
Current protocols and outcomes of ABO-incompatible kidney transplantation based on a single-center experience
Introduction
Although the brain death law was established in Japan, the proportion of kidney transplant donations has not increased over the previous two decades, and there is a serious shortage of deceased donor kidneys (1-3). ABO-incompatible living kidney transplantation (ABO-ILKT) has been used in our institution since 1989 to widen donor utilization for living donor kidney transplantation (3), and we have already reported successful short-term and long-term (≤10 years) outcomes of ABO-ILKT (3-8). During the past two decades, desensitization protocols for ABO-ILKT have been changed according to newly developed immunosuppressive agents (3,9) and to minimize pretransplant conditioning in order to achieve better graft survival and fewer adverse events (5-8). Herein, we review the history, therapeutic strategy, pathology, and future directions of ABO-ILKT.
History of ABO-incompatible kidney transplantation
According to Landsteiner’s theory (10), anti-blood group A and B antibodies (isohemagglutinins) recognize A and B blood group antigens, respectively. In the 1960s, there were concerns that blood group incompatibility between the donor and recipient might cause graft injury. The exact mechanism of graft injury in ABO-incompatible allografts was uncertain, and the blood group antibodies were suspected to cause agglutination of erythrocytes in the graft blood vessels of the donor.
After investigating the early functional outcomes of 30 renal allografts, Porter et al. reported that two of three grafts that failed to function were in cases of ABO-ILKT. Histological evaluation revealed that interstitial hemorrhage and sludging of erythrocytes occurred in these grafts (11). The results of ABO-ILKT in 12 recipients were analyzed in 1969; three of the kidneys were considered to have been immediately rejected, and six were rejected within 3 months. The pathology of those rejected kidneys revealed arterial thrombosis and parenchymal necrosis, and blood group compatibility between donors and recipients was considered as a prerequisite for successful kidney transplantation (12).
In the early 1970s, A2 donors were considered for transplantation in blood group O recipients. As the expression of A2 antigens was reportedly much weaker and less than that in erythrocytes of A1 individuals (13), a clinical trial with transplantation from A2 renal grafts to O recipients was conducted in 1974. Although 8 of 20 transplants were lost within 1-month post-transplantation, 12 grafts functioned in the long term with the recipients receiving standard immunosuppression without additional treatment (14). This clinical trial ended in 1988, and the longest survival time was 22 years. Subsequently, the concept of A2 grafting to O recipients was adopted by other groups. Seven of 9 grafts with a low titer of anti-blood group antibodies less than 32 survived more than 1 year, whereas three of four grafts with a high titer of more than 64 were lost (15). On the basis of this study, A2 incompatible kidney transplantation could be a good and safe option for O recipients. Nelson et al. (16) reported their 10-year experience with 50 A2 incompatible transplantations in 1998, and their outcome was as follows: the 1-month and 2-year graft survival rates were 94% and 94%, respectively.
Slapak et al. reported the first A1 incompatible kidney transplantation with selective immunoadsorption or plasmapheresis pretreatment in 1984 (17), and surprisingly, the overall 1-year graft survival rate was 87% (13/16). Alexandre et al. also reported that A1 incompatible kidney transplantation was performed successfully with splenectomy and plasmapheresis (18).
In 1989, we performed the first case of ABO-ILKT in our institution, and so far, we have experienced more than 500 cases of ABO-ILKT. In recent years, our procedure has been performed worldwide (19) and in Japan (9).
