
Machine preservation of donor kidneys in transplantation
Introduction
For decades, kidneys have been preserved in static cold storage (SCS) as a means of reducing cellular metabolism and improving viability during storage prior to reperfusion during organ transplantation. However, prolonged anoxia and cold injury lead to preservation-related damage, which is associated with delayed graft function, inferior graft function and reduced graft survival (1-4). Machine perfusion has been designed to reduce organ damage and recently, contemporary hypothermic pulsatile pumps have been shown to improve the clinical outcomes in patients receiving donor graft of inferior quality. Ultimately these machines may also increase the donor pool for patients who are on the waiting list by allowing assessment and utilization of grafts that would have previously been deemed unusable.
Hypothermic machine perfusion (HMP) vs. cold storage
Donor organs are a scarce commodity. Efforts to increase the donor pool prompted more critical evaluation and use of expanded criteria donor (ECD) organs. The resulting increasing in available organs came with a cost. ECD organs show increase rates of ischemia reperfusion injury, early graft dysfunction, and reduced long-term graft survival. This prompted investigators to seek methods to reduce renal injury in improve outcomes in this population. Compared to SCS, HMP has been shown to outperform SCS in the donation after cardiac death (DCD) and ECD graft pool (5).
Although HMP technology has been utilized for years (6), few studies compared HMP vs. SCS in a meaningful controlled manner until the past decade. A Canadian single centre study was performed to compare performance of HMP vs. SCS using a paired kidney analysis. In addition to showing improved post-operative doppler resistive indices in the HMP group, estimated GFR were compared and found to be significantly improve with HMP. This effect was more pronounced in the DCD subgroup (62.9 vs. 41.75, P=0.004) (7).
A large retrospective study of 54,136 patients undergoing kidney transplant compared SCS with HMP. There was a significant benefit seen in the incidence of delayed graft function in the donation after brain death (DBD) (OR 0.59, 0.56–0.63) as well as the DCD patient groups (OR 0.70, 0.61–0.80) with HMP. The effect was greatest in DCD grafts with prolonged cold ischemic times utilizing HMP compared with SCS (8). Furthermore, UNOS data utilizing a cohort of 26,586 patients demonstrated that delayed graft function rates were significantly reduced with machine perfusion (OR 0.64, P<0.001). The authors calculated that 12.5 kidneys would need to be pumped to avoid one episode of delayed graft function. However, HMP did not seem to improve graft survival at 1, 3 and 5 years nor did patient length of stay change when compared with SCS. A second analysis using paired kidneys, involving 2,290 donors confirmed that HMP leads to improved DGF (OR 0.61, P<0.001) without benefit to graft survival and length of stay (9).
A European randomized controlled trial compared HMP with SCS perfusion in 82 consecutive DCD donors. Delayed graft function was significantly improved with HMP (OR 0.43, P=0.025). Additionally, DGF lasted fewer days and recipients of HMP required fewer dialysis treatments. Graft survival at 1 year was not different between the two groups (93.9% vs. 95.1%) (10). By contrast, a UK trial of similar design showed no improvement in delayed graft function rates with HMP (58% vs. 56%). Delays in initiation of machine perfusion or variation in warm ischemic times were believed to account for these differences between studies (11).
Beyond the randomized controlled trials, a meta-analysis (12) was carried out to evaluate if HMP could improve outcomes in ECD kidneys compared to SCS. A total of seven studies were included (13-17) including three randomized trials (18,19). The results showed that delayed graft function was significantly improved with HMP (OR 0.59, P<0.0001), in addition to superior 1-year graft survival (OR 1.12, P=0.005). Similarly, a meta-analysis evaluated the effects of HMP on DCD kidneys with regards to delayed graft function and 1-year graft survival. The results showed an overall beneficial effect with HMP on delayed graft function with an OR of 0.64 (0.43–0.95, I2=63%) (20). However, there was not a demonstrable benefit with regards to 1-year graft survival. Overall, HMP seems to have a small but limited benefit in 1 year graft survival rates in the less than ideal graft groups. However, DGF rates are universally improved in these subgroups.
Perfusion parameters as predictors of outcome
Machine perfusion provides an opportunity to evaluate grafts prior to transplantation. Prediction of graft outcomes based on pump perfusion characteristics has been evaluated. A single center study with 155 DCD kidney transplants employed calculation of perfusion flow index (PFI) at hour three of perfusion in order to predict outcomes. This parameter is calculated by dividing the flow rate by resistance and expressed as mL/min/mmHg per 100 g of kidney. A cut-off PFI of 1.08 showed significant differences in graft survival with a sensitivity of 71% and specificity of 38% (21).
