
Optimisation of shock wave lithotripsy: a systematic review of technical aspects to improve outcomes
Introduction
Shock wave lithotripsy (SWL) was first introduced in the 1980s for the management of stone disease. It has since become an important treatment option for either renal or ureteric stone stones. The European Association of Urology (EAU) currently recommends the use of SWL in renal stones up to 20 mm in size, in the absence of unfavourable factors for lower pole stones (1). However, recent technological progress and improvements in minimally invasive endourological techniques, and associated high success rates, have reduced the overall use of SWL (2).
Studies have since searched for the optimal treatment strategies to optimise SWL and improve stone-free outcomes and success rates. Multiple studies have identified patient factors associated with SWL success. These include, but are not limited to stone density, skin-to-stone distance, stone burden, and stone location, and allow for improved patient selection and counselling (1).
The aim of this study is to review the technical aspects of SWL treatment strategies, with a view to improving and optimising patient outcomes.
Materials and methods
The systematic review was performed as per the preferred reporting items for systematic review and meta-analysis (PRISMA) statement. The search strategy was conducted to find relevant studies on the PubMed database from January 1984 to present (November 2018), using the search terms ‘shock wave lithotripsy’ and ‘stone’ as keywords (Figure 1).
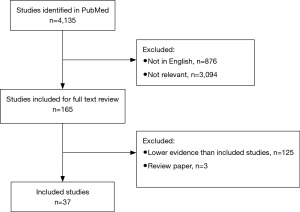
Only English language articles were included. Studies were deemed relevant if they addressed technical aspects of SWL for renal or ureteric stones in humans. All study designs were included. Records were screened independently by two authors; discrepancies were resolved with mutual agreement and consensus with the senior author.
In total, 4,135 articles were identified, of which 3,259 were English language articles. Abstracts were reviewed following the identification of 165 relevant titles. The summaries and full text manuscripts of relevant articles were reviewed in order to select the studies with the best level of evidence in each theme covered during the review. Regarding the technical aspects of SWL, themes were selected to include: shock wave generation, patient positioning, number of shocks, rate, energy ramping, coupling, and targeting. For these included SWL themes, relevant and selected studies with the highest level of evidence are described below.
Technical principles of SWL
SWL fragments calculi using pulsed acoustic waves at high intensity and low frequency. These waves are directed using an external power source, known as a lithotripter. It is fundamental that the shockwave can pass through the body and hit the stone with minimal loss of energy. Technical factors must be taken into consideration to optimise results, including the type of machine, patient position, number, rate and energy of shocks, stone targeting, and patient analgesia. A summary of favourable technical factors is shown in Table 1, including Grading of Recommendations, Assessment, Development and Evaluations (GRADE) ratings.
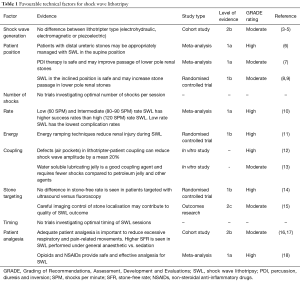
Full table
Shock wave generation
There are currently four types of shock wave generation: electrohydraulic, electromagnetic, and piezoelectric. Electrohydraulic generators generate shock waves when a spark is discharged between two electrodes, producing a vaporisation bubble. This bubble expands and then collapses, producing a pressure wave, which is then focussed by an ellipsoid reflector (19). Electroconductive generators are a variant of this method. The generator similarly uses two electrodes, but sit within a conductive solution, resulting in lower spark variation (20).
Electromagnetic generators utilise an electromagnetic coil that either sits on a flat surface with an overlying conductive membrane, or surrounds a cylinder with a spherical cap. The magnetic field causes repulsion of the membrane or cap, producing a shock wave which is focused by an acoustic lens or parabolic reflector (21). In piezoelectric generators, piezoelectric ceramics or crystals, within a water-filled container, are stimulated via high-frequency electrical pulses. The stress/strain changes in the material create ultrasonic vibrations, which are positioned on a reflector (22).