Evolution of therapeutic strategies for successful ABO-incompatible kidney transplantation in Japan and other countries
In the last decade, many transplant teams started ABO-ILKT in Japan and other countries (20-25). Several protocols allow successful ABO-ILKT; however, no single method has emerged as being superior to the others. We attempted to establish whether changes in the immunosuppressive regimen result in better outcomes (7,26). Cyclosporine (CSA), azathioprine (AZA), methylprednisolone (MP), antilymphocyte globulin, and deoxyspergualin were used as standard immunosuppressive agents between 1989 and 1997 for ABO-ILKT recipients in our institution. During this period, graft survival of ABO-ILKT was significantly worse than that in ABO-compatible living kidney transplantation (ABO-CLKT) recipients because of early graft loss caused by acute antibody-mediated rejection (ABMR) (3). We started using tacrolimus (TAC) as a standard immunosuppressant in 1998, and graft survival in ABO-ILKT recipients improved greatly in the TAC era compared to the CSA era (4). In 2001, we started using mycophenolate mofetil (MMF) or ABO-ILKT because MMF has been available in Japan since 2000. MMF was administered from the day of transplantation, and the short-term graft survival rate improved in the MMF era compared with the CSA/AZA-based or TAC/AZA-based immunosuppressive era. Since MMF takes about 7–10 days to obtain a therapeutic concentration of MPA to inhibit T-cell and B-cell proliferation (27,28), we began using three immunosuppressive drugs 7 days before transplantation with basiliximab (8). The suppression of B-cells by TAC, MMF, and MP seems to be the most crucial factor for antibody suppression and eventual suppression of acute ABMR in ABO-ILKT recipients (29). The combination of TAC, MMF, and MP significantly reduced the incidence rate of acute rejection and provided excellent survival of grafts in ABO-ILKT recipients (7). Thereafter, we have performed transplantations successfully across the blood group barrier with plasmapheresis and splenectomy performed at the same time of transplantation in cases of B-cell depletion (3,30,31).
Instead of splenectomy, a Swedish team began using rituximab, a chimeric anti-CD20 antibody, to suppress anti-blood group antibody production (32). The authors reported excellent short-term outcomes for ABO-ILKT (20). Johns Hopkins (33) and Mayo Clinic (22) teams also reported that excellent short-term outcomes for ABO-ILKT were achieved under various induction protocols that included rituximab. We have now accepted that there is a growing consensus that splenectomy is no longer necessary for desensitization in ABO-ILKT (34).
We started using rituximab as a substitute for splenectomy in ABO-ILKT since 2005 (8), as reported by the Swedish team (20). We found that rituximab could deplete B cell lineage in a dose-dependent manner (35). Marked reduction of B cells in the white pulp of the spleen was observed in all recipients, compared with the controls, after the administration of rituximab. We also compared the clinical outcomes between two different dosages of rituximab, 200 and 500 mg/body (36). This study showed similar excellent graft function, the same incidence of acute rejection, and a low incidence of adverse events in the 200 and 500 mg/body groups (37).
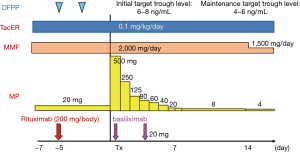
Figure 1 shows our current preconditioning regimen, which comprises 1–3 sessions of plasmapheresis, a low-dose rituximab injection, and three standard immunosuppressive drugs (basiliximab induction, intravenous immunoglobulin, or prophylactic post-transplant plasmapheresis) (27,28,36). The current graft survival rate (2005–2013) is more than 90% in ABO-ILKT at the 9-year follow-up, which was comparable to the rate for ABO-CLKT (26). Figure 2 provides the updated results for our protocol of 142 ABO-incompatible and 335 ABO-compatible kidney transplant recipients. The 10-year cumulative patient survival rate and graft survival rate (non-censored for death) in the same period were 99.1% and 95.9%, and 90.2% and 88.3% for the ABO-ILKT and ABO-CLKT groups, respectively; the differences were not significant.
The most recent analyses of 10 years of data from 95 ABO-incompatible kidney transplant recipients from a Germen group confirmed an excellent median graft survival rate of 94% with no significant difference from that reported in 245 ABO-compatible kidney transplant recipients. These findings are supported by up-to-date data from the Collaborative Transplant Study, which showed the 3-year outcomes for 1,420 ABO-incompatible kidney transplantations from 101 centers (19).
In the United States, 738 ABO-incompatible kidney transplantations were analyzed from 280 transplant centers between 1995 and 2009. The graft survival rates were 94.1%, 89.6%, 82.6%, and 72.9% at 1, 3, 5, and 10 years of follow-up, respectively (38). Overall graft survival rates have improved recently. In John Hopkins Medical Institutions, between 1999 and 2007, 28 of 60 patients did not receive rituximab or splenectomy, as the protocol was changed during the follow-up period. ABMR and the graft loss rate did not increase on the basis of these data (24).
