-RPLND was associated with significant difference in 5-year recurrence-free survival vs. primary RPLND (98% vs. 79%, P<0.001), but with no difference in 5-year cancer specific survival. Median cycles were also reduced in the upfront RPLND cohort (1.4 vs. 4.2, P<0.001). Furthermore, only 13% of patients in the primary RPLND group required full induction chemotherapy and 51% avoided chemotherapy altogether (41). Another study compared 109 patients who underwent RPLND followed by 2 cycles of adjuvant chemotherapy and 78 patients treated with induction chemotherapy, of which 33% required secondary RPLND. After 36-month follow-up, 7% patients in the primary RPLND group had relapsed compared to 11% in induction chemotherapy group (40). For patients wishing to minimize the lifetime risk of exposure to chemotherapy, primary RPLND remains an option for clinical stage IIA NSGCT.
Management of postchemotherapy residual masses ≤1 cm
After induction chemotherapy for CS II patients, further management depends on the size of residual masses. There is a clear consensus that for masses ≥1 cm, post-chemotherapy is recommended as there is a significant rate of both viable malignancy (11–17%) and teratoma (39–42%) (45,46). However, the management of residual masses <1 cm after induction chemotherapy is controversial as the histology of PC-RPLND masses <1 cm is 71% fibronecrosis, 24% teratoma, and 4% viable malignancy (47). Though imaging with FDG PET plays a role in the management of seminoma, a prospective trial demonstrated a false negative rate of 40% in NSGCT and furthermore, all cases of teratoma resulted in false-negative PET scans (48). Both surveillance and nerve-sparing RPLND are options for residual masses <1 cm according to the NCCN (10).
Some groups advocate nerve-sparing bilateral RPLND regardless of size of residual mass after induction chemotherapy arguing that surgery is curative in 45–77% of patients with viable malignancy and 75–90% with teratoma (49-51). By omitting pc-RPLND, nearly all future recurrences will subject patients to salvage chemotherapy with only 25% of patients having long-term survival (52,53). Other studies note that failure to control the retroperitoneum is a risk factor for late relapse (54-56). In a large series consisting of 252 patients with CS II NSGCT, of which 116 received induction chemotherapy and underwent PC-RPLND regardless of residual mass size, Stephenson et al. found the histology of resected PC-RPLND specimens remained consistent over time (necrosis 50–64%, viable malignancy 6–13%, teratoma 31–50%, P=0.030) despite an increasing use of induction chemotherapy. Furthermore, in 36 patients with residual masses ≤5 mm, 6% (95% CI, 0–13%) had viable malignancy and 25% (95% CI, 10–40%) had teratoma (41). Another study examined 87 patients who underwent PC-RPLND, 62% had a residual mass ≤1 cm with 33% of cases having teratoma or vital tumor (57).
Others argue that subjecting all patients with residual masses regardless of size results in overtreatment for most patients. A large retrospective series of patients from Indiana (n=141, median follow-up 15.5 years) who underwent post chemotherapy surveillance showed 12 patients (9%) experienced relapse, 6 of whom relapsed outside the retroperitoneum. Notably, although 34% of these patients had teratoma in the orchiectomy specimen, the overall estimated 15 years RFS and CSS were 90% and 97% (58). Another series examined 161 patients who were complete responders after chemotherapy; 10 experienced relapse (6%), 8 of which were managed with PC-RPLND and an overall CSS of 100% (59). Post-chemotherapy-RPLND carries some risks that are not trivial. One series reported post-operative and late complications of 32% and 7% respectively (29). Even at tertiary care and high referral centers, reported rates of retrograde ejaculation are as high as 15–20% (60-62). One study noted a NNT (Number needed to treat) of 108 pc-RPLND to prevent 1 death in patients with a radiographically negative retroperitoneum (63).
Management of CS IIB NSGCT
Intermediate volume retroperitoneal disease (clinical stage IIB) precludes the use of AS, and AT is recommended by both the NCCN and EAU. Further management is depending on disease burden and staging. For patients with persistently elevated tumor markers; induction chemotherapy with 3 cycles of BEP or 4 cycles of EP are preferred. Furthermore, chemotherapy is recommended for patients with negative markers and multifocal metastases (10).
