
Can serum 17-hydroxyprogesterone and insulin-like factor 3 be used as a marker for evaluation of intratesticular testosterone?
Introduction
Currently there exists no reliable and readily available serum biomarker for measurement of intratesticular testosterone (ITT) other than invasive testicular aspiration. The identification of a serum biomarker may allow further elucidation into the causes of male infertility, monitoring patients on therapy for male infertility (e.g., clomiphene citrate), as well as the development and monitoring of an effective male contraceptive. The adult testis serves two primary functions: the production of spermatozoa and the secretion of testosterone (T) (1). T is known to play a vital role in the initiation and maintenance of spermatogenesis (2,3). Healthy testicular function is dependent upon hormonal control of spermatogenesis through the intratesticular activity of the pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (2). LH stimulates Leydig cells of the testicular interstitium to synthesize and secrete T (2). FSH activity is also critical for normal spermatogenesis through its action on Sertoli cells, though its specific role is unclear (1,4,5). Withdrawal of gonadotropin stimulation, namely LH, results in a decrease in ITT, which then decreases sperm production (5). It is believed that ITT directly stimulates spermatogenesis in men, however, the amount of ITT required to initiate spermatogenesis has yet to be elucidated (5). Additionally, it is important to note that ITT concentrations in normal men are approximately 100 times greater than T in serum, which suggests that serum T may not be an accurate marker for ITT (3). A 2004 study examined the relationship between intratesticular fluid and serum T and found that ITT levels comparable with serum T were not sufficient to support normal spermatogenesis (4). A reliable biomarker for ITT can help elucidate the currently obscure intratesticular hormonal milieu.
While ITT is thought to serve as a central role to spermatogenesis, the relationship between LH, ITT and spermatogenesis is far from understood (6). This gap in literature is most likely attributed to the difficulty in assessing the intratesticular microenvironment, especially with repeated measurements of individuals during hormonal manipulation (6). Existing options for evaluating ITT include repeat surgical testicular biopsies, which risk testicular injury. A safe and accurate method is bedside testicular aspiration with local anesthesia. Although minimally invasive, there are risks associated with the procedure including pain, bleeding, infection and injury to the testicle (7). However, due to the risks and overall difficulty involved, ITT concentrations are rarely evaluated in men (3,4,6). In clinical practice, men are treated with human chorionic gonadotropin, recombinant FSH and clomiphene citrate to increase ITT to either initiate spermatogenesis (i.e., hypogonadotropic hypogonadism, anabolic steroid abuse), maintain spermatogenesis (i.e., preserve spermatogenesis in men on testosterone replacement) or to improve spermatogenesis (i.e., idiopathic oligospermia). Identification of a serum biomarker has the potential to identify men deficient of ITT who may respond to therapy and to serve as a means of monitoring these patients for a therapeutic response (7-9).
Control of the intratesticular hormonal environment
The hypothalamus and the pituitary gland are the primary regulators of the intratesticular microenvironment through negative feedback of T (2,10). Exogenous T administration at both physiologic and supraphysiologic doses can dramatically suppress gonadotropin release (11,12). This can lead to a decrease in sperm in 65% of men to levels sufficient for contraception (13,14). Treatment with the LH receptor agonist, human chorionic gonadotropin (hCG), stimulates Leydig cells in a similar fashion as LH (6). The resultant increase in ITT stimulates spermatogenesis in men with hypogonadotropic hypogonadism from hypothalamic and/or pituitary failure and in men with experimentally induced gonadotropin deficiency (2). Therefore, treatment with hCG, usually in conjunction with recombinant FSH can be useful to treat infertility as it leads to initiation of spermatogenesis (15). Thus, the identification of serum biomarker to potentially predict response and monitoring of therapy would provide a benefit to infertility patients. Despite the fact that ITT concentration is key to induce spermatogenesis, the dose of hCG is often titrated to serum T levels. Given the poor association between serum T and ITT, the situation may arise where administration of hCG may normalize serum T levels without normalizing ITT (16). This occurrence is also consistent in normal men with experimentally-induced hypogonadotropic hypogonadism (3).