No randomised trials have been conducted to compare these forms of shock wave generation. Multiple cohort studies have been conducted, particularly in comparing electromagnetic and electrohydraulic, finding no significant difference in treatment outcomes between the methods of shock wave generation (3-5).
Patient positioning
Patient positioning is important to both reduce the distance shock waves must travel, and to prevent interference from skeletal elements, including the transverse processes, ribs, sacroiliac bones and pelvis. Stones located in the angle between the spine and pelvic brim, and below the sacroiliac joint can be particularly problematic as these bony structures may reduce shockwave strength.
Positioning of patients in these distal ureteric stones has been problematic. Initial SWL patients managed supine were thought to have reduced efficacy due to bony pelvis interference. SWL in the prone position was proposed in 1998, and additional studies established this position to be both safe and effective. However, prone patients suffer from discomfort, increased intraabdominal pressure and reduced lung capacity. A number of urologists moved back to the supine position, targeting stones through gaps in the bony pelvis, such as the sciaticum majus foramen and sciaticum minus foramen.
In 2016, Li et al. (6) performed a systematic review and meta-analysis of studies comparing supine and prone positioning in distal ureteric stones. Pooled data from 647 patients across 1 randomised controlled trial and 3 case control studies was associated with significantly higher stone-free rate (SFR) for supine SWL. This was consistent for both the first (OR 4.17) and final (OR 3.02) session. This meta-analysis was followed by a larger randomized controlled trial (RCT) of 160 patients by Choo et al. (23), again showing higher SFR in the supine group (72.6% vs. 54.7%, OR 2.413), with no sciatic nerve injuries. This evidence suggests that patients with distal ureteric stones may be appropriately managed supine.
Further theories exist regarding lower pole stones. Due to their position, it has been hypothesised that stones in the lower pole are less likely to clear due to poorer drainage of these dependent calyces. Adjuvant therapy has been proposed in the form of percussion, diuresis and inversion (PDI) therapy to help improve clearance (8). PDI therapy can help improve stone clearance in 3 ways: (I) increasing urine production to ‘flush out’ fragments; (II) using gravity to aid stone fragment passage by placing the patient in steep, prone Trendelenburg position; (III) using manual flank percussion to dislodge stone fragments through vibration. Patients typically undergo multiple sessions after SWL treatment.
Two small RCTs found improved SFR in the PDI group vs. the control group of SWL only (40% vs. 3% and 62.5% vs. 35.4%) (24,25). A Cochrane review summarised these findings to be potentially safe and effective therapies to assist lower pole stone clearance; however, given the limited evidence and small trials, further adequately powered studies are required (7). Further studies have performed SWL in an inclined position. A large trial of 740 patients found significantly greater SFR when SWL was performed in an inclined position (81% vs. 73%) (9). while a smaller trial of 140 patients a small increase in SFR, although this was not significant (76% vs. 72%) (8). There was no increase in complications in the inclined group. SWL in the inclined position may aid fragment passage in lower pole stones at no additional cost.
The positioning of patients during SWL is important to achieve adequate fragmentation. The majority of patients can be managed in the supine position, including distal ureteric stones, where the transgluteal approach is safe and effective. The use of an inclined position in lower poles may aid stone passage, in a safe and cost-effective manner.
Number of shocks
Excessive shockwave administration may result in either renal injury or injuries to other organs. No past or current trials specifically investigate the ideal number of shocks per session. Furthermore, no specific recommendations for the total number of shockwaves per treatment have been given in SWL guidelines (1); however, each manufacturer provides advice for both maximum shockwave number and energy (26). The general upper limit for number of shocks is 4,000, although this number should be adjusted relative to the energy level used (26). Once fragmentation occurs, further disintegration may be limited due to attenuation from surrounding stone fragments.
