
Heavy metal imaging in fibrotic human kidney tissue using the synchrotron X-ray fluorescence microprobe
Introduction
The health problem
Unusually high rates of chronic kidney disease (CKD) of unknown or uncertain etiology (CKDu) have been noted in the North Central Province of Sri Lanka for more than a decade, with formal recognition of the problem given in a baseline study of the region‘s adult population in 2003 (1) and in many other studies since, some of which are referenced here (2-6) including in children of the region (7). In the North Central Province of Sri Lanka, male rice-paddy farmers, often smokers, appear to be at highest risk of developing CKDu. The disease is of international significance and interest and has been likened to other clusters of CKDu in nations such as those of the Balkan region, China, India, El Salvador and Guatemala (8-12). In Sri Lanka, fine needle renal biopsies of those affected revealed inflammatory lesions with gross tubulointerstitial and glomerular fibrosis, and it was estimated that up to 8% of males in the predominant occupation group were at risk of developing CKDu (4). The severity and uniqueness of CKDu in the North Central Province of Sri Lanka have made its investigation a priority to the Sri Lankan Ministry of Health. In addition, the World Health Organization has acknowledged this as an important issue in Sri Lanka, and by virtue of the potential applicability to other disease clusters, in other countries as well. CKD is associated with significant morbidity and progression to end-stage kidney disease (ESKD) and death. The socio-economic cost of CKD and ESKD in any society is important, but in developing countries where the health budget is severely limited per head of population, the ramifications of this situation are paramount.
Synchrotron X-ray fluorescence microscopy to study trace metals
Synchrotrons are particle accelerators. They can accelerate electrons, almost to the speed of light, and deflect the electrons through magnetic fields in a fixed closed-loop path. In doing this, they create extremely bright light that is then channelled through beamlines to be detected and measured for different research applications (13). The magnetic field bends the particle beam into its closed path, increasing during the accelerating process and “synchronizing” to increasing and specific particulate kinetic energy. Spatial correlation between elemental accumulation and specific pathology can be carried out using tissue samples and a specific synchrotron application, microscopy using the X-ray fluorescence microprobe (XFM) (14). Sub-micron spatial resolution of trace elements may be achieved, and sensitivity may be in the order of parts per million or better, for a large number of elements (15,16). One example of a correlation between elemental accumulation and pathology was reported for brain tissue from Alzheimer’s disease patients (16). Synchrotron XFM indicated that the tissue contained “hot spots” of accumulated metal ions, specifically copper and zinc, with a strong spatial correlation between these two ions and the β-amyloid plaques seen in Alzheimer’s disease, emphasizing at least a spatial association of metal ions with amyloid formation.
Specific application of synchrotron elemental XFM for CKDu in Sri Lanka
One of the hypotheses for development of CKDu in Sri Lanka was the involvement of heavy metal toxicity in the kidney (17). Elements of particular interest were those known for renal toxicity potential (18-21) and those with potential relevance for the Sri Lankan North Central Province region. There has been heavy use of agro-chemicals in the region over decades. In particular, cadmium (Cd) was investigated (17). This heavy metal is present in superphosphate fertilizer and so enters the food chain (22). Cd is an environmental pollutant, and is a known renal toxicant (23-25). Once absorbed, Cd preferentially accumulates in the kidney proximal tubular epithelial cells where it can cause renal dysfunction, epithelial cell death, and CKD. Experimental studies have demonstrated that kidney proximal tubular epithelial cells are particularly susceptible to Cd-induced autophagy and apoptosis (26,27).
This manuscript reports some pilot data from an investigation of localization of kidney fibrosis in CKDu with selected heavy metals including cadmium. At the time of the synchrotron analysis, fine-needle renal biopsies had been collected from 45 CKDu cases in Sri Lanka. The biopsies were fixed in 10% buffered formalin, dehydrated through a series of alcohols, cleared in xylene, and then embedded in paraffin wax for histological sectioning and pathology. After viewing hematoxylin and eosin stained sections microscopically, several biopsies with clear tubulointerstitial fibrosis were selected to progress to XFM elemental analyses. Normal kidney specimens accessed from kidney cancer nephrectomies at Princess Alexandra Hospital, Brisbane, Australia, similarly prepared for microscopy, were used as non-fibrotic kidney samples. Two synchrotrons were utilized: the Australian Synchrotron in Melbourne, Australia and the SPring8 Synchrotron in Hyogo Prefecture, Japan. The investigation planned to provide the first elemental analyses of renal tissue from the CKDu affected region in Sri Lanka with very high sensitivity and excellent spatial resolution.