17-hydroxyprogesterone (17-OHP) as a marker of ITT
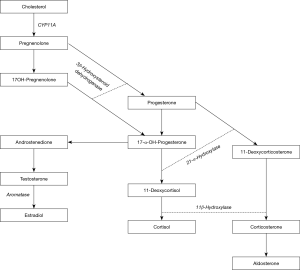
Intratesticular steroids are comprised by approximately 70% T, 20% 17-OHP, and smaller percentages of other hormones (6). Approximately 70% of T and 17-OHP are thought to be of testicular origin (17,18). The remainder of 17-OHP production is thought to be adrenal origin (16). Figure 1 illustrates the synthesis of 17-OHP from its steroid precursors. Amory et al. suggested that serum 17-OHP strongly reflects ITT concentrations in normal men receiving gonadotropin suppression and hCG (6). This study assessed ITT concentration by testicular aspiration before and after treatment through the random allocation of healthy men receiving exogenous T into hCG dosage groups of either 0, 125, 250, or 500 IU every other day for 3 weeks. It was found that serum 17-OHP decreased by about 60% in men receiving placebo and increased by 70% in men at the highest dose of hCG. Their results found 17-OHP did not correlate to ITT at baseline, but was very strongly correlated during treatment, specifically when taking into consideration change from baseline and post-treatment measurements. While the overall correlation between ITT and serum 17-OHP was strong, the ITT increased much more than the serum 17-OHP in the lowest dose hCG group. This difference implies that serum 17-OHP may not be as sensitive to changes in ITT mediated by lower doses of hCG stimulation as it is to the higher doses of hCG (6). The authors highlighted the limitations of their study findings which included being a small, single institution study with a large variance in the measurement of ITT. They further discuss how suppression of the hypothalamic-pituitary-gonadal axis with the use of exogenous testosterone may not reflect accurate measurement of ITT. Nonetheless, their results are compelling and suggest further evaluation into this biomarker.
A recent paper by Roth et al. sought to evaluate the minimal amount of hCG required to stimulate ITT in men with experimental gonadotropin deficiency. They treated 37 men with a GnRH antagonist to induce gonadotropin insufficiency and subsequently randomized them to receive 1 of 4 low dose hCG treatments (0, 15, 60 or 125 IU SQ every other day) or to 7.5 g of daily testosterone gel for 10 days. Their results found minimal amount of hCG was necessary for the production of ITT likely between 15–60 IU. Their results found very little correlation between ITT and 17-OHP. However, as the authors state the dose of hCG was quite minimal to their previous work and the incongruity in results is likely due to the lower doses of hCG used by Roth et al., which in turn would have induced dramatically lower ITT concentrations. As previously stated this may indicate that serum 17-OHP correlates with ITT in the presence of normal or near-normal hCG stimulation (16).
Insulin-like factor 3 (INSL3) as a marker for ITT
INSL3 is a peptide hormone produced by mature Leydig cells in mammals and is another promising serum biomarker of ITT. Prior data by Bay et al. investigated INSL3 through the delivery of gonadotropin suppression with exogenous testosterone and progestin and then assessment of spontaneous or assisted recovery with exogenous gonadotropins (15). They found no increase in INSL3 after administration of supraphysiologic hCG in normal men which suggests that INSL3 may be maximally produced in men with normal testicular physiology (15). Their results corroborate their prior finding of differential expression of T and INSL3 (20). INSL3 also declined drastically after gonadotropin deprivation and was responsive to hCG in the short-term (4 days). This finding provides some evidence to support INSL3 as a marker for ITT, however, serum T recovered significantly better (80% baseline) compared with serum INSL3 (38.9% baseline) in the presence of fully recovered serum LH, signifying that INSL3 is more sensitive than T to impairment in Leydig cell function (15). Interestingly, INSL3 has been suggested to be a marker of Leydig cell differentiation since it is constitutively secreted and thus not subject to variability like testosterone (21). INSL3 has the capability to reflect the number of Leydig cells present which is evidently unique from testosterone, which is homeostatically regulated by the hypothalamic-pituitary-gonadal axis (15). Other studies have reported stimulation of INSL3 production in men with hypogonadotropic hypogonadism treated with high doses of hCG monotherapy who would have had normally suppressed levels (15). Roth et al. built upon the findings of Bay et al. and delivered a co-treatment of acyline, a GnRH antagonist, with very low-dose hCG and found that serum INSL3 concentrations in normal men effectively decrease with gonadotropin suppression and increase in a dose-response relationship with low-dose hCG stimulation, strongly correlating with ITT and serum T concentrations (16). This finding provides evidence that serum INSL3 might be useful as a biomarker for the effect of hCG therapy and LH-like stimulation of Leydig cells. The utility of INSL3 as an alternative and superior serum marker to serum T might allow for more accurate monitoring of hCG therapy in infertile men, however further research is required to validate its reflection of ITT and Leydig cell function.