Rate
Application of optimal shock wave rates can both improve stone fragmentation and reduce surrounding tissue damage (27,28). A number of randomised controlled trials have performed, comparing SWL frequencies. These have been aggregated by Kang et al. (10) in a systematic review and network meta-analysis comparing low [60 shocks per min (SPM)], intermediate (80–90 SPM) and high (120 SPM) lithotripsy rates. Thirteen RCTs were included, showing that success rates of low- (OR 2.2) or intermediate-frequency SWL (OR 2.5) were greater than high-frequency SWL. There was no significant difference in SWL success rate for low- and intermediate-frequency SWL (OR 0.87).
By rank probability testing, intermediate-frequency SWL had highest success, followed by low- and high- frequency SWL. For complications, low-frequency SWL had the lowest complication rate, followed by high- and intermediate-frequency. Based on available evidence, intermediate- and low-frequency SWL have comparable outcomes, as compared to high-frequency SWL, when comparing both success and complications. One must consider this in view of the longer treatment times required for these lower frequency treatments.
Energy ramping
The SWL procedure typically starts at a low energy level, and is gradually increased. Firstly, this allows the patient to accommodate to the sensation of SWL. Secondly, EAU guidelines suggest that power ramping is associated with less renal damage, with level 1b evidence. The largest of these randomised trials, by Skuginna et al. (11), included 418 patients, randomised to a stepwise voltage ramping protocol (power 7 to 9), versus a fixed power group (level 9). Ramping protocol induced statistically fewer ultrasound-detected renal haematomas (5.6%), compared with fixed power (13%). It is unclear how many of these haematomas were clinically significant. Lambert et al. (29) performed a smaller study of 45 patients, finding urinary macroglobulin and β2-microglobulin levels, indices of renal injury, were significantly lower 1 week post-SWL in the ramping protocol group. Honey et al. (30) compared immediate vs. delayed energy ramping, and found no difference in clinical complication rates between the two groups.
Although evidence of long-term and structural effects of lithotripsy is limited, it is known that haemorrhage may promote an inflammatory response, leading to nephron disruption, interstitial oedema, fibrosis and renal scarring. It is thought that ramping promotes vasoconstriction; these stiffer vessels are less likely to bleed, preventing renal injury (31). The largest randomised trial on energy ramping in SWL suggests a higher haematoma rate in fixed energy level SWL, although it is unclear as to how many of these manifest clinically. As lithotripsy is performed for a benign condition, often repeatedly and in younger patients; energy ramping limits renal damage, potentially preserving future renal function.
Evidence that energy ramping improves stone comminution is unclear. Two small trials found benefit in SFR with ramping, 96% vs. 72% (32) and 81% vs. 48% (29), with a third trial finding the opposite effect (54.5% vs. 72.5%) (30), and a fourth showing no statistical difference (82% vs. 90%) (33). However, the large Skuginna et al.’s trial found no difference in SFR at 3 months, regardless of stone-free definition used (no fragments or clinically insignificant residual fragments) (11). A meta-analysis of these trials has not been performed, given the variation in energy ramping protocols and follow-up including measurement of SFR and renal damage.
There is strong evidence that energy ramping during SWL has a renoprotective effect. However, there is no evidence that stone fragmentation is increased.
Coupling
Patient coupling is essential maximise energy transmission. In modern lithotripters, the gap between the dry lithotripter head and the patient must be bridged, as shock waves do not propagate through air (21). The presence of air in the path shock waves reduces transmission, and is inversely proportional to transmission. Pishchalnikov et al. (12) observed and photographed air pockets and found the process of coupling can produced air pockets ranging from 1.5% to 19% of the coupling surface area, reducing shock wave amplitude by a mean of 20%. Furthermore, patient repositioning can introduce further 57% reduction in energy.
To try and reduce this interference, a transmission medium is required, allowing shock waves to pass to the targeted calculus. A wide range of coupling media have been used, including silicon oil, castor oil, ultrasound gel, petroleum jelly, and other water-soluble lubricating jellies. Cartledge et al. (13) performed an in vitro study comparing five coupling agents: petroleum jelly, ultrasound jelly, eutectic mixture of local anaesthetic (EMLA) cream, lidocaine jelly (Instillagel), and commercial water-soluble lubricating jelly. The number of shocks required to achieve stone fragmentation varied greatly, with water-soluble lubricating jellies requiring the fewest shocks, and petroleum jelly the most.