For patients with negative markers and lymph node metastases within the primary landing zones, both primary chemotherapy or nerve-sparing RPLND are options per NCCN guidelines (10). A prospective study by Weissbach et al. compared RPLND or primary chemotherapy in CS IIA/B patients and found no difference in relapse rates at 36 months. No differences in quality of life were noted between groups, and 12% of patients treated with RPLND were reclassified as pathologic stage I disease (40). Therefore, upfront RPLND remains an option for patients wishing to avoid or unable to tolerate chemotherapy. Importantly, the cure rate for CS IIB disease is high (with survival from 88–95%) (26,64,65) with either RPLND or induction chemotherapy
Surveillance after AT
Current recommendations for follow-up of NSGCT include the use of the serum tumor markers alpha feto-protein (AFP), beta-human chorionic gonadotropin hormone (B-HGG), and lactate dehydrogenase (LDH) both in AS and after AT. One limitation with current tumor markers is that up to 35% of relapsing patients have normal levels of tumor markers (66). MicroRNAS (miRNAs) are a promising novel biomarker for GCT. In a landmark study, Dieckmann et al. demonstrated the utility of mIR-371a-3p with a sensitivity of 88.7% and specificity of 92.5% (67). Terbuch et al. examined mIR-371a-3p levels in 10 patients at different periods during disease relapse and salvage chemotherapy, and found microRNA levels were 13.65 fold higher than patients without evidence of disease (P=0.014) (68). In a large, prospective multicenter study with 616 patients with GCT, miR-371a-3p levels showed a sensitivity of 90.1%, a specificity of 94.0% with a positive predictive value of 97.2%. In the same study, combined sensitivity of traditional tumor markers were under 50% for seminoma and 80% for NSGCT (69).
The mIR-371a-3p test shows remarkable promise in detecting and monitoring the recurrence for NSGCT. Possible future considerations are its use in further stratifying/selecting and monitoring patients on AS; goal directed number of treatment cycles of chemotherapy; and management of post chemotherapy retroperitoneal masses.
Conclusions
Though GCT is the most common solid tumor in young men, it is a highly-treatable cancer for which multimodal cancer treatments provide high cure rate. For stage I NSGCT, patients have options of AS, adjuvant chemotherapy, or primary RPLND; with studies showing excellent long-term outcomes with a 99% long-term cancer-specific survival rate. The cornerstone of treatment for stage IS remains induction chemotherapy. For clinical stage II NSGCT, options are nuanced and range from nerve-sparing RPLND, primary chemotherapy (followed by surveillance or post chemotherapy surgery depending on response and size of residual mass); with cures achievable in 95–99% of patients. The discovery of microRNA as a novel biomarker for GCT may lead to future improvements in risk stratification, selection of therapies, and monitoring of treatment.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- SEER cancer statistics factsheets: Testis Cancer. National Cancer Institute, Bethesda, MD. 2018. Available online: http://seer.cancer.gov/statfacts/html/testis.html
- Stang A, Rusner C, Trabert B, et al. Incidence of testicular tumor subtypes according to the updated WHO classification, North Rhine-Westphalia, Germany, 2008-2013. Andrology 2018. [Epub ahead of print].
- Tandstad T, Stahl O, Hakansson U, et al. One course of adjuvant BEP in clinical stage I nonseminoma mature and expanded results from the SWENOTECA group. Ann Oncol 2014;25:2167-72. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th edition. New York: Springer, 2017.
- Hudolin T, Kastelan Z, Knezevic N, et al. Correlation between retroperitoneal lymph node size and presence of metastases in nonseminomatous germ cell tumors. Int J Surg Pathol 2012;20:15-8. [Crossref] [PubMed]
- Leibovitch L, Foster RS, Kopecky KK, et al. Improved accuracy of computerized tomography based clinical staging in low stage nonseminomatous germ cell cancer using size criteria of retroperitoneal lymph nodes. J Urol 1995;154:1759-63. [Crossref] [PubMed]
- Klepp O, Olsson AM, Henrikson H, et al. Prognostic factors in clinical stage I nonseminomatous germ cell tumors of the testis: multivariate analysis of a prospective multicenter study. Swedish-Norwegian Testicular Cancer Group. J Clin Oncol 1990;8:509-18. [Crossref] [PubMed]
- Hermans BP, Sweeney CJ, Foster RS, et al. Risk of systemic metastases in clinical stage I nonseminoma germ cell testis tumor managed by retroperitoneal lymph node dissection. J Urol 2000;163:1721-4. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Testicular Cancer (Version 1.2019). 2018.