Collection of pilot data
Institutional Human Research Ethics Committee (HREC) approvals were available from the University of Peradeniya, Sri Lanka (details in 4) and University of Queensland and Princess Alexandra Hospital Approval HREC (HREC/12/QPAH/125 Vesey: Utilisation of Fresh Human Kidney Tissue for Research into Kidney Disease). Informed consent was available from all patients. Males were selected for this study, to avoid effects of gender differences (28). Fourteen biopsies were selected from the formalin-fixed, paraffin-embedded renal fine needle aspiration biopsies from the Sri Lankan CKD patients. Normal fixed and paraffin-embedded kidney from renal cancer nephrectomies performed in Brisbane, Australia, confirmed with no kidney fibrosis and preserved and processed by the same method as the Sri Lankan biopsies (N=6), were also used. Mean ages were 47.9 years (CKD patients) and 42.3 years (non-CKD kidney) (P=0.28). Histochemistry using haematoxylin and eosin (for structure) and Masson’s trichrome (for collagen and fibrosis) was carried out on 4 µm sections for routine light microscopy. The sections for synchrotron analysis were prepared at 10 µm thickness onto silicon nitride windows (Silson, Blisworth, UK) or Kapton film windows, in a manner to minimize metal contamination of the specimens (QIMR Histology Facility, Brisbane, Australia). Foundation Investor time from The University of Queensland and Monash University was used for the analysis at the Australian Synchrotron. 2D elemental mapping of the sections was first carried out using the Australian Synchrotron’s scanning fluorescence XFM beamline with KB mirror microprobe and Maia 384 detector. The Maia detector offered fast readout and quantitative spectral analysis with separation of overlapping peaks. Beam flux was monitored with the ionization chamber. For the current investigation, selected samples were analyzed with 16 keV for Ti to Sr (Table 1).

Full table
Minimum detection limit of 100 ppb (parts per billion) was required for most elements. Scanning speed was up to 20 mm/s per sample, possible with the XYZ stage at the Australian Synchrotron. GeoPIXE software (trace element imaging and analysis) was used to determine elemental presence and intensity. The SPring-8 Synchrotron in Japan was then utilized to investigate Cd which could be analyzed only with some difficulty at this time at the Australian Synchrotron. Funding from the Centre for Chronic Disease, The University of Queensland, under the directorship of Prof Wendy Hoy, was used to carry out the SPring-8 analyses and we acknowledge the assistance of the assigned SPring-8 technician. Sections on silicon nitride holders were each subjected to high-energy X-ray fluorescence synchrotron radiation. Samples were stabilized on the XYZ stage. Bulk measurements of Cd were followed by micro measurements of selected areas approximately 1 µm2 (large scan). Bulk measurements were used to assist localization of morphologic structures. Micro measurements (microbeam) used a monochromated beam focused onto the approximately 0.5 µm2 area, and a K-B mirror was used with a focal length of 350 mm. For analysis of the SPring8 XFM output, a data file of elemental counts per pixel and a corresponding heat map were produced for each scan of the samples. The selected regions of the tissue section were approximate, and were based on group consensus after viewing the maps and images. Corresponding pixels in the data files were identified using coordinates. Within the section area, some pixels were set to “missing” if they were out of the section area or were over holes in the section, or, in microbeam scans only, were judged to be “hotspots” due to artefact or possible contamination. Data files were analysed in Stata statistical software (StataCorp. 2011. Release 12. College Station, TX: StataCorp LP).
Synchrotron analyses
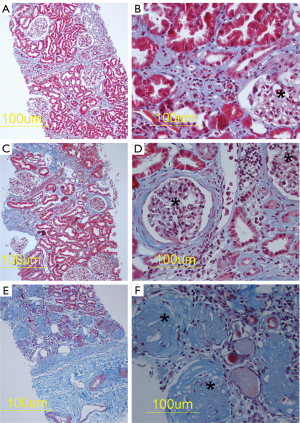
Figure 1 shows examples of histopathology of CKDu kidneys from Sri Lanka with clear fibrotic lesions. Masson’s trichrome histochemistry was used to demonstrate mild (Figure 1A,B), moderate (Figure 1C,D) and extensive renal fibrosis (Figure 1E,F) which is shown as blue in the Figure. In Figure 1E and 1F there is marked glomerulosclerosis. The healthy kidney tissue from Australian nephrectomy samples showed no abnormal fibrosis. The aim of our synchrotron analyses with the XFM was to match localisation of toxic metals with the fibrotic areas.