Coupling is an important facet of SWL, and care must be taken to use an appropriate transmission medium, reduce patient movement and repositioning, and ensure an absence of air in the shock wave path.
Targeting
Targeting of the calculus to be treated is crucial to successful SWL, and requires accurate three-dimensional targeting of the stone within the lithotripter focal zone. A number of factors can affect targeting, including user error, poor shockwave alignment, stone movement during treatment, and patient movement due to discomfort or respiration (34).
Fluoroscopy and ultrasound are commonly used for targeting, although the latter is limited to stones within the kidney. Fluoroscopy targeting is familiar to urologists and allows for convenient stone localisation, at the expense of radiation exposure to the operator and patient (35). Pulsed or coned fluoroscopy may reduce radiation exposure. The addition of ultrasound allows for identification of radiolucent stones and fragmentation can be monitored real-time using Doppler ultrasound. Both techniques are useful for SWL. Van Besien et al. (14) randomised 114 patients to have stones targeted using ultrasound and fluoroscopy; no significant difference was observed, as SFR was 52% in the ultrasound-guided group vs. 42% in the fluoroscopy-guided group. Similar results were seen in a retrospective cohort study by Smith et al. (36), showing no difference in SFR.
Targeting should be confirmed at regular intervals during the treatment. A retrospective study analysing treatment results across 12 surgeons, with a single surgeon experiencing significantly better outcomes; this surgeon treated greatest number of patients, used the highest number of shocks and had the longest fluoroscopy time (15). This long fluoroscopy time may highlight increased confirmation of targeting throughout the procedure, although this data comes from a retrospective series.
Stones within the kidney or proximal ureter may move substantially during respiration, by as much as 50 mm (37). This movement can cause up to 40% of shock waves to miss the stone (38). To minimise this, the stone should be targeted during the longer expiratory phase. There is some evidence that the use of a plate or belt across the upper abdomen can counteract stone movement during respiration (39).
Timing of sessions
No prospective clinical study has been performed to assess the timing of repeated SWL sessions. The EAU guidelines suggest that clinical experience indicates that repeat sessions are feasible (within 1 day for ureteral stones) (1).
We note a study by Schnabel et al. (40) assessing the incidence and risk factors of renal haematoma, after assessing 1,300 SWL treatments. Haematoma developed in seven patients, with 3 symptomatic. In 2 of these 7 patients, haematomas developed after 3 SWL treatments across 7 days. A comment by Adanur et al. (41) highlights this short interval as a possible contributing factor for renal haematoma. Small haematomas may regress within weeks; given this, it has been suggested that at least a week should pass before repeating SWL treatments.
Current evidence for the timing of SWL is weak. Further studies are required to assess this from a perspective of patient safety and SWL efficacy.
Analgesia
Adequate procedural analgesia falls under both a patient and technical factor (42). By increasing patient tolerability during the procedure through analgesia, improvements can be gained from multiple areas. Firstly, by reducing patient movement, targeting can be better focussed on the stone, with more shocks reaching the calculus and fewer peri-procedural readjustments required. Second, energy ramping may be easier; with patients tolerating a higher maximum energy level.
For the majority of patients, the treatment procedure may be completed with oral analgesics only. Alternatively, sedation or general anaesthesia may be used. Both awake and general anaesthetic SWL have benefits and disadvantages (43). Avoiding general anaesthetic has advantages in reducing morbidity and allowing treatment on an outpatient basis. However, general anaesthesia allows for more controlled respiration, leading to greater stone targeting and fragmentation. Two cohort studies comparing general anaesthetic vs. sedation lithotripsy found higher success rate in the general anaesthesia group (78% vs. 51% and 87% vs. 55%) (16,17). This increase may be related to the variations in sedation, which may result in patient movement and breathing variations. No RCTs have compared general anaesthesia SWL to awake lithotripsy with oral analgesia.
