
Targeting the adipose tissue to fight prostate cancer
Hypertrophic fat deposits, as seen in obesity, have shown to be a critical factor in the development of multiple types of malignancies, including prostate cancer (1). This link is not yet completely understood and, considering the epidemic proportions of obesity worldwide, an increased prevalence of cancer can be foreseen in the immediate future.
In order to curb the morbidity associated with obesity, and the increased incidence of different types of cancer worldwide, it is imperative to dissect the molecular pathophysiological links behind this interplay.
In recent years, it has become clear that the white adipose tissue (WAT) of overweight individuals displays a distinct histology and a secretome that differs from the one seen with lean and healthy fat deposits. Adipocytes, the fat containing cells constituting the majority of the WAT, are differentiated cells derived from a mesenchymal progenitor known as adipose stromal cell (ASC). In obese, but not in healthy individuals, there is an increased number of resident and circulating ASCs which have shown to secrete adipokines that favour both malignant growth and metastatic dissemination (2,3).
Recently, Su et al. (4) have shown how ASCs from the WAT surrounding the prostate are not only promoting epithelial-mesenchymal transition in prostate cancer cells, but they are also conferring chemoresistance in vitro and in vivo in mice models.
The epithelial-to-mesenchymal transition (EMT) is a physiological process normally undertaken by different cells during embryonic development for the creation of different tissues and organs. In adults, the EMT is a process normally seen just in wound repair, tissue regeneration and organ fibrosis.
A transition to a more mesenchymal phenotype is known to be associated with an increased plasticity, proliferation, chemoresistance, and invasiveness, supporting the dissemination into distant secondary foci of metastasis. A new approach has emerged in recent years trying to tackle different malignancies targeting this process; a recent success comes from a study that exploited this increased cellular plasticity of transitioning cells, committing breast cancer cells to differentiate into adipocytes in a mouse model (5).
Despite these promising results, the molecular players behind the EMT in the context of different malignancies are still largely unknown.
The intriguing study of Su et al. builds upon this concept. The authors showed how two different prostate cancer cell lines, PC3 and LNCaP, when co-cultured with primary ASCs, transition to a more mesenchymal phenotype displaying an increased motility in a scratch wound assay. Further to this, a Boyden chamber trans-well migration assay confirmed the increased invasiveness which, in particular, was shown to be dependent on the soluble adipokines secreted by the ASCs (Figure 1).
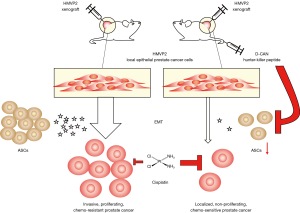
Additionally, the ASCs-induced mesenchymal phenotype of Prostate cancer cells conferred protection from docetaxel, cabazitaxel, and cisplatin treatment. LNCaP cells co-cultured and exposed to the adipokines of ASCs are protected from the cisplatin-induced increase in reactive oxygen species (ROS), possibly indicating the involvement of the intracellular antioxidant machinery or, alternatively, a mere secondary effect of the increased mesenchymal phenotype as reported for breast cancer (6).
These in vitro findings were then corroborated in an obese mouse model bearing a xenograft of the murine prostate cancer cell line HMVP2. Targeting and disrupting ASCs hampered the tumour growth and invasiveness.
To target ASCs, they used the hunter-killer peptide D-CAN, which was developed in a previous study by Daquinag et al. (7). Treatment of the mice models with D-CAN 1.6 mg/kg was indeed capable to not only reduce the xenografts growth, but also to increase their chemosensitivity to Cisplatin 0.5 mg/kg, a dose normally insufficient to elicit any effect.
Surprisingly, the specific ablation of ASCs was not affecting the overall WAT mass and was effective especially in combination with chemotherapy, in animals fed either a chow or high-fat diet.
The authors corroborated this novel ASC targeting approach in immune-deficient mice bearing a human xenograft of LNCaP cells, confirming the inter-species translatability of these findings. This new model proposed by the authors, as summarized in Figure 1, is in agreement with a previous study that unveiled the pro-metastatic capabilities of ASCs in a murine xenograft model of breast cancer (3).