Additionally, flow and resistance parameters have been used to predict delayed graft function. A retrospective cohort of 190 DCD donor grafts utilized pump parameters obtained within the first two hours of pump perfusion. A high intra-renal resistance at 2 hours predicated delayed graft function, and high initial resistance predicted graft survival at 1-year. Despite limitations in accuracy, these studies show that HMP indices at 2–3 hours may help to select organs for discard in certain situations (22).
Normothermic machine perfusion (NMP)
Originally, NMP had been utilized as a method to briefly recondition kidneys during periods of cold storage. Following prolonged 20 hr HMP, Hosgood et al. utilized a 2-hour period of NMP in a porcine DCD model to recondition the kidneys. This NMP system utilized a pediatric cardiopulmonary by-pass machine to provide oxygenated blood to the grafts (23). Although few functional differences were seen between NMP and control groups, lipid peroxidation was lower in the group with 2 hours of NMP following 20-hour HMP. However, improvements in graft function were not demonstrated when NMP times were increased to 4 hours (24). In the same year, the same group performed the first kidney transplant in human using a cold storage followed by brief NMP approach. The recipient had excellent outcomes with no DGF and good renal function at 3 months (25).
Following the success of the pilot case, Nicholson presented the first clinical series of ex vivo NMP in 2013 utilizing the same cardiopulmonary bypass pump system. After procurement, the grafts were stored with SCS until induction of anesthesia at the time of transplantation. The kidney would be perfused using the NMP device using a supplemented plasma-free red cell suspension for approximately 60 minutes. Following perfusion, the kidneys were place back in SCS until ready for implantation. The results showed a dramatic reduction in DGF from 36.2% to 5.6% (P=0.014). Although the study was underpowered to evaluated patient or graft survival, this pivotal study showed that a short period of NMP after prolonged SCS could reduce DGF and provide a period of assessment of marginal grafts (26).
In order to evaluate marginal kidneys, Hosgood et al. developed an assessment score using discarded kidneys and 60-minute perfusion with NMP. Macroscopic appearance, urine output and mean renal blood flow were evaluated and the grafts given a score of 1–5 (27). After evaluation of 36 kidneys and utilizing grafts with scores under 3, the authors concluded that discarded kidneys that score between 1–3 can be safely transplanted (28). The first salvage of a previously discarded kidney was performed using this technique in 2016 (29).
The initial success of brief periods of NMP prompted the evaluation of longer periods of normothermia vs. cold storage times by a Canadian group (30). This group utilized a porcine DCD porcine model. Briefly, a 30-minute warm ischemia time was induced followed by 8 hours of NMP or SCS and auto transplantation was performed. Compared with SCS, kidneys undergoing NMP showed favorable pump characteristics, decreasing lactate levels and minimal evidence of renal graft injury along with superior early renal function. A comparison of brief NMP vs. prolonged NMP was performed using this model as well. Contrary to the results of the Hosgood experiments, increasing NMP: SCS ratio improved graft function and reduced levels of renal injury markers (31). Furthermore, significantly prolonged periods of NMP at 16 and 8 hours showed improved outcomes compared to 60 minutes of NMP (32). The benefits of prolonged NMP must be balanced with the increased effort required to survey and maintain the NMP device over long periods of time.
Subnormothermic machine perfusion
With increasing evidence that therapeutic whole body hypothermia between 34–35 °C may be beneficial to renal preservation in deceased kidney donors (33), the ideal temperature for ex vivo perfusion has become controversial. The first group to evaluate room temperature subnormothermic ex vivo perfusion showed that their 20 °C system provided renal preservation at least equivalent to 5 °C hypothermia in a porcine model (34).
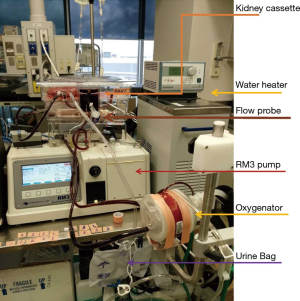
Adams et al. recently demonstrated that brief 1 hr NMP was functionally superior to 32 °C MP when either condition was utilized as an adjunct with 24-hour SCS (35). By contrast, utilizing a modified clinical RM3 (Waters Instrument Inc., Rochester, MN, USA) pump in a DCD porcine model (Figure 1), our group demonstrated that while 4-hour NMP provide superior results compared with SCS, the levels of kidney damage measured by urinary KIM-1, NGAL along with histologic assessment of apoptosis (TUNEL) and acute tubular necrosis were further reduced with 4-hour subnormothermic (22 °C) blood-based ex vivo machine perfusion compared to 5 and 37 °C perfusion (36). These later experiments did not involve prolonged cold storage conditions in addition to the utilization of normothermic or subnormothermic machine perfusion.