For units that perform SWL with oral analgesia, Rasmussen et al. (44) performed a double-blinded study on analgesic requirements during SWL. All patients received naproxen suppositories and subcutaneous lidocaine. In addition, half received 2 mL (0.1 mg) Fentanyl, while a control group only received 2 mL normal saline. Pain scores and the need for supplementary analgesia were not significantly different between the two groups, highlighting that IV analgesia and opioids is not required during SWL.
For this oral analgesia, Aboumarzouk et al. (18) performed a meta-analysis of RCTs comparing opioids, non-steroidal anti-inflammatory drugs (NSAIDs) and simple analgesics (paracetamol). After identifying 4 clinical trials comparing NSAIDs and opioids, the authors found both to provide both safe and effective analgesia. There were no significant differences in pain scores for NSAIDs or opioids in 3 of these studies; however, 2 studies found adequate analgesia was more likely to be achieved with opioids than NSAIDs. Patients tolerated both opioids and NSAIDs well, with no differences in post-operative nausea and vomiting.
SWL can be safely performed with oral analgesia and general anaesthesia. While general anaesthesia may allow for improved stone targeting, oral analgesia provides advantages by allowing for truly minimally invasive and ambulatory stone management. Multiple studies have identified oral opioids and NSAIDs to be appropriate in reducing pain from SWL.
Conclusions
SWL has good outcomes in the treatment of upper urinary tract stones. It remains the only truly non-invasive stone treatment. While SFR might not be equivalent to ureteroscopy or percutaneous nephrolithotomy outcomes, SWL can be optimised by changing several technical factors, including type of machine, patient position, number, rate and energy of shocks, stone targeting, and patient analgesia. With low complication rates, SWL, paired with these improved technical factors and appropriate patient selection, remains an excellent treatment option in 2019.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Türk C, Petřík A, Sarica K, et al. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol 2016;69:475-82. [Crossref] [PubMed]
- Geraghty RM, Jones P, Somani BK. Worldwide Trends of Urinary Stone Disease Treatment Over the Last Two Decades: A Systematic Review. J Endourol 2017;31:547-56. [Crossref] [PubMed]
- Alanee S, Ugarte R, Monga M. The effectiveness of shock wave lithotripters: a case matched comparison. J Urol 2010;184:2364-7. [Crossref] [PubMed]
- Bhojani N, Mandeville JA, Hameed TA, et al. Lithotripter outcomes in a community practice setting: comparison of an electromagnetic and an electrohydraulic lithotripter. J Urol 2015;193:875-9. [Crossref] [PubMed]
- Bianchi G, Marega D, Knez R, et al. Comparison of an electromagnetic and an electrohydraulic lithotripter: Efficacy, pain and complications. Arch Ital Urol Androl 2018;90:169-71. [Crossref] [PubMed]
- Li T, Gao L, Chen P, et al. Supine versus Prone Position during Extracorporeal Shockwave Lithotripsy for Treating Distal Ureteral Calculi: A Systematic Review and Meta-Analysis. Urol Int 2016;97:1-7. [Crossref] [PubMed]
- Liu LR, Li QJ, Wei Q, et al. Percussion, diuresis, and inversion therapy for the passage of lower pole kidney stones following shock wave lithotripsy. Cochrane Database Syst Rev 2013.CD008569. [PubMed]
- Leong WS, Liong ML, Liong YV, et al. Does simultaneous inversion during extracorporeal shock wave lithotripsy improve stone clearance: a long-term, prospective, single-blind, randomized controlled study. Urology 2014;83:40-4. [Crossref] [PubMed]