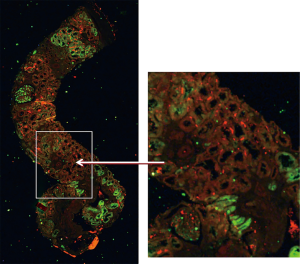
The XFM two-dimensional mapping experiments performed at the Australian Synchrotron combined horizontal continuous scanning with vertical step-mode scanning. Spatial resolution was 1–2 µm. Elements finally analyzed and mapped included metals or metalloids chromium, iron, copper, zinc, arsenic, strontium and lead, and the non-metals selenium and bromine. Elements with marked renal distribution were iron and zinc (Fe and Zn). Figure 2 demonstrates the structure of the sectioned renal biopsy visible with XFM, at low and high power, with renal distribution of Fe and Zn. Neither element localised with areas of renal fibrosis, which is the morphological evidence for CKD.
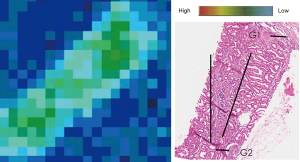
The results from the SPring-8 Synchrotron indicated the presence of Cd in the Sri Lankan CKD group, but surprisingly also in the normal healthy kidney samples from Australia. Geometric mean of Cd was significantly lower in the Sri Lankan than Australian kidney samples (52.48 and 134.90 ppm respectively, P=0.0008) (Table 2). Cd mapping did not indicate localized distribution within any kidney structures (Figure 3). Geometric means in the glomeruli or tubules were not significantly different between the two groups (P=0.77, P=0.81). However, the nature of the relationship of the pathological characteristics in question (tubulointerstitial fibrosis, glomerular sclerosis) and Cd was not able to be elucidated.

Full table
Perspective on pilot data
This was the first direct analysis of co-localisation of fine structural characteristics of CKD (for example, tubulointerstitial fibrosis, glomerulosclerosis) with elements that might cause pathologies or negatively modify metabolism in the Sri Lankan CKD biopsies. Elements with marked renal distribution were Fe and Zn. However, in comparison with normal kidney specimens of non-Sri Lankan origin that had been prepared similarly, there were not many marked differences in Fe and Zn distribution. Although evidence was lacking for a causative link between any particular element and the loss of functioning renal mass in the biopsies, the synchrotron elemental assay is proving to be an emerging tool for such analyses.
Cd was present at detectable levels in both the Sri Lankan and Australian specimens, but there was no co-localization with fibrotic kidney lesions. Higher tissue Cd in healthy subjects may relate to their normal iron stores (29). The fact that Cd was not localized to any specific structural characteristic in the Australian or Sri Lankan kidney specimens in our SPring8 investigation, but there was no fibrosis where Fe and Zn were localized, could indicate a protective antagonistic effect of the two metals toward Cd that was unavailable in fibrotic locations where Fe and Zn were not specifically localized. Richter and colleagues (30) note that Fe and Zn, amongst other elements, are antagonistic toward Cd toxicity under different circumstances.
Without standards and samples from Sri Lankans without CKD from the same locality in Sri Lanka, interpretation of any relationship between Cd and CKD structural characteristics was not possible. Other limitations of this pilot study were considerable and included potential introduction of contaminants; modification of sample constituents during biopsy preparation and preservation; small sample size; lack of morphometric descriptors other than age; and the unexplained, broad range of Cd concentrations seen in the Australian samples. We were intrigued by the similarity in Cd levels between the Australian non-CKD controls and the Sri Lankan CKD samples. There is no evidence that Cd pollution or dietary Cd intake is at all elevated in the population around Brisbane, Australia (31), from which the samples were sourced. One explanation may be that the tubular epithelial atrophy evident in the Sri Lankan CKD samples masks the increase in Cd that could have caused the atrophy. There is a precedent for lower kidney Cd in people from high Cd areas compared with areas with low Cd pollution. Uetani et al. (32) reported on Cd in the kidney cortex of patients with Itai-itai disease and suspected cases in Japan. These levels were much lower than in residents of Cd non-polluted areas. The lower kidney Cd from people in Cd-polluted areas may indicate that the kidneys have lost Cd due to advanced damage, autophagy and apoptosis of the renal tubular epithelium under the influence of Cd toxicity, as reported previously (26,27). XFM mapping was also insensitive to fluorine, for which there is a high degree of etiologic suspicion for CKDu (33).