Apheresis therapy for ABO-incompatible kidney transplantation
Plasma exchange and plasmapheresis can remove antibodies from the recipient’s blood stream in a non-specific manner. Double filtration plasmapheresis uses two hollow fiber filters with varying pore sizes. During extracorporeal circulation, the first filter filtrates and isolates the plasma component from whole blood. Then, the patient receives the cell-rich blood. The second plasma filter has smaller pores than the first. The albumin-spared plasma is mixed with cell-rich blood in an extracorporeal line, and the remainder of the filtrate is discarded (39). Using plasma exchange, Alexandre et al. (18) removed isohemagglutinins from 26 recipients, allowing transplantation of ABO-incompatible renal grafts. Two grafts were lost because of acute rejection at 7 and 19 days after transplantation. Overall, the graft survival rate approached that seen in ABO-compatible transplants. Rebound of antibody titers after plasma exchange is commonly observed. Rebound of depleted antibody levels can be inhibited by the administration of cytotoxic agents, such as cyclophosphamide. However, even with immunosuppressive medications, rebound of antibody levels may be challenging to control. In our institute, dual filtration plasma exchange (DFPP) or plasma exchange was performed only during pretransplantation, and the number of DFPP or plasma exchanges was determined according to the anti-blood antibody titer. Patients with low antibody titers were treated by DFPP just one time before transplantation.
To deplete anti-blood antibodies, Bannett et al. (40) used synthetic carbohydrate antigens immobilized on solid-phase columns before ABO-incompatible transplantation. In the first series, the blood of six patients was immunoabsorbed with matrix-bearing synthetic blood antigens. In five patients, hemagglutination titers were reduced by only two-fold, and plasmapheresis was added to these patients’ regimens. Five of the patients had good allografts function in the follow-up period. The kidney transplanted into the patient in whom absorption had failed was rejected within just 7 days.
Pathology in ABO-incompatible transplanted kidneys
C4d staining is not always a useful marker for diagnosing ABMR because C4d is commonly observed in most ABO-incompatible cases without any sign of ABMR. In a retrospective study, we investigated the histological findings and C4d staining in 89 protocol biopsies obtained from 48 ABO-incompatible transplant recipients and 250 controls (133 ABO-CLKT) (41). C4d deposition in the peritubular capillaries in ABO-ILKT was found in 94% of cases, with diffuse staining in 66%. On the basis of our results, we concluded that C4d deposition did not correlate with ABMR, and it may not have any diagnostic or therapeutic relevance. We reported that acute ABMR has a strong impact on long-term outcomes, and that preoperative donor-specific anti-HLA antibodies (DSA) have a more substantial association with poor graft outcomes than anti-blood group antibody titers, including in ABO-ILKT (42). Pathological findings in ABO-incompatible transplanted kidneys showed the negative impact of microvascular inflammation (MVI) on the graft survival rate and graft function (43). One hundred forty-eight ABO-incompatible kidney transplant recipients with no preformed nor de novo DSA were divided into two groups according to the degree of MVI. The rate of graft survival was significantly lower in the severe MVI group than in its counterpart, and graft function was significantly inferior at 1 and 10 years after transplantation in the severe MVI group compared with its counterpart.
Preoperative anti-blood group antibodies titers and postoperative rebound titers
In 2000, we identified the relationship between preoperative anti-blood group antibody titers and the graft survival rate (44,45). We concluded that titers for the anti-blood group antibody of more than 64 times the baseline level are a risk factor for graft loss. Yet, all patients evaluated in these studies were assessed before the TAC and MMF era. In 2005, after TAC and MMF were introduced, we found no correlation between the preoperative and postoperative anti-blood group antibody titers and rate of graft survival (46).
Some institutions recommend the use of postoperative therapeutic plasma exchange for preventing ABMR. Additionally, Tobian et al. (47) from Johns Hopkins reported that the incidence of ABMR was significantly higher in recipients with high post-transplant titers for the anti-blood group antibody of more than 64. Ishida et al. also evaluated the relevance of postoperative anti-ABO titer rebound and acute rejection in ABO-incompatible kidney transplantation in our institute (48). Postoperative anti-blood group antibody rebound was not correlated with the incidence of acute rejection; thus, it was concluded that no treatment is required for rebound of anti-blood group antibodies.