- Cakiroglu B, Sinanoglu O, Tas T, et al. The effect of inclined position on stone free rates in patients with lower caliceal stones during SWL session. Arch Ital Urol Androl 2015;87:38-40. [Crossref] [PubMed]
- Kang DH, Cho KS, Ham WS, et al. Comparison of High, Intermediate, and Low Frequency Shock Wave Lithotripsy for Urinary Tract Stone Disease: Systematic Review and Network Meta-Analysis. PLoS One 2016;11:e0158661. [Crossref] [PubMed]
- Skuginna V, Nguyen DP, Seiler R, et al. Does Stepwise Voltage Ramping Protect the Kidney from Injury During Extracorporeal Shockwave Lithotripsy? Results of a Prospective Randomized Trial. Eur Urol 2016;69:267-73. [Crossref] [PubMed]
- Pishchalnikov YA, Neucks JS, VonDerHaar RJ, et al. Air pockets trapped during routine coupling in dry head lithotripsy can significantly decrease the delivery of shock wave energy. J Urol 2006;176:2706-10. [Crossref] [PubMed]
- Cartledge JJ, Cross WR, Lloyd SN, et al. The efficacy of a range of contact media as coupling agents in extracorporeal shockwave lithotripsy. BJU Int 2001;88:321-4. [Crossref] [PubMed]
- Van Besien J, Uvin P, Hermie I, et al. Ultrasonography Is Not Inferior to Fluoroscopy to Guide Extracorporeal Shock Waves during Treatment of Renal and Upper Ureteric Calculi: A Randomized Prospective Study. Biomed Res Int 2017;2017:7802672.
- Logarakis NF, Jewett MA, Luymes J, et al. Variation in clinical outcome following shock wave lithotripsy. J Urol 2000;163:721-5. [Crossref] [PubMed]
- Eichel L, Batzold P, Erturk E. Operator experience and adequate anesthesia improve treatment outcome with third-generation lithotripters. J Endourol 2001;15:671-3. [Crossref] [PubMed]
- Sorensen C, Chandhoke P, Moore M, et al. Comparison of intravenous sedation versus general anesthesia on the efficacy of the Doli 50 lithotriptor. J Urol 2002;168:35-7. [Crossref] [PubMed]
- Aboumarzouk OM, Hasan R, Tasleem A, et al. Analgesia for patients undergoing shockwave lithotripsy for urinary stones - a systematic review and meta-analysis. Int Braz J Urol 2017;43:394-406. [Crossref] [PubMed]
- Elmansy HE, Lingeman JE. Recent advances in lithotripsy technology and treatment strategies: A systematic review update. Int J Surg 2016;36:676-80. [Crossref] [PubMed]
- Rassweiler JJ, Knoll T, Kohrmann KU, et al. Shock wave technology and application: an update. Eur Urol 2011;59:784-96. [Crossref] [PubMed]
- Lingeman JE, McAteer JA, Gnessin E, et al. Shock wave lithotripsy: advances in technology and technique. Nat Rev Urol 2009;6:660-70. [Crossref] [PubMed]
- Ng CF, McLornan L, Thompson TJ, et al. Comparison of 2 generations of piezoelectric lithotriptors using matched pair analysis. J Urol 2004;172:1887-91. [Crossref] [PubMed]
- Choo MS, Han JH, Kim JK, et al. The transgluteal approach to shockwave lithotripsy to treat distal ureter stones: a prospective, randomized, and multicenter study. World J Urol 2018;36:1299-306. [Crossref] [PubMed]
- Pace KT, Tariq N, Dyer SJ, et al. Mechanical percussion, inversion and diuresis for residual lower pole fragments after shock wave lithotripsy: a prospective, single blind, randomized controlled trial. J Urol 2001;166:2065-71. [Crossref] [PubMed]
- Chiong E, Hwee ST, Kay LM, et al. Randomized controlled study of mechanical percussion, diuresis, and inversion therapy to assist passage of lower pole renal calculi after shock wave lithotripsy. Urology 2005;65:1070-4. [Crossref] [PubMed]