Conclusions
There did not appear to be a correlation between localisation of heavy metals in tissue and kidney fibrosis. Future investigations will need to include non-CKD kidney biopsies from the same area in Sri Lanka as the CKD biopsies were sourced.
Acknowledgments
We acknowledge other participants in this project: Dr. David Paterson, and Dr. Daryl Howard, Australian Synchrotron, Melbourne, Australia; Dr. Tilak Abeysekera, Nephrology Unit, General Hospital, Kandy; Dr. TNC Athuraliya, Dept Pharmacology, Faculty of Medicine, Univ of Peradeniya, Sri Lanka; Dr. Thor Bostrom, School of Physics, QUT, Brisbane, Australia; Dr. Martin Cholewa, Monash Centre for Synchrotron Science, Monash Univ, Australian Synchrotron; Dr. Jamie Laird, CSIRO, Univ of Melbourne, Melbourne, Australia; Dr. Hideaki Ishii, Tokyo Medical University, Japan; Prof. Soisungwan Satarug, Centre for Kidney Disease Research and Centre for Health Services Research, Faculty of Medicine, Translational Research Institute, Univ Queensland, Brisbane, Australia; and technician Dr. Y Terada, Spring-8 Synchrotron, Hyogo Prefecture, Japan. This research was undertaken on the XFM Beamline of the Australian Synchrotron, part of ANSTO.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Athuraliya TNC, Abeysekera T, Amarasinghe PH. A baseline study on early renal disease in a selected community of the North-Central Province of Sri Lanka. April 2003 - July 2003, The Faculties of Medicine and Science, University of Peradeniya; Renal Unit, General Hospital Kandy; Provincial Ministry of Health, North-Central Province, Sri Lanka.
- Athuraliya TN, Abeysekera DT, Amerasinghe PH, et al. Prevalence of chronic kidney disease in two tertiary care hospitals: high proportion of cases with uncertain aetiology. Ceylon Med J 2009;54:23-5. [Crossref] [PubMed]
- Bandara JM, Senevirathna DM, Dasanayake DM, et al. Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia). Environ Geochem Health 2008;30:465-78. [Crossref] [PubMed]
- Nanayakkara S, Komiya T, Ratnatunga N, et al. Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ Health Prev Med 2012;17:213-21. [Crossref] [PubMed]
- Wijetunge S, Ratnatunga NV, Abeysekera DT, et al. Retrospective analysis of renal histology in asymptomatic patients with probable chronic kidney disease of unknown aetiology in Sri Lanka. Ceylon Med J 2013;58:142-7. [Crossref] [PubMed]
- Wijetunge S, Ratnatunga NV, Abeysekera TD, et al. Endemic chronic kidney disease of unknown etiology in Sri Lanka: Correlation of pathology with clinical stages. Indian J Nephrol 2015;25:274-80. [Crossref] [PubMed]
- Agampodi SB, Amarasinghe GS, Naotunna PGCR, et al. Early renal damage among children living in the region of highest burden of chronic kidney disease of unknown etiology (CKDu) in Sri Lanka. BMC Nephrol 2018;19:115. [Crossref] [PubMed]
- Hanjangsit K, Dimitrov PS, Zhang H, et al. Incidence and predictive factors of Balkan endemic nephropathy: a longitudinal study. Saudi J Kidney Dis Transpl 2014;25:343-52. [Crossref] [PubMed]
- Wesseling C, Crowe J, Hogstedt C, et al. Resolving the enigma of the mesoamerican nephropathy: a research workshop summary. First International Research Workshop on the Mesoamerican Nephropathy. Am J Kidney Dis 2014;63:396-404. [Crossref] [PubMed]
- Gifford FJ, Gifford RM, Eddleston M, et al. Endemic nephropathy around the World. Kidney Int Rep 2017;2:282-92. [Crossref] [PubMed]
- Grollman AP, Jelakovic B. Role of environmental toxins in Endemic (Balkan) Nephropathy. J Am Soc Nephrol 2007;18:2817-23. [Crossref] [PubMed]
- Lunyera J, Mohottige D, Von Isenburg M, et al. CKD of uncertain etiology: A Systematic Review. Clin J Am Soc Nephrol 2016;11:379-85. [Crossref] [PubMed]
- Hasnain SS. Synchrotron techniques for metalloproteins and human disease in post genome era. J Synchrotron Radiat 2004;11:7-11. [Crossref] [PubMed]