Graft accommodation and the effect of blood group antigens on the graft endothelium
The mechanism responsible for graft accommodation is unknown, but many possible mechanisms have been hypothesized. Platt and colleagues showed that graft accommodation might be explained by the following mechanisms: (I) an alteration in the functional properties of anti-donor antibodies; (II) variation in the antigens; and (III) the organ developed a resistance to humoral injury. Investigations in rodents focused on the likelihood that the organ may acquire resistance to humoral injury by the expression of anti-apoptotic genes (49). However, studies of tissues from ABO-incompatible kidney transplant recipients have suggested that other mechanisms, e.g., the expression of complement regulatory proteins, may also be important in graft accommodation (50). Accommodated grafts would lack signs of injury notwithstanding the presence of anti-donor antibody and/or complement components.
A previous study demonstrated that blood group antigenicity would decrease over the long term after ABO-incompatible kidney transplantation (51). In this prior study, we examined changes in the expression of blood group antigens on the grafts over the long term after ABO-ILKT with A-antigen or B-antigen incompatibility (A to B, and B to A). As a result, the expression of the recipient’s blood group antigens on the endothelium of the graft decreased, and it was considered as one of the mechanisms underlying the graft accommodation that had occurred over the long term after ABO-incompatible kidney transplantation.
Moreover, we previously investigated the presence of chimerism in renal allografts of ABO-incompatible kidney transplantation recipients by immunohistochemical detection of blood group A and B antigens in a biopsy sample to assess the association among chimerism, clinical course, and histopathological changes (52). Twelve of 49 patients exhibited endothelium chimerism. Among them, seven had acute and chronic active ABMR, and two had severe calcineurin inhibitor toxicity. The graft survival rate was significantly lower in patients with chimerism than in those without chimerism.
Infection in ABO-incompatible kidney transplantation
There are conflicting results about infectious complications after ABO-ILKT in previous literature. Habicht et al. reported that the frequency of viral infection, such as cytomegalovirus, Herpes simplex virus, Varicella zoster virus, and polyoma virus infection, was higher in ABO-incompatible recipients than in ABO-compatible recipients (53). In a study of Johns Hopkins group, the frequency of BK virus nephropathy among ABO-incompatible patients was about three times higher than that among HLA-incompatible patients (17.7% versus 5.9%) (54). In our institute, the rate of CMV infection was higher in ABO-incompatible kidney transplant patients before 2004. However, after 2005, minimizing the use of immunosuppressive agents and pretransplant conditioning, ABO-ILKT was shown to reduce the risk of CMV infection from 30.1% to 21.5%, with no difference compared with ABO-CLKT (27). Lentine et al. reported that ABO-ILKT was associated with a higher risk of pneumonia, urinary tract infection, and wound infections (55). The patients in our institute had a relatively low risk of such infections (27). The low prevalence of bacterial infections could be accredited to the use of preemptive antibiotics and pneumococcal vaccines. These results may explain how we could change our immunosuppressive regimen and desensitization protocol without increasing the risk of infectious adverse events.
Future directions for ABO-incompatible kidney transplantation
In 2011, we published data concerning differences in the rates of graft survival and chronic ABMR between ABO-compatible and ABO-incompatible kidney transplantation with induction treatment (56). Between 2001 and 2004, recipients in the ABO-incompatible group (ABOi-SPX, n=45) received pretransplant DFPP and splenectomy on the transplant day. From 2005 to 2009, low-dose rituximab was administered instead of performing splenectomy (ABOi-RIT, n=57). We used 83 cases of ABO-compatible kidney transplantation as a control between 2001 and 2007 (ABOc). The chronic ABMR rates 6 months postoperatively were 8.8%, 3.5%, and 28.9%, and the de novo DSA positive rates were 2.2%, 1.7%, and 18.1% in the ABOi-SPX, ABOi-RIT, and ABOc groups, respectively. The ABOc group showed the highest rate of chronic ABMR and de novo DSA. B-cell depletion protocols, e.g., splenectomy or the administration of rituximab, seem to reduce chronic ABMR after kidney transplantation. Subramanian et al. (57) also reported that at 1 year, the glomerular filtration rate in ABO-ILKT patients was significantly better than that in ABO-CLKT patients. De-novo DSA and the response to single antigen beads are reduced or absent in ABO-ILKT, which is proposed to lead to less acute rejection and better long-term function thereafter. Some researchers have challenged the long-lasting suppression of B-cell lineage due to rituximab, but the observation period was too short to draw this conclusion (58).