- Vergouwe Y, Steyerberg EW, Eijkemans MJ, et al. Predictors of occult metastasis in clinical stage I nonseminoma: a systematic review. J Clin Oncol 2003;21:4092-9. [Crossref] [PubMed]
- Lago-Hernandez CA, Feldman H, O'Donnell E, et al. A refined risk stratification scheme for clinical stage 1 NSGCT based on evaluation of both embryonal predominance and lymphovascular invasion. Ann Oncol 2015;26:1396-401. [Crossref] [PubMed]
- Albers P, Albrecht W, Algaba F, et al. European Association of Urology Guidlines on Testicular Cancer. 2018. Available online: 2018.https://uroweb.org//guideline/testicular-cancer Accessed 02/01/2018
- Kollmannsberger C, Tandstad T, Bedard PL, et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol 2015;33:51-7. [Crossref] [PubMed]
- Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol 2013;31:3490-3. [Crossref] [PubMed]
- Weiner AB, Pearce SM, Eggener SE. Management trends for men with early-stage nonseminomatous germ cell tumors of the testicle: An analysis of the National Cancer Database. Cancer 2017;123:245-52. [Crossref] [PubMed]
- Beyer J, Albers P, Altena R, et al. Maintaining success, reducing treatment burden, focusing on survivorship: highlights from the third European consensus conference on diagnosis and treatment of germ-cell cancer. Ann Oncol 2013;24:878-88. [Crossref] [PubMed]
- Yu HY, Madison RA, Setodji CM, et al. Quality of surveillance for stage I testis cancer in the community. J Clin Oncol 2009;27:4327-32. [Crossref] [PubMed]
- Cullen MH, Stenning SP, Parkinson MC, et al. Short-course adjuvant chemotherapy in high-risk stage I nonseminomatous germ cell tumors of the testis: a Medical Research Council report. J Clin Oncol 1996;14:1106-13. [Crossref] [PubMed]
- Huddart RA, Norman A, Shahidi M, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol 2003;21:1513-23. [Crossref] [PubMed]
- van den Belt-Dusebout AW, de Wit R, Gietema JA, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol 2007;25:4370-8. [Crossref] [PubMed]
- Albers P, Siener R, Krege S, et al. Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I Nonseminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group. J Clin Oncol 2008;26:2966-72. [Crossref] [PubMed]
- Tandstad T, Dahl O, Cohn-Cedermark G, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. J Clin Oncol 2009;27:2122-8. [Crossref] [PubMed]
- Eggener SE, Carver BS, Sharp DS, et al. Incidence of disease outside modified retroperitoneal lymph node dissection templates in clinical stage I or IIA nonseminomatous germ cell testicular cancer. J Urol 2007;177:937-42; discussion 942-3. [Crossref] [PubMed]
- Stephenson AJ, Bosl GJ, Motzer RJ, et al. Retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer: impact of patient selection factors on outcome. J Clin Oncol 2005;23:2781-8. [Crossref] [PubMed]
- Kondagunta GV, Sheinfeld J, Mazumdar M, et al. Relapse-free and overall survival in patients with pathologic stage II nonseminomatous germ cell cancer treated with etoposide and cisplatin adjuvant chemotherapy. J Clin Oncol 2004;22:464-7. [Crossref] [PubMed]
- de Wit R, Bosl GJ. Optimal management of clinical stage I testis cancer: one size does not fit all. J Clin Oncol 2013;31:3477-9. [Crossref] [PubMed]
- Baniel J, Foster RS, Rowland RG, et al. Complications of primary retroperitoneal lymph node dissection. J Urol 1994;152:424-7. [Crossref] [PubMed]
- Subramanian VS, Nguyen CT, Stephenson AJ, et al. Complications of open primary and post-chemotherapy retroperitoneal lymph node dissection for testicular cancer. Urol Oncol 2010;28:504-9. [Crossref] [PubMed]
- Donohue JP, Thornhill JA, Foster RS, et al. Clinical stage B non-seminomatous germ cell testis cancer: the Indiana University experience (1965-1989) using routine primary retroperitoneal lymph node dissection. Eur J Cancer 1995;31A:1599-604. [Crossref] [PubMed]
- Davis BE, Herr HW, Fair WR, et al. The management of patients with nonseminomatous germ cell tumors of the testis with serologic disease only after orchiectomy. J Urol 1994;152:111-3; discussion 114. [Crossref] [PubMed]