- Kopittke PM, Punshon T, Paterson DJ, et al. Synchrotron-based X-Ray fluorescence microscopy as a technique for imaging of elements in plants. Plant Physiol 2018;178:507-23. [Crossref] [PubMed]
- Wang P, Menzies NW, Lombi E, et al. Quantitative determination of metal and metalloid spatial distribution in hydrated and fresh roots of cowpea using synchrotron-based X-ray fluorescence microscopy. Sci Total Environ 2013;463-4:131-9. [Crossref] [PubMed]
- Miller LM, Wang Q, Telivala TP, et al. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer's disease. J Struct Biol 2006;155:30-7. [Crossref] [PubMed]
- Bandara JM, Wijewardena HV, Liyanege J, et al. Chronic renal failure in Sri Lanka caused by elevated dietary cadmium: Trojan horse of the green revolution. Toxicol Lett 2010;198:33-9. [Crossref] [PubMed]
- Rajapakse S, Shivanthan MC, Selvarajah M. Chronic kidney disease of unknown etiology in Sri Lanka. Int J Occup Environ Health 2016;22:259-64. [Crossref] [PubMed]
- Wanigasuriya K, Jayawardene I, Amarasiriwardena C, et al. Novel urinary biomarkers and their association with urinary heavy metals in chronic kidney disease of unknown aetiology in Sri Lanka: a pilot study. Saudi J Kidney Dis Transpl 2014;25:343-52.
- Shiroishi K, Kjellström T, Kubota K, et al. Urine analysis for detection of cadmium-induced renal changes, with special reference to beta2-microglobulin. A cooperative study between Japan and Sweden. Environ Res 1977;13:407-24. [Crossref] [PubMed]
- Satarug S, Nishijo M, Ujjin P, et al. Effects of chronic exposure to low-level cadmium on renal tubular function and CYP2A6-mediated coumarin metabolism in healthy human subjects. Toxicol Lett 2004;148:187-97. [Crossref] [PubMed]
- Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect 2004;112:1099-103. [Crossref] [PubMed]
- Satarug S, Vesey DA, Gobe GC. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem Toxicol 2017;106:430-45. [Crossref] [PubMed]
- Satarug S, Vesey DA, Gobe GC. Health risk assessment of dietary cadmium intake: Do current guidelines indicate how much is safe? Environ Health Perspect 2017;125:284-8. [Crossref] [PubMed]
- Satarug S, Garrett SH, Sens MA, et al. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 2010;118:182-90. [Crossref] [PubMed]
- Fujiwara Y, Lee JY, Tokumoto M, Satoh M. Cadmium renal toxicity via apoptotic pathways. Biol Pharm Bull 2012;35:1892-7. [Crossref] [PubMed]
- Lee WK, Probst S, Santoyo-Sánchez MP, et al. Initial autophagic protection switches to disruption of autophagic flux by lysosomal instability during cadmium stress accrual in renal NRK-52E cells. Arch Toxicol 2017;91:3225-45. [Crossref] [PubMed]
- Nishijo M, Satarug S, Honda R, et al. The gender differences in health effects of environmental cadmium exposure and potential mechanisms. Mol Cell Biochem 2004;255:87-92. [Crossref] [PubMed]
- Apinan R, Satarug S, Ruengweerayut R, et al. The influence of iron stores on cadmium body burden in a Thai population. Environ Geochem Health 2010;32:237-42. [Crossref] [PubMed]
- Richter P, Faroon O, Pappas RS. Cadmium and cadmium/zinc ratios and tobacco-related morbidities. Int J Environ Res Public Health 2017;14:1154. [Crossref] [PubMed]
- Satarug S, Baker JR, Urbenjapol S, et al. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 2003;137:65-83. [Crossref] [PubMed]
- Uetani M, Kobayashi E, Suwazono Y, et al. Tissue cadmium (Cd) concentrations of people living in a Cd polluted area, Japan. Biometals 2006;19:521-5. [Crossref] [PubMed]
- Wickramarathna S, Balasooriya S, Diyabalanage S, et al. Tracing environmental aetiological factors of chronic kidney diseases in the dry zone of Sri Lanka-A hydrogeochemical and isotope approach. J Trace Elem Med Biol 2017;44:298-306. [Crossref] [PubMed]