Perfusion solutions and additives
The perfusion solution is a vital component in the machine perfusion environment. This solution may carry free radical scavengers, nutrients, vasodilators, cell membrane stabilizers and other metabolic precursors. Importantly, there is no standard perfusion solution and various groups have described center specific solutions with a unique blend of these types of additives (5). Recently, we have compared HMP with HTK vs. subnormothermic perfusion with bovine hemoglobin-based oxygen carrier in our modified RM3 pump-circuit system. Using our standard DCD porcine model of donation, we showed that bovine hemoglobin (Hemopure, Cambridge, MA, USA) provided superior degrees of organ protection with regards to graft injury (ATN and TUNEL) as well as renal damage markers (KIM1) after 4-hour machine perfusion compared to hypothermic perfusion (Figure 2).

In addition to reduction of ischemia reperfusion injury as well as damage as a result of prolonged cold storage conditions, subnormothermic and NMP provide platforms in which additives can be utilized to reduce vascular spasm, inflammation and cell death in a physiologically relevant manner. Small endogenous molecules like carbon monoxide (CO) and their carrying molecules have shown promises in CO’s role in vasoregulation and amelioration of apoptosis, and inflammation (37,38). A new generation of carrying molecules, Carbon monoxide releasing molecule 401 (CORM-401), was evaluated in a NMP study at our center. Importantly, these molecules function ideally at physiologic temperatures and not at hypothermic temperatures in which metabolism will cease. Kidneys perfused with CORM-401 showed significantly less apoptosis on histology and molecular evaluation. Markers of acute tubular ischemia (KIM-1 and NGAL) were also significantly reduced in the CORM-401 treated kidney. Renal blood flow rates, urine output and creatinine clearance were also significantly improved in the treated kidneys (39).
Similarly, Hydrogen Sulfide (H2S) has anti-inflammatory, anti-apoptotic and vasodilatory properties at physiologic concentrations and temperatures. This molecule has been shown to reduce the effects of ischemic injury on renal function (40-42) and was evaluated in a transplant models. When donor kidneys were perfused with University of Wisconsin solution with H2S, graft survival and function was significantly improved in the H2S treatment group. Early ATN scores and KIM-1 histologic expression was significantly improved with H2S treatment. This demonstrated that H2S protects renal grafts against the effects of cold induced ischemic reperfusion injury (43,44). Subsequent studies evaluating H2S in our subnormothermic machine perfusion model have also shown beneficial effect compared with machine perfusion alone. Together, these studies show that machine perfusion techniques benefit from small endogenous molecules that protect the graft against ischemia reperfusion injury.
Summary
In summary, the development of machine perfusion has provided a vehicle in which the kidney can be protected under physiologic conditions in order to attenuate ischemia reperfusion injury. Additionally, the kidney can further be protected by administration of a host of agents that target ischemia reperfusion injury pathways. This may be extended to the future assessment of high concentrations of antivirals, antibiotics, and genetic modifiers in order to protect both the kidney and the recipient from disease in order to limit systemic toxicity, increase organ utilization and extend graft survival.