Clinical implications
Identification of the minimum concentration of ITT necessary for spermatogenesis in men has important clinical implications. The ability for a serum biomarker to accurately reflect ITT concentrations has the potential to identify men deficient of ITT who may respond to therapy to initiate spermatogenesis (i.e., hypogonadotropic hypogonadism), maintain spermatogenesis (i.e., men on testosterone replacement) and improve spermatogenesis (i.e., idiopathic oligospermia). Furthermore, it may also identify men with impaired sperm production who may not benefit from hormonal manipulation (i.e., men with normal ITT pre-treatment with oligospermia). The development of an effective male hormonal contraceptive could be possible with identification of the minimum concentration of ITT necessary for spermatogenesis. Some men do not respond to gonadotropic suppression, despite maximal suppression (2). The cause behind this is unknown, which presents a consequential barrier in the production of a male contraceptive (22,23). Through the use of a biomarker for ITT, more information about the intratesticular hormonal microenvironment can be discerned which can fill gaps in knowledge that are currently preventing the development of male contraceptive agents. Not only this, but a serum biomarker would lead to safer and more reliable methods for repeated sampling of the intratesticular environment. However, even in the absence of LH or hCG, ITT concentrations are still 100 times higher than normal serum T (16). These concentrations are sufficient to support spermatogenesis which must be accommodated for when developing a male hormonal contraceptive. Perhaps, T biosynthesis inhibitors, such as ketoconazole, may be useful to efficaciously decrease ITT concentrations (3). A serum biomarker for ITT would be of great value to investigate this phenomenon as a method to assess the associations between low ITT concentrations and spermatogenesis. A clearer understanding of the interactions between intratesticular hormones and spermatogenesis can be advantageous to overcome hurdles in the development of a male contraceptive.
Conclusions
Both the minimal and optimal concentrations of ITT necessary for spermatogenesis remain unknown. A serum biomarker to evaluate ITT could be useful to investigate the intratesticular hormonal milieu. A serum ITT marker would also be clinically useful in conjunction with serum T measurements to identify the ideal dosage of hCG required to treat infertility in men with hypogonadism. Finally, the identification of an appropriate serum marker for ITT has the potential to overcome the obstacles of and aid future research in developing effective hormonal male contraceptives.
Acknowledgements
None.
Footnote
Conflicts of Interest: R Ramasamy: Coloplast—Consultant; Boston Scientific—Investigator; Endo—Investigator, Advisory Board; Aytu Biosciences—Investigator, Advisory Board; Direx—Investigator. P Patel—Advisory board; Aytu Biosciences. The other authors have no conflicts of interest to declare.