West et al. reported that several immunologic factors contribute to ABH tolerance after ABO-incompatible heart transplantation in young children, including paucity of immunoglobulin M + CD27 B cells at the time of transplantation and persistent scarcity post-transplantation. Their study provided confirmation for the memory nature of this type of cell and for the important role of these cells in T-independent B-cell responses. Determining the amount of different types of memory B cells at the time of ABO-incompatible transplantation may be useful in decision-making regarding the suitability of the procedure. Interventions that target memory B cells may allow the extension of ABO-incompatible tolerance and superior post-transplantation survival of small children compared with older children and adults (59-61).
Conclusions
Currently, graft survival and safety in ABO-ILKT are comparable to those in ABO-CLKT in our institute. ABO-ILKT is acceptable for treating patients with end-stage renal disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Takahashi K, Saito K, Takahara S, et al. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant 2004;4:1089-96. [Crossref] [PubMed]
- Takahashi K, Saito K. Present status of ABO-incompatible kidney transplantation in Japan. Xenotransplantation 2006;13:118-22. [Crossref] [PubMed]
- Tanabe K, Takahashi K, Sonda K, et al. Long-term results of ABO-incompatible living kidney transplantation: a single-center experience. Transplantation 1998;65:224-8. [Crossref] [PubMed]
- Tanabe K, Tokumoto T, Ishida H, et al. ABO-incompatible renal transplantation at Tokyo Women's Medical University. Clin Transpl 2003.175-81. [PubMed]
- Tanabe K, Tokumoto T, Ishida H, et al. Excellent outcome of ABO-incompatible living kidney transplantation under pretransplantation immunosuppression with tacrolimus, mycophenolate mofetil, and steroid. Transplant Proc 2004;36:2175-7. [Crossref] [PubMed]
- Tanabe K. Japanese experience of ABO-incompatible living kidney transplantation. Transplantation 2007;84:S4-7. [Crossref] [PubMed]
- Ishida H, Miyamoto N, Shirakawa H, et al. Evaluation of immunosuppressive regimens in ABO-incompatible living kidney transplantation--single center analysis. Am J Transplant 2007;7:825-31. [Crossref] [PubMed]
- Tanabe K, Ishida H, Shimizu T, et al. Evaluation of two different preconditioning regimens for ABO-incompatible living kidney donor transplantation. A comparison of splenectomy vs. rituximab-treated non-splenectomy preconditioning regimens. Contrib Nephrol 2009;162:61-74. [Crossref] [PubMed]
- Takahashi K, Saito K. ABO-incompatible kidney transplantation. Transplant Rev (Orlando) 2013;27:1-8. [Crossref] [PubMed]
- Owen R. Karl Landsteiner and the first human marker locus. Genetics 2000;155:995-8. [PubMed]
- Porter KA. Morphological Aspects of Renal Homograft Rejection. Br Med Bull 1965;21:171-5. [Crossref] [PubMed]
- Wilbrandt R, Tung KS, Deodhar SD, et al. ABO blood group incompatibility in human renal homotransplantation. Am J Clin Pathol 1969;51:15-23. [Crossref] [PubMed]
- Economidou J, Hughes-Jones NC, Gardner B. Quantitative measurements concerning A and B antigen sites. Vox Sang 1967;12:321-8. [Crossref] [PubMed]
- Rydberg L, Breimer ME, Samuelsson BE, et al. Blood group ABO-incompatible (A2 to O) kidney transplantation in human subjects: a clinical, serologic, and biochemical approach. Transplant Proc 1987;19:4528-37. [PubMed]
- Welsh KI, van Dam M, Koffman CG, et al. Transplantation of blood group A2 kidneys into O or B recipients: the effect of pretransplant anti-A titers on graft survival. Transplant Proc 1987;19:4565-7. [PubMed]
- Nelson PW, Landreneau MD, Luger AM, et al. Ten-year experience in transplantation of A2 kidneys into B and O recipients. Transplantation 1998;65:256-60. [Crossref] [PubMed]
- Slapak M, Naik RB, Lee HA. Renal transplant in a patient with major donor-recipient blood group incompatibility: reversal of acute rejection by the use of modified plasmapheresis. Transplantation 1981;31:4-7. [Crossref] [PubMed]
