
Should we spare neoadjuvant chemotherapy in low-risk muscle-invasive bladder cancer patients scheduled for radical cystectomy?
There is an ongoing debate whether every eligible patient suffering from muscle-invasive bladder cancer should undergo cisplatin-based neoadjuvant chemotherapy (NAC) before definitive treatment by radical cystectomy. Supporters of this approach refer to level 1 evidence given by high quality meta-analyses indicating a survival benefit of at least 5% improvement in 5-year overall survival by NAC (1-5) (Table 1). Given that most patients relapsing from bladder cancer will present with distant metastases rather than local recurrence (6,7), NAC is thought to tackle micrometastatic disease. Further, NAC results also in tumor-downstaging which has also been identified as a surrogate for improved survival (8-10). Their opponents refer to data indicating that a substantial number of patients will not benefit from NAC after all, which has also been confirmed by recent molecular analysis (11,12). In these patients, a worsening of the prognosis is at least debatable due to delay of radical cystectomy (9). Further, they point towards the option of adjuvant chemotherapy, which has also been shown to be an effective option, though data is not as strong as compared to NAC. In this context, several models have been proposed to identify patients likely to benefit from neoadjuvant chemotherapy and to exclude those who might not necessarily need to receive upfront neoadjuvant chemotherapy for an optimal treatment after all (8,13-15). One elaborated model is the M.D. Anderson Model (proposed by Culp et al. in 2014) (15). This model defines high-risk (HR) patients likely to benefit from NC by clinical features including the presence of hydronephrosis, cT3b–cT4a disease and/or histological evidence of lymphovascular invasion, micropapillary or neuroendocrine features as opposed to low-risk (LR) patients missing these features in which an immediate cystectomy should not impede oncological outcome.
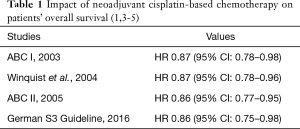
Full table
In this context, Lyon et al. validated the M.D. Anderson model with a special focus on the question whether in LR patients NAC could be reliably omitted (16). In their institutional database, they identified 1,025 low-risk and 906 high-risk patients; 104 low-risk and 196 high-risk patients having been treated by neoadjuvant chemotherapy. Of note, patients treated by NC were younger, had fewer comorbidities (Charlson Conorbodity index) and a worse clinical stage as compared to patients with immediate radical cystectomy. Further, more lymph-nodes were removed in patients who underwent neoadjuvant chemotherapy.
As expected, high-risk patients undergoing immediate radical cystectomy had both a worse cancer-specific as well as a worse overall survival when compared to low-risk patients undergoing immediate radical cystectomy (39% vs. 56%, P=0.001; 50% vs. 68%, P=0.001). Focussing on low-risk patients, a higher likelihood of cancer downstaging (pT0: OR 3.05, 95% CI: 1.89–4.93, P<0.001; < pT2: OR 2.53, 95% CI: 1.64–3.89, P<0.001) was observed in those, who underwent neoadjuvant chemotherapy. Though downstaging resulted in both improved OS and CSS in low-risk patients, administration of neoadjuvant chemotherapy did either improve CSS nor OS in low-risk patients.
When following those low-risk patients (n=921), who did not receive neoadjuvant therapy, the authors identified 293 patients would having pathological non-organ-confined (≥ pT3a, pN+) and who would have been eligible for cisplatin-based neoadjuvant chemotherapy. However, of these only 81 patients received adjuvant chemotherapy. The authors where either ineligible for cisplatin-based adjuvant chemotherapy following radical cystectomy (78/293) or where not submitted to undergo adjuvant therapy by other reasons (patient’s/health-care provider’s decision).
Following these results, is it reasonable to spare neoadjuvant chemotherapy in “low-risk” patients, as in those a survival benefit has not been observed? In line with the conclusion of Lyon and co-workers, I don’t think so.
First, also in low-risk patients, neoadjuvant chemotherapy will result in downstaging in a significant number of patients. In my opinion, this is the most relevant and reliable oncological outcome being evaluated by Lyon and co-workers. Tumor downstaging is an accepted surrogate marker for efficacy of neoadjuvant therapy and a strong predictor for long-term survival of patients (8-10). Both in randomised trials as well as in the meta-analysis, patients with pathological downstaging (especially those with complete pathological response) exhibited a far more favourable outcome. Comparable results regarding 5-year PFS and OS comparing low-risk patients with or without neoadjuvant chemotherapy in this study are rather due to selection bias than to a treatment effect. For example, lymphovascular invasion was more frequent and preoperative clinical tumor stage was generally higher in the neoadjuvant population of this study. Unfortunately, the authors did not detail patient characteristics depending on treatment modality and risk status.
Second, despite the risk of overtreatment, avoiding neoadjuvant chemotherapy will result in a significant number of patients who would likely benefit from a perioperative systemic therapy but do not receive it. One third (32%) of low-risk patients, who underwent immediate radical cystectomy, had non-organ confined disease which should definitively trigger adjuvant chemotherapy. However more than 70% of these patients did not receive adjuvant chemotherapy after all, either because of being ineligible to undergo adjuvant therapy following surgery or due to patient/provider choice.
Third, a selection based on clinicopathological features is rather inappropriate to reliably identify patients that are not likely to benefit from a multimodal treatment. Clinicopathological patient characteristics are prone to multiple biases, especially regarding preoperative tumor staging. Among patients with muscle invasive bladder cancer, clinical understaging of the primary tumor occurs in 50% of patients and overstaging in another 20% of patients (17). Accuracy of CT in predicting local tumor stage is only 50% with a significant inter-observer variability and limited ability to detect extravesical extension and lymph node metastases (18,19). While all-stage accuracy of MRI is slightly better than CT at 63%, MRI is thought to be able to distinguish between pT2 and pT3 with an accuracy of 89% (20). Reported accuracy of CT and MRI in lymphatic staging is similarly poor, with a sensitivity of only 30–50% despite a specificity of 70–100% (21-24). With current methodology, only 50% of patients with lymphonodal involvement will be identified pre-operatively. PET scanning may improve the diagnostic accuracy of lymphonodal staging, but it remains under investigation and has not been widely utilized (22,25,26).