- Saxman SB, Nichols CR, Foster RS, et al. The management of patients with clinical stage I nonseminomatous testicular tumors and persistently elevated serologic markers. J Urol 1996;155:587-9. [Crossref] [PubMed]
- Culine S, Theodore C, Terrier-Lacombe MJ, et al. Primary chemotherapy in patients with nonseminomatous germ cell tumors of the testis and biological disease only after orchiectomy. J Urol 1996;155:1296-8. [Crossref] [PubMed]
- Dash A, Carver BS, Stasi J, et al. The indication for postchemotherapy lymph node dissection in clinical stage IS nonseminomatous germ cell tumor. Cancer 2008;112:800-5. [Crossref] [PubMed]
- Lv ZJ, Wu S, Dong P, et al. Clinical outcomes in patients with stage I non-seminomatous germ cell cancer. Asian J Androl 2013;15:558-63. [Crossref] [PubMed]
- Mezvrishvili Z, Managadze L. Three cycles of etoposide and cisplatin chemotherapy in clinical stage IS nonseminomatous testicular cancer. Int Urol Nephrol 2006;38:621-4. [Crossref] [PubMed]
- Foster RS, Baniel J, Leibovitch I, et al. Teratoma in the orchiectomy specimen and volume of metastasis are predictors of retroperitoneal teratoma in low stage nonseminomatous testis cancer. J Urol 1996;155:1943-5. [Crossref] [PubMed]
- Motzer RJ, Amsterdam A, Prieto V, et al. Teratoma with malignant transformation: diverse malignant histologies arising in men with germ cell tumors. J Urol 1998;159:133-8. [Crossref] [PubMed]
- Logothetis CJ, Samuels ML, Trindade A, et al. The growing teratoma syndrome. Cancer 1982;50:1629-35. [Crossref] [PubMed]
- Weissbach L, Bussar-Maatz R, Flechtner H, et al. RPLND or primary chemotherapy in clinical stage IIA/B nonseminomatous germ cell tumors? Results of a prospective multicenter trial including quality of life assessment. Eur Urol 2000;37:582-94. [Crossref] [PubMed]
- Stephenson AJ, Bosl GJ, Motzer RJ, et al. Nonrandomized comparison of primary chemotherapy and retroperitoneal lymph node dissection for clinical stage IIA and IIB nonseminomatous germ cell testicular cancer. J Clin Oncol 2007;25:5597-602. [Crossref] [PubMed]
- Stephenson AJ, Bosl GJ, Bajorin DF, et al. Retroperitoneal lymph node dissection in patients with low stage testicular cancer with embryonal carcinoma predominance and/or lymphovascular invasion. J Urol 2005;174:557-60; discussion 60. [Crossref] [PubMed]
- Stephenson AJ, Klein EA. Surgical management of low-stage nonseminomatous germ cell testicular cancer. BJU Int 2009;104:1362-8. [Crossref] [PubMed]
- Behnia M, Foster R, Einhorn LH, et al. Adjuvant bleomycin, etoposide and cisplatin in pathological stage II non-seminomatous testicular cancer. the Indiana University experience. Eur J Cancer 2000;36:472-5. [Crossref] [PubMed]
- Spiess PE, Brown GA, Pisters LL, et al. Viable malignant germ cell tumor in the postchemotherapy retroperitoneal lymph node dissection specimen: can it be predicted using clinical parameters? Cancer 2006;107:1503-10. [Crossref] [PubMed]
- Steyerberg EW, Keizer HJ, Fossa SD, et al. Prediction of residual retroperitoneal mass histology after chemotherapy for metastatic nonseminomatous germ cell tumor: multivariate analysis of individual patient data from six study groups. J Clin Oncol 1995;13:1177-87. [Crossref] [PubMed]
- Ravi P, Gray KP, O'Donnell EK, et al. A meta-analysis of patient outcomes with subcentimeter disease after chemotherapy for metastatic non-seminomatous germ cell tumor. Ann Oncol 2014;25:331-8. [Crossref] [PubMed]
- Pfannenberg AC, Oechsle K, Bokemeyer C, et al. The role of [(18)F] FDG-PET, CT/MRI and tumor marker kinetics in the evaluation of post chemotherapy residual masses in metastatic germ cell tumors--prospects for management. World J Urol 2004;22:132-9. [Crossref] [PubMed]
- Toner GC, Panicek DM, Heelan RT, et al. Adjunctive surgery after chemotherapy for nonseminomatous germ cell tumors: recommendations for patient selection. J Clin Oncol 1990;8:1683-94. [Crossref] [PubMed]