References
- McLachlan RI, O'Donnell L, Meachem SJ, et al. Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J Androl 2002;23:149-62. [PubMed]
- Coviello AD, Matsumoto AM, Bremner WJ, et al. Low-Dose Human Chorionic Gonadotropin Maintains Intratesticular Testosterone in Normal Men with Testosterone-Induced Gonadotropin Suppression. J Clin Endocrinol Metab 2005;90:2595-602. [Crossref] [PubMed]
- Roth MY, Page ST, Lin K, et al. Dose Dependent Increase in Intratesticular Testosterone by Very LowDose Human Chorionic Gonadotropin in Normal Men with Experimental Gonadotropin Deficiency. J Clin Endocrinol Metab 2010;95:3806-13. [Crossref] [PubMed]
- Coviello AD, Bremner WJ, Matsumoto AM, et al. Intratesticular Testosterone Concentrations Comparable With Serum Levels Are Not Sufficient to Maintain Normal Sperm Production in Men Receiving a Hormonal Contraceptive Regimen. J Androl 2004;25:931-8. [Crossref] [PubMed]
- Bremner WJ, Matsumoto AM, Sussman AM, et al. Follicle-stimulating hormone and human spermatogenesis. J Clin Invest 1981;68:1044-52. [Crossref] [PubMed]
- Amory JK, Coviello AD, Page ST, et al. Serum 17-hydroxyprogesterone strongly correlates with intratesticular testosterone in gonadotropin-suppressed normal men receiving various dosages of human chorionic gonadotropin. Fertil Steril 2008;89:380-6. [Crossref] [PubMed]
- Hsieh TC, Pastuszak AW, Hwang K, et al. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol 2013;189:647-50. [Crossref] [PubMed]
- Depenbusch M, von Eckardstein S, Simoni M, et al. Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone. Eur J Endocrinol 2002;147:617-24. [Crossref] [PubMed]
- Bridges N, Trofimenko V, Fields S, et al. Male factor infertility and clomiphene citrate: a meta-analysis – The effect of clomiphene citrate on oligospermia. Urol Pract 2015;2:199-205. [Crossref]
- Amory JK, Bremner WJ. Regulation of testicular function in men: implications for male hormonal contraceptive development. J Steroid Biochem Mol Biol 2003;85:357-61. [Crossref] [PubMed]
- Anderson RA, Wallace AM, Wu FC. Comparison between testosterone enanthate-induced azoospermia and oligozoospermia in a male contraceptive study. III. Higher 5_-reductase activity in oligozoospermic men administered supraphysiological doses of testosterone. J Clin Endocrinol Metab 1996;81:902-8. [PubMed]
- Matsumoto AM. Effects of chronic testosterone administration in normal men: safety and efficacy of high dosage testosterone and parallel dose-dependent suppression of luteinizing hormone, follicle-stimulating hormone, and sperm production. J Clin Endocrinol Metab 1990;70:282-7. [Crossref] [PubMed]
- Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet 1990;336:955-9. [Crossref] [PubMed]
- World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligospermia in normal men. Fertil Steril 1996;65:821-9. [Crossref] [PubMed]
- Bay K, Matthiesson K, McLachlan R, et al. The Effects of Gonadotropin Suppression and Selective Replacement on Insulin-Like Factor 3 Secretion in Normal Adult Men. J Clin Endocrinol Metab 2006;91:1108-11. [Crossref] [PubMed]
- Roth MY, Lin K, Bay K, et al. Serum INSL3 is highly correlated with intratesticular testosterone in normal men with acute, experimental gonadotropin deficiency stimulated with low-dose hCG: a randomized-controlled trial. Fertil Steril 2013;99:132-9. [Crossref] [PubMed]
- Stege R, Eriksson A, Henriksson P, et al. Orchidectomy or oestrogen treatment in prostatic cancer: effects on serum levels of adrenal androgens and related steroids. Int J Androl 1987;10:581-7. [Crossref] [PubMed]
- Carlström K, Stege R. Adrenocortical function in prostatic cancer patients: effects of orchidectomy or different modes of estrogen treatment on basal steroid levels and on the response to exogenous adrenocorticotropic hormone. Urol Int 1990;45:160-3. [Crossref] [PubMed]
- Deaton MA, Glorioso JE, McLean DB. Congenital adrenal hyperplasia: not really a zebra. Am Fam Physician 1999;59:1190-6. [PubMed]
- Bay K, Hartung S, Ivell R, et al. Insulin-like factor 3 (INSL3) serum levels in 135 normal men and 85 men with testicular disorders: relationship to the LH-testosterone axis. J Clin Endocrinol Metab 2005;90:3410-8. [Crossref] [PubMed]
- Ivell R, Wade JD, Anand-Ivell R, et al. INSL3 as a Biomarker of Leydig Cell Functionality. Biol Reprod 2013;88:147. [Crossref] [PubMed]
- McLachlan RI, Robertson DM, Pruysers E, et al. Relationship between serum gonadotropins and spermatogenic suppression in men undergoing steroidal contraceptive treatment. J Clin Endocrinol Metab 2004;89:142-9. [Crossref] [PubMed]
- Amory JK, Anawalt BD, Bremner WJ, et al. Daily Testosterone and Gonadotropin Levels Are Similar in Azoospermic and Nonazoospermic Normal Men Administered Weekly Testosterone: Implications for Male Contraceptive Development. J Androl 2001;22:1053-60. [Crossref] [PubMed]