In line with the thoughts of the authors, I do think that neoadjuvant chemotherapy should definitively be offered both in low and high-risk patients. In addition, and in my opinion quite more important, this study points out another relevant conclusion: our current clinical measures are not sufficient to adequately tailor treatment to the clinical need of our patients. Hopefully, a more decent work-up of the individual tumor biology of our patients will help us to decide who will benefit most from which therapy (precision oncology). For example, The Cancer Genome Atlas consortium proposed an according framework for therapeutic decisions in the setting of neoadjuvant chemotherapy based on tumor biology only recently (11). Prospective validation has not been performed yet but retrospective data strongly support this proposal already. Whether validation of this proposed model will be successful is yet unknown, but this is definitively a step in the right direction.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, D.K., AWMF). S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Harnblasenkarzinoms, Langversion 1.1. 2016. Available online: http://leitlinienprogramm-onkologie.de/Harnblasenkarzinom.92.0.html
- Leow JJ, Martin-Doyle W, Fay AP, et al. A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol 2014;66:529-41. [Crossref] [PubMed]
- Winquist E, Kirchner TS, Segal R, et al. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol 2004;171:561-9. [Crossref] [PubMed]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202-5; discussion 205-6. [Crossref] [PubMed]
- Advanced Bladder Cancer Meta-analysis C. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927-34. [Crossref] [PubMed]
- Hautmann RE, de Petriconi RC, Pfeiffer C, et al. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol 2012;61:1039-47. [Crossref] [PubMed]
- Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol 2003;21:690-6. [Crossref] [PubMed]
- Lavery HJ, Stensland KD, Niegisch G, et al. Pathological T0 following radical cystectomy with or without neoadjuvant chemotherapy: a useful surrogate. J Urol 2014;191:898-906. [Crossref] [PubMed]
- Bhindi B, Frank I, Mason RJ, et al. Oncologic Outcomes for Patients with Residual Cancer at Cystectomy Following Neoadjuvant Chemotherapy: A Pathologic Stage-matched Analysis. Eur Urol 2017;72:660-4. [Crossref] [PubMed]
- Peyton CC, Tang D, Reich RR, et al. Downstaging and Survival Outcomes Associated With Neoadjuvant Chemotherapy Regimens Among Patients Treated With Cystectomy for Muscle-Invasive Bladder Cancer. JAMA Oncol 2018;4:1535-42. [Crossref] [PubMed]
- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017;171:540-56.e25. [Crossref] [PubMed]
- Seiler R, Ashab HAD, Erho N, et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur Urol 2017;72:544-54. [Crossref] [PubMed]
- Niegisch G, Lorch A, Droller MJ, et al. Neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer: which patients benefit? Eur Urol 2013;64:355-7. [Crossref] [PubMed]
- Mitra AP, Skinner EC, Miranda G, et al. A precystectomy decision model to predict pathological upstaging and oncological outcomes in clinical stage T2 bladder cancer. BJU Int 2013;111:240-8. [Crossref] [PubMed]
- Culp SH, Dickstein RJ, Grossman HB, et al. Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. J Urol 2014;191:40-7. [Crossref] [PubMed]
- Lyon TD, Frank I, Sharma V, et al. A risk-stratified approach to neoadjuvant chemotherapy in muscle-invasive bladder cancer: implications for patients classified with low-risk disease. World J Urol 2018. World J Urol 2018. [Epub ahead of print]. [Crossref]
- Svatek RS, Shariat SF, Novara G, et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int 2011;107:898-904. [Crossref] [PubMed]
- Tritschler S, Mosler C, Tilki D, et al. Interobserver Variability Limits Exact Preoperative Staging by Computed Tomography in Bladder Cancer. Urology 2012;79:1317-21. [Crossref] [PubMed]
- Paik ML, Scolieri MJ, Brown SL, et al. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol 2000;163:1693-6. [Crossref] [PubMed]
- Rajesh A, Sokhi HK, Fung R, et al. Bladder cancer: evaluation of staging accuracy using dynamic MRI. Clin Radiol 2011;66:1140-5. [Crossref] [PubMed]
- Baltaci S, Resorlu B, Yagci C, et al. Computerized tomography for detecting perivesical infiltration and lymph node metastasis in invasive bladder carcinoma. Urol Int 2008;81:399-402. [Crossref] [PubMed]
- Lodde M, Lacombe L, Friede J, et al. Evaluation of fluorodeoxyglucose positron-emission tomography with computed tomography for staging of urothelial carcinoma. BJU Int 2010;106:658-63. [Crossref] [PubMed]
- Picchio M, Treiber U, Beer AJ, et al. Value of 11C-choline PET and contrast-enhanced CT for staging of bladder cancer: correlation with histopathologic findings. J Nucl Med 2006;47:938-44. [PubMed]
- Saokar A, Islam T, Jantsch M, et al. Detection of lymph nodes in pelvic malignancies with Computed Tomography and Magnetic Resonance Imaging. Clin Imaging 2010;34:361-6. [Crossref] [PubMed]
- Apolo AB, Riches J, Schoder H, et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol 2010;28:3973-8. [Crossref] [PubMed]
- Swinnen G, Maes A, Pottel H, et al. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol 2010;57:641-7. [Crossref] [PubMed]