Acknowledgements
This work was supported by grants from the Canadian Institute of Health Research-Canadian National Transplantation Research Program (CIHR-CNTRP) to PP Luke, Physician Services Incorporated (PP Luke, RN Bhattacharjee), Academic Medical Organization of Southwestern Ontario (AMOSO) innovation fund (PP Luke, RN Bhattacharjee) and Internal Research Fund (RN Bhattacharjee, PP Luke).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kwiatkowski A, Wszola M, Perkowska-Ptasinska A, et al. Influence of preservation method on histopathological lesions of kidney allografts. Ann Transplant 2009;14:10-3. [PubMed]
- Dragun D, Hoff U, Park JK, et al. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int 2001;60:1173-81. [Crossref] [PubMed]
- Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant 2011;11:2279-96. [Crossref] [PubMed]
- Mikhalski D, Wissing KM, Ghisdal L, et al. Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation 2008;85:S3-9. [Crossref] [PubMed]
- Henry SD, Guarrera JV. Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev (Orlando) 2012;26:163-75. [Crossref] [PubMed]
- De Deken J, Kocabayoglu P, Moers C. Hypothermic machine perfusion in kidney transplantation. Curr Opin Organ Transplant 2016;21:294-300. [Crossref] [PubMed]
- Dion MS, McGregor TB, McAlister VC, et al. Hypothermic machine perfusion improves Doppler ultrasonography resistive indices and long-term allograft function after renal transplantation: a single-centre analysis. BJU Int 2015;116:932-7. [Crossref] [PubMed]
- Lodhi SA, Lamb KE, Uddin I, et al. Pulsatile pump decreases risk of delayed graft function in kidneys donated after cardiac death. Am J Transplant 2012;12:2774-80. [Crossref] [PubMed]
- Cannon RM, Brock GN, Garrison RN, et al. To pump or not to pump: a comparison of machine perfusion vs cold storage for deceased donor kidney transplantation. J Am Coll Surg 2013;216:625-33; discussion 633-4. [Crossref] [PubMed]
- Jochmans I, Moers C, Smits JM, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg 2010;252:756-64. [Crossref] [PubMed]
- Watson CJ, Wells AC, Roberts RJ, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant 2010;10:1991-9. [Crossref] [PubMed]
- Jiao B, Liu S, Liu H, et al. Hypothermic machine perfusion reduces delayed graft function and improves one-year graft survival of kidneys from expanded criteria donors: a meta-analysis. PLoS One 2013;8:e81826. [Crossref] [PubMed]
- Bon D, Chatauret N, Giraud S, et al. New strategies to optimize kidney recovery and preservation in transplantation. Nat Rev Nephrol 2012;8:339-47. [Crossref] [PubMed]
- Buchanan PM, Lentine KL, Burroughs TE, et al. Association of lower costs of pulsatile machine perfusion in renal transplantation from expanded criteria donors. Am J Transplant 2008;8:2391-401. [Crossref] [PubMed]
- Stratta RJ, Moore PS, Farney AC, et al. Influence of pulsatile perfusion preservation on outcomes in kidney transplantation from expanded criteria donors. J Am Coll Surg 2007;204:873-82; discussion 882-4. [Crossref] [PubMed]
- Matsuoka L, Shah T, Aswad S, et al. Pulsatile perfusion reduces the incidence of delayed graft function in expanded criteria donor kidney transplantation. Am J Transplant 2006;6:1473-8. [Crossref] [PubMed]
- Sedigh A, Tufveson G, Backman L, et al. Initial experience with hypothermic machine perfusion of kidneys from deceased donors in the Uppsala region in Sweden. Transplant Proc 2013;45:1168-71. [Crossref] [PubMed]
- Treckmann J, Moers C, Smits JM, et al. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl Int 2011;24:548-54. [Crossref] [PubMed]
- Gallinat A, Moers C, Treckmann J, et al. Machine perfusion versus cold storage for the preservation of kidneys from donors >/= 65 years allocated in the Eurotransplant Senior Programme. Nephrol Dial Transplant 2012;27:4458-63. [Crossref] [PubMed]
- Bathini V, McGregor T, McAlister VC, et al. Renal perfusion pump vs cold storage for donation after cardiac death kidneys: a systematic review. J Urol 2013;189:2214-20. [Crossref] [PubMed]
- Sevinc M, Stamp S, Ling J, et al. Ex Vivo Perfusion Characteristics of Donation After Cardiac Death Kidneys Predict Long-Term Graft Survival. Transplant Proc 2016;48:3251-60. [Crossref] [PubMed]
- Patel SK, Pankewycz OG, Nader ND, et al. Prognostic utility of hypothermic machine perfusion in deceased donor renal transplantation. Transplant Proc 2012;44:2207-12. [Crossref] [PubMed]