- Stenning SP, Parkinson MC, Fisher C, et al. Postchemotherapy residual masses in germ cell tumor patients: content, clinical features, and prognosis. Medical Research Council Testicular Tumour Working Party. Cancer 1998;83:1409-19. [Crossref] [PubMed]
- Fizazi K, Oldenburg J, Dunant A, et al. Assessing prognosis and optimizing treatment in patients with postchemotherapy viable nonseminomatous germ-cell tumors (NSGCT): results of the sCR2 international study. Ann Oncol 2008;19:259-64. [Crossref] [PubMed]
- McCaffrey JA, Mazumdar M, Bajorin DF, et al. Ifosfamide- and cisplatin-containing chemotherapy as first-line salvage therapy in germ cell tumors: response and survival. J Clin Oncol 1997;15:2559-63. [Crossref] [PubMed]
- Kondagunta GV, Bacik J, Donadio A, et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol 2005;23:6549-55. [Crossref] [PubMed]
- Lutke Holzik MF, Hoekstra HJ, Mulder NH, et al. Non-germ cell malignancy in residual or recurrent mass after chemotherapy for nonseminomatous testicular germ cell tumor. Ann Surg Oncol 2003;10:131-5. [Crossref] [PubMed]
- Baniel J, Foster RS, Gonin R, et al. Late relapse of testicular cancer. J Clin Oncol 1995;13:1170-6. [Crossref] [PubMed]
- Sharp DS, Carver BS, Eggener SE, et al. Clinical outcome and predictors of survival in late relapse of germ cell tumor. J Clin Oncol 2008;26:5524-9. [Crossref] [PubMed]
- Oldenburg J, Alfsen GC, Lien HH, et al. Postchemotherapy retroperitoneal surgery remains necessary in patients with nonseminomatous testicular cancer and minimal residual tumor masses. J Clin Oncol 2003;21:3310-7. [Crossref] [PubMed]
- Ehrlich Y, Brames MJ, Beck SD, et al. Long-term follow-up of Cisplatin combination chemotherapy in patients with disseminated nonseminomatous germ cell tumors: is a postchemotherapy retroperitoneal lymph node dissection needed after complete remission? J Clin Oncol 2010;28:531-6. [Crossref] [PubMed]
- Kollmannsberger C, Daneshmand S, So A, et al. Management of disseminated nonseminomatous germ cell tumors with risk-based chemotherapy followed by response-guided postchemotherapy surgery. J Clin Oncol 2010;28:537-42. [Crossref] [PubMed]
- Coogan CL, Hejase MJ, Wahle GR, et al. Nerve sparing post-chemotherapy retroperitoneal lymph node dissection for advanced testicular cancer. J Urol 1996;156:1656-8. [Crossref] [PubMed]
- Miki T, Mizutani Y, Nakamura T, et al. Post-chemotherapy nerve-sparing retroperitoneal lymph node dissection for advanced germ cell tumor. Int J Urol 2009;16:379-82. [Crossref] [PubMed]
- Pettus JA, Carver BS, Masterson T, et al. Preservation of ejaculation in patients undergoing nerve-sparing postchemotherapy retroperitoneal lymph node dissection for metastatic testicular cancer. Urology 2009;73:328-31; discussion 331-2. [Crossref] [PubMed]
- Daneshmand S, Stephenson AJ, Sheinfeld J, et al. The management of subcentimeter residual mass in NSGCT: pcRPLND vs. observation. Urol Oncol 2011;29:842-7. [Crossref] [PubMed]
- Donohue JP, Thornhill JA, Foster RS, et al. The role of retroperitoneal lymphadenectomy in clinical stage B testis cancer: the Indiana University experience (1965 to 1989). J Urol 1995;153:85-9. [Crossref] [PubMed]
- Horwich A, Norman A, Fisher C, et al. Primary chemotherapy for stage II nonseminomatous germ cell tumors of the testis. J Urol 1994;151:72-7; discussion 77-8. [Crossref] [PubMed]
- Mir MC, Pavan N, Gonzalgo ML. Current Clinical Applications of Testicular Cancer Biomarkers. Urol Clin North Am 2016;43:119-25. [Crossref] [PubMed]
- Dieckmann KP, Radtke A, Spiekermann M, et al. Serum Levels of MicroRNA miR-371a-3p: A Sensitive and Specific New Biomarker for Germ Cell Tumours. Eur Urol 2017;71:213-20. [Crossref] [PubMed]
- Terbuch A, Adiprasito JB, Stiegelbauer V, et al. MiR-371a-3p Serum Levels Are Increased in Recurrence of Testicular Germ Cell Tumor Patients. Int J Mol Sci 2018;19. [Crossref] [PubMed]
- Dieckmann KP, Radtke A, Geczi L, et al. Serum Levels of MicroRNA-371a-3p (M371 Test) as a New Biomarker of Testicular Germ Cell Tumors: Results of a Prospective Multicentric Study. J Clin Oncol 2019;37:1412-23. [Crossref] [PubMed]