- Alexandre GP, Squifflet JP, De Bruyere M, et al. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc 1987;19:4538-42. [PubMed]
- Opelz G, Morath C, Susal C, et al. Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: results from 101 centers. Transplantation 2015;99:400-4. [Crossref] [PubMed]
- Tyden G, Kumlien G, Genberg H, et al. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant 2005;5:145-8. [Crossref] [PubMed]
- Segev DL, Simpkins CE, Warren DS, et al. ABO incompatible high-titer renal transplantation without splenectomy or anti-CD20 treatment. Am J Transplant 2005;5:2570-5. [Crossref] [PubMed]
- Gloor JM, Cosio FG, Rea DJ, et al. Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant 2006;6:1841-7. [Crossref] [PubMed]
- Tyden G, Donauer J, Wadstrom J, et al. Implementation of a Protocol for ABO-incompatible kidney transplantation--a three-center experience with 60 consecutive transplantations. Transplantation 2007;83:1153-5. [PubMed]
- Montgomery RA, Locke JE, King KE, et al. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation 2009;87:1246-55. [Crossref] [PubMed]
- Genberg H, Kumlien G, Wennberg L, et al. Isoagglutinin adsorption in ABO-incompatible transplantation. Transfus Apher Sci 2010;43:231-5. [Crossref] [PubMed]
- Okumi M, Toki D, Nozaki T, et al. ABO-Incompatible Living Kidney Transplants: Evolution of Outcomes and Immunosuppressive Management. Am J Transplant 2016;16:886-96. [Crossref] [PubMed]
- van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation 1999;68:261-6. [Crossref] [PubMed]
- Gonin JM. Maintenance immunosuppression: new agents and persistent dilemmas. Adv Ren Replace Ther 2000;7:95-116. [Crossref] [PubMed]
- Ishida H, Tanabe K, Furusawa M, et al. Mycophenolate mofetil suppresses the production of anti-blood type anitbodies after renal transplantation across the abo blood barrier: ELISA to detect humoral activity. Transplantation 2002;74:1187-9. [Crossref] [PubMed]
- Takahashi K, Yagisawa T, Sonda K, et al. ABO-incompatible kidney transplantation in a single-center trial. Transplant Proc 1993;25:271-3. [PubMed]
- Toma H, Tanabe K, Tokumoto T. Long-term outcome of ABO-incompatible renal transplantation. Urol Clin North Am 2001;28:769-80. [Crossref] [PubMed]
- Tyden G, Kumlien G, Fehrman I. Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation 2003;76:730-1. [Crossref] [PubMed]
- Sonnenday CJ, Warren DS, Cooper M, et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant 2004;4:1315-22. [Crossref] [PubMed]
- Gloor JM, Stegall MD. ABO incompatible kidney transplantation. Curr Opin Nephrol Hypertens 2007;16:529-34. [Crossref] [PubMed]
- Toki D, Ishida H, Horita S, et al. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients. Transpl Int 2009;22:447-54. [Crossref] [PubMed]
- Shirakawa H, Ishida H, Shimizu T, et al. The low dose of rituximab in ABO-incompatible kidney transplantation without a splenectomy: a single-center experience. Clin Transplant 2011;25:878-84. [Crossref] [PubMed]
- Takagi T, Ishida H, Shirakawa H, et al. Evaluation of low-dose rituximab induction therapy in living related kidney transplantation. Transplantation 2010;89:1466-70. [Crossref] [PubMed]
- Montgomery JR, Berger JC, Warren DS, et al. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation 2012;93:603-9. [PubMed]
- Tanabe K. Double-filtration plasmapheresis. Transplantation 2007;84:S30-2. [Crossref] [PubMed]
- Bannett AD, McAlack RF, Raja R, et al. Experiences with known ABO-mismatched renal transplants. Transplant Proc 1987;19:4543-6. [PubMed]
- Setoguchi K, Ishida H, Shimmura H, et al. Analysis of renal transplant protocol biopsies in ABO-incompatible kidney transplantation. Am J Transplant 2008;8:86-94. [PubMed]
- Toki D, Ishida H, Setoguchi K, et al. Acute antibody-mediated rejection in living ABO-incompatible kidney transplantation: long-term impact and risk factors. Am J Transplant 2009;9:567-77. [Crossref] [PubMed]