- Kroczak T, Scotland KB, Chew B, et al. Shockwave lithotripsy: techniques for improving outcomes. World J Urol 2017;35:1341-6. [Crossref] [PubMed]
- Anglada-Curado FJ, Campos-Hernandez P, Carrasco-Valiente J, et al. Extracorporeal shock wave lithotripsy for distal ureteral calculi: improved efficacy using low frequency. Int J Urol 2013;20:214-9. [Crossref] [PubMed]
- Chacko J, Moore M, Sankey N, et al. Does a slower treatment rate impact the efficacy of extracorporeal shock wave lithotripsy for solitary kidney or ureteral stones? J Urol 2006;175:1370-3; discussion 1373-4. [Crossref] [PubMed]
- Lambert EH, Walsh R, Moreno MW, et al. Effect of escalating versus fixed voltage treatment on stone comminution and renal injury during extracorporeal shock wave lithotripsy: a prospective randomized trial. J Urol 2010;183:580-4. [Crossref] [PubMed]
- Honey RJ, Ray AA, Ghiculete D, et al. Shock wave lithotripsy: a randomized, double-blind trial to compare immediate versus delayed voltage escalation. Urology 2010;75:38-43. [Crossref] [PubMed]
- Handa RK, Bailey MR, Paun M, et al. Pretreatment with low-energy shock waves induces renal vasoconstriction during standard shock wave lithotripsy (SWL): a treatment protocol known to reduce SWL-induced renal injury. BJU Int 2009;103:1270-4. [Crossref] [PubMed]
- Demirci D, Sofikerim M, Yalcin E, et al. Comparison of conventional and step-wise shockwave lithotripsy in management of urinary calculi. J Endourol 2007;21:1407-10. [Crossref] [PubMed]
- Rabah DM, Mabrouki MS, Farhat KH, et al. Comparison of escalating, constant, and reduction energy output in ESWL for renal stones: multi-arm prospective randomized study. Urolithiasis 2017;45:311-6. [Crossref] [PubMed]
- Bohris C, Bayer T, Gumpinger R. Ultrasound monitoring of kidney stone extracorporeal shockwave lithotripsy with an external transducer: does fatty tissue cause image distortions that affect stone comminution? J Endourol 2010;24:81-8. [Crossref] [PubMed]
- Karlin G, Marino C, Badlani G, et al. Benefits of an ultrasound-guided ESWL unit. Arch Esp Urol 1990;43:579-81. [PubMed]
- Smith HE, Bryant DA. Extracorporeal shockwave lithotripsy without radiation: Ultrasound localization is as effective as fluoroscopy. Urol Ann 2016;8:454-7. [Crossref] [PubMed]
- Schwartz LH, Richaud J, Buffat L, et al. Kidney mobility during respiration. Radiother Oncol 1994;32:84-6. [Crossref] [PubMed]
- Sorensen MD, Bailey MR, Shah AR, et al. Quantitative assessment of shockwave lithotripsy accuracy and the effect of respiratory motion. J Endourol 2012;26:1070-4. [Crossref] [PubMed]
- Bohris C, Stief CG, Strittmatter F. Improvement of SWL Efficacy: Reduction of the Respiration-Induced Kidney Motion by Using an Abdominal Compression Plate. J Endourol 2016;30:411-6. [Crossref] [PubMed]
- Schnabel MJ, Gierth M, Chaussy CG, et al. Incidence and risk factors of renal hematoma: a prospective study of 1,300 SWL treatments. Urolithiasis 2014;42:247-53. [Crossref] [PubMed]
- Adanur S, Ziypak T, Yapanoglu T, et al. What should be the ideal time interval between repeated extracorporeal shock wave lithotripsy sessions for renal stone treatment? Urolithiasis 2014;42:471. [Crossref] [PubMed]
- Chaussy CG, Tiselius HG. How can and should we optimize extracorporeal shockwave lithotripsy? Urolithiasis 2018;46:3-17. [Crossref] [PubMed]
- Gupta NP, Kumar A. Analgesia for pain control during extracorporeal shock wave lithotripsy: Current status. Indian J Urol 2008;24:155-8. [Crossref] [PubMed]
- Rasmussen YH, Dahl C. Analgesic requirements for ESWL treatment. A double blind study. Scand J Urol Nephrol 1994;28:225-7. [Crossref] [PubMed]