The study of Su et al. provides a new strategy to tackle the observed increased cancer risk in obese patients.
Targeting ASCs with the D-CAN peptide appears to have no cytotoxic effects, at least in the short term, displaying encouraging synergistic activity with Cisplatin in both obese and lean animals.
The authors conclude showing how adipocytes differentiated from periprostatic ASCs are still maintaining their chemo-protectant properties, indicating how their secretome is at least partially maintained, although the identity of the molecular species behind this capability, are yet unknown.
They suggest how the chemokine CXCL12, highly expressed by ASCs, is likely responsible for its EMT inducing properties, a known pharmacological target for multiple cancers (8).
Nonetheless, in order to reconcile our overall understanding of the adipose tissue, it is important to reconsider the role of individual cellular components of this complex endocrine organ.
Beyond ASCs, as shown in this and previous studies (2,9), the more differentiated adipocytes retain their pro-tumoral properties, and an obesogenic microenvironment is known to suppress immune players (10). Other authors have also unveiled some important anti-tumoral targets such as p62 (11). Indeed, this is an important factor to weigh in with this strategy because targeting ASCs might not be sufficient if the differentiated Adipocytes retain their chemo-protectant and possibly pro-metastatic properties.
Our understanding of the biology of ASCs in lean and obese patients is still incomplete and needs to be dissected in the pathological and healthy WAT for a more tailored treatment of prostate cancer.
Addressing these questions will give us a rationale to target specific pro-metastatic adipokines and ultimately curb cancer mortality, given that malignant morbidity is primarily due to chemoresistance and invasive dissemination.
Acknowledgments
Funding: Funding was provided by Prostate Cancer UK (grant PG13-029 and PG12-23), Diabetes Australia and Avner Pancreatic Cancer Foundation. SP and MF acknowledge the School of Pharmacy and Biomedical Sciences (CHIRI), Faculty of Health Sciences of Curtin University, for the staff, and infrastructure support. SP also recognizes the support of the Curtin University Health Sciences Faculty International Research Scholarships.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol 2019;15:139-54. [Crossref] [PubMed]
- Zhang T, Tseng C, Zhang Y, et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat Commun 2016;7:11674. [Crossref] [PubMed]
- Rowan BG, Gimble JM, Sheng M, et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One 2014;9:e89595. [Crossref] [PubMed]
- Su F, Ahn S, Saha A, et al. Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance. Oncogene 2019;38:1979-88. [Crossref] [PubMed]
- Ishay-Ronen D, Diepenbruck M, Kalathur RK, et al. Gain Fat-Lose Metastasis: Converting Invasive Breast Cancer Cells into Adipocytes Inhibits Cancer Metastasis. Cancer Cell 2019;35:17-32.e6. [Crossref] [PubMed]
- Dong C, Yuan T, Wu Y, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 2013;23:316-31. [Crossref] [PubMed]
- Daquinag AC, Dadbin A, Snyder B, et al. Non-glycanated Decorin Is a Drug Target on Human Adipose Stromal Cells. Mol Ther Oncolytics 2017;6:1-9. [Crossref] [PubMed]
- Zhou Y, Cao HB, Li WJ, et al. The CXCL12 (SDF-1)/CXCR4 chemokine axis: Oncogenic properties, molecular targeting, and synthetic and natural product CXCR4 inhibitors for cancer therapy. Chin J Nat Med 2018;16:801-10. [PubMed]
- Jung Y, Kim JK, Shiozawa Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun 2013;4:1795. [Crossref] [PubMed]
- Michelet X, Dyck L, Hogan A, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 2018;19:1330-40. [Crossref] [PubMed]
- Huang J, Duran A, Reina-Campos M, et al. Adipocyte p62/SQSTM1 Suppresses Tumorigenesis through Opposite Regulations of Metabolism in Adipose Tissue and Tumor. Cancer Cell 2018;33:770-84.e6. [Crossref] [PubMed]