- Hosgood SA, Barlow AD, Yates PJ, et al. A pilot study assessing the feasibility of a short period of normothermic preservation in an experimental model of non heart beating donor kidneys. J Surg Res 2011;171:283-90. [Crossref] [PubMed]
- Hosgood SA, Mohamed IH, Bagul A, et al. Hypothermic machine perfusion after static cold storage does not improve the preservation condition in an experimental porcine kidney model. Br J Surg 2011;98:943-50. [Crossref] [PubMed]
- Hosgood SA, Nicholson ML. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation 2011;92:735-8. [Crossref] [PubMed]
- Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant 2013;13:1246-52. [Crossref] [PubMed]
- Hosgood SA, Barlow AD, Dormer J, et al. The use of ex-vivo normothermic perfusion for the resuscitation and assessment of human kidneys discarded because of inadequate in situ perfusion. J Transl Med 2015;13:329. [Crossref] [PubMed]
- Hosgood SA, Barlow AD, Hunter JP, et al. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br J Surg 2015;102:1433-40. [Crossref] [PubMed]
- Hosgood SA, Saeb-Parsy K, Hamed MO, et al. Successful Transplantation of Human Kidneys Deemed Untransplantable but Resuscitated by Ex Vivo Normothermic Machine Perfusion. Am J Transplant 2016;16:3282-5. [Crossref] [PubMed]
- Kaths JM, Echeverri J, Goldaracena N, et al. Eight-Hour Continuous Normothermic Ex Vivo Kidney Perfusion Is a Safe Preservation Technique for Kidney Transplantation: A New Opportunity for the Storage, Assessment, and Repair of Kidney Grafts. Transplantation 2016;100:1862-70. [Crossref] [PubMed]
- Kaths JM, Echeverri J, Chun YM, et al. Continuous Normothermic Ex Vivo Kidney Perfusion Improves Graft Function in Donation After Circulatory Death Pig Kidney Transplantation. Transplantation 2017;101:754-63. [Crossref] [PubMed]
- Kaths JM, Cen JY, Chun YM, et al. Continuous Normothermic Ex Vivo Kidney Perfusion Is Superior to Brief Normothermic Perfusion Following Static Cold Storage in Donation After Circulatory Death Pig Kidney Transplantation. Am J Transplant 2017;17:957-69. [Crossref] [PubMed]
- Niemann CU, Feiner J, Swain S, et al. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N Engl J Med 2015;373:405-14. [Crossref] [PubMed]
- Hoyer DP, Gallinat A, Swoboda S, et al. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model. Transpl Int 2014;27:1097-106. [Crossref] [PubMed]
- Adams TD, Patel M, Hosgood SA, et al. Lowering Perfusate Temperature From 37 degrees C to 32 degrees C Diminishes Function in a Porcine Model of Ex Vivo Kidney Perfusion. Transplant Direct 2017;3:e140. [Crossref] [PubMed]
- Bhattacharjee M, Richard-Mohamed M, Ruthirakanthan A. Comparison of temperatures for optimal preservation of donor kidneys during oxygenated pulsatile perfusion. Am J Transplant 2017.17.
- Caumartin Y, Stephen J, Deng JP, et al. Carbon monoxide-releasing molecules protect against ischemia-reperfusion injury during kidney transplantation. Kidney Int 2011;79:1080-9. [Crossref] [PubMed]
- Sener A, Tran KC, Deng JP, et al. Carbon monoxide releasing molecules inhibit cell death resulting from renal transplantation related stress. J Urol 2013;190:772-8. [Crossref] [PubMed]
- Bhattacharjee RN, Richard-Mohamed M, Sun Q, et al. CORM-401 reduces ischemia reperfusion injury in an ex vivo renal porcine model of the donation after cardiac death. Transplantation 2018;102:1066-74. [Crossref] [PubMed]
- Hosgood SA, Nicholson ML. Hydrogen sulphide ameliorates ischaemia-reperfusion injury in an experimental model of non-heart-beating donor kidney transplantation. Br J Surg 2010;97:202-9. [Crossref] [PubMed]
- Hunter JP, Hosgood SA, Patel M, et al. Effects of hydrogen sulphide in an experimental model of renal ischaemia-reperfusion injury. Br J Surg 2012;99:1665-71. [Crossref] [PubMed]
- Snijder PM, van den Berg E, Whiteman M, et al. Emerging role of gasotransmitters in renal transplantation. Am J Transplant 2013;13:3067-75. [Crossref] [PubMed]
- Lobb I, Mok A, Lan Z, et al. Supplemental hydrogen sulphide protects transplant kidney function and prolongs recipient survival after prolonged cold ischaemia-reperfusion injury by mitigating renal graft apoptosis and inflammation. BJU Int 2012;110:E1187-95. [Crossref] [PubMed]
- Lobb I, Davison M, Carter D, et al. Hydrogen Sulfide Treatment Mitigates Renal Allograft Ischemia-Reperfusion Injury during Cold Storage and Improves Early Transplant Kidney Function and Survival Following Allogeneic Renal Transplantation. J Urol 2015;194:1806-15. [Crossref] [PubMed]