- Ishihara H, Ishida H, Unagami K, et al. Evaluation of Microvascular Inflammation in ABO-Incompatible Kidney Transplantation. Transplantation 2017;101:1423-32. [Crossref] [PubMed]
- Ishida H, Koyama I, Sawada T, et al. Anti-AB titer changes in patients with ABO incompatibility after living related kidney transplantations: survey of 101 cases to determine whether splenectomies are necessary for successful transplantation. Transplantation 2000;70:681-5. [Crossref] [PubMed]
- Shimmura H, Tanabe K, Ishikawa N, et al. Role of anti-A/B antibody titers in results of ABO-incompatible kidney transplantation. Transplantation 2000;70:1331-5. [Crossref] [PubMed]
- Shimmura H, Tanabe K, Ishida H, et al. Lack of correlation between results of ABO-incompatible living kidney transplantation and anti-ABO blood type antibody titers under our current immunosuppression. Transplantation 2005;80:985-8. [Crossref] [PubMed]
- Tobian AA, Shirey RS, Montgomery RA, et al. ABO antibody titer and risk of antibody-mediated rejection in ABO-incompatible renal transplantation. Am J Transplant 2010;10:1247-53. [Crossref] [PubMed]
- Ishida H, Kondo T, Shimizu T, et al. Postoperative rebound of antiblood type antibodies and antibody-mediated rejection after ABO-incompatible living-related kidney transplantation. Transpl Int 2015;28:286-96. [Crossref] [PubMed]
- Bach FH, Ferran C, Hechenleitner P, et al. Accommodation of vascularized xenografts: expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nat Med 1997;3:196-204. [Crossref] [PubMed]
- Platt JL. C4d and the fate of organ allografts. J Am Soc Nephrol 2002;13:2417-9. [Crossref] [PubMed]
- Tanabe T, Ishida H, Horita S, et al. Decrease of blood type antigenicity over the long-term after ABO-incompatible kidney transplantation. Transpl Immunol 2011;25:1-6. [Crossref] [PubMed]
- Tanabe T, Ishida H, Horita S, et al. Endothelial chimerism after ABO-incompatible kidney transplantation. Transplantation 2012;93:709-16. [Crossref] [PubMed]
- Habicht A, Broker V, Blume C, et al. Increase of infectious complications in ABO-incompatible kidney transplant recipients--a single centre experience. Nephrol Dial Transplant 2011;26:4124-31. [Crossref] [PubMed]
- Sharif A, Alachkar N, Bagnasco S, et al. Incidence and outcomes of BK virus allograft nephropathy among ABO- and HLA-incompatible kidney transplant recipients. Clin J Am Soc Nephrol 2012;7:1320-7. [Crossref] [PubMed]
- Lentine KL, Axelrod D, Klein C, et al. Early clinical complications after ABO-incompatible live-donor kidney transplantation: a national study of Medicare-insured recipients. Transplantation 2014;98:54-65. [Crossref] [PubMed]
- Kohei N, Hirai T, Omoto K, et al. Chronic antibody-mediated rejection is reduced by targeting B-cell immunity during an introductory period. Am J Transplant 2012;12:469-76. [Crossref] [PubMed]
- Subramanian V, Gunasekaran M, Gaut JP, et al. ABO incompatible renal transplants and decreased likelihood for developing immune responses to HLA and kidney self-antigens. Hum Immunol 2016;77:76-83. [Crossref] [PubMed]
- Ashimine S, Watarai Y, Yamamoto T, et al. Neither pre-transplant rituximab nor splenectomy affects de novo HLA antibody production after renal transplantation. Kidney Int 2014;85:425-30. [Crossref] [PubMed]
- Urschel S, Ryan LA. Development of B-cell memory in early childhood and the impact on antigen-specific tolerance after heart transplantation. J Heart Lung Transplant 2016;35:491-9. [Crossref] [PubMed]
- Jeyakanthan M, Meloncelli PJ, Zou L, et al. ABH-Glycan Microarray Characterizes ABO Subtype Antibodies: Fine Specificity of Immune Tolerance After ABO-Incompatible Transplantation. Am J Transplant 2016;16:1548-58. [Crossref] [PubMed]
- West LJ. Neonatal tolerance: applicability to solid organ transplantation. Curr Opin Organ Transplant 2016;21:66-73. [Crossref] [PubMed]



