
Impact of advanced paternal age on the intracytoplasmic sperm injection (ICSI) outcomes in donor egg cycles
Introduction
The last two decades have been characterized by a steady increase of paternal and maternal age (1). This phenomenon is likely to be secondary to a variety of factors, including increased life expectancy, the changing role of women in society, the absence of supportive family policies, economic uncertainty and the availability of assisted reproductive technologies (ARTs) (2).
While the impact of advancing female age on reproduction is widely recognized, very little is known regarding the influence of paternal age (3,4). Advanced maternal age is proved to be associated with decreased fertilization and implantation rates, just like higher miscarriages rates. This could be a consequence of the increase of chromosomal abnormalities in oocytes and embryos (5-8). Also increased rates of pregnancy complications, congenital anomalies or perinatal mortality were reported in literature (9,10). On the other hand, the effect of paternal age on both sperm characteristics and reproductive outcomes has not been adequately investigated.
The exact knowledge of paternal age role is fundamental for the correct counselling of older couples, in order to predict the successful chances of an ART treatment.
Previous studies have demonstrated that the quality of the semen in terms of volume (11), total sperm count (12), sperm motility (13), sperm morphology (14) or sperm DNA integrity (15) is inversely related with male age. This aspect can be possibly explained by sperm DNA damage due to methylation (16) and to exposure to reactive oxygen species (ROS) (17-19). Some recent papers indicate a direct correlation between men age and risk of DNA damage (20), spontaneous abortion (21), stillbirth (22), foetal death (23), birth defects (24), low birth weight, impaired neurocognitive development, epilepsy, schizophrenia, and several types of malignancies (25).
Although the evidence shows that age negatively affects seminal parameters, results of the impact of male age on the outcomes of assisted reproduction are conflicting (26,27).
This is because it can be difficult to assess the impact of paternal age by examining the reproductive outcomes in the whole population or in couples undergoing autologous in vitro fertilization (IVF) cycles. In this regard, maternal age and ovarian hyperstimulation, as well as embryo development represent possible bias (28). For this reason, the ovum donation model represents a better model since all the oocytes are collected from young women, reducing the confounding factor associated with the oocyte quality. Nevertheless, it is not possible to eliminate the adverse effects caused by endometrial aging or uterine factors (29).
Overall, the majority of reports assessing the effect of male aging on reproductive outcomes have not found statistically significant correlation between male age and rate of fertilization, implantation, pregnancy, miscarriage, or live birth (30-34).
Thus, the aim of our study was to assess the impact of paternal age on the oocyte-donation outcomes in intracytoplasmic sperm injection (ICSI) cycles, after selecting only cycles with overall oocyte survival rate greater than 85%.
Methods
Study population
Data of 278 couples who underwent fertility treatment at the ARTs Centre of the University of Florence (Italy) between January 2016 and December 2017 were retrospectively reviewed. All the patients gave a written consent to agree on having their data included into the study. All couples had a history of primary infertility with a severe female infertility factor, including diminished ovarian reserve, poor response to ovarian stimulation, chromosome aberration, repeated failure of IVF cycles or premature ovarian failure. In all cases, a donor egg was utilized to perform an ICSI cycle using ejaculated spermatozoa by fresh. Only couples with a donor egg program were included in this series in order to standardize female characteristics and to reduce all possible confounding factors associated with egg quality.
All anonymous oocyte donors were <25 years of age (range, 18–25 years) at the time of participation and fulfilled the standard screening criteria as outlined by the American Society of Reproductive Medicine (ASRM). All these women were healthy, had normal menstrual cycles and no gynaecological pathologies.
Inclusion criteria were: infertility from almost one year, presence of normal or sub-fertile seminal parameters in almost two seminal analyses (according to 2010 WHO guidelines), an overall oocyte survival rate after thawing greater than 85%. Only couples who underwent ICSI with fresh sperm were included in this series. Azoospermia, hypogonadism, varicocele, retractile testes or previous testicular torsion, concomitant urogenital infections, previous chemotherapy or radiotherapy, hematologic disorders, chronic inflammatory disease and drug therapy were exclusion criteria.
All couples performed a complete medical work-up including gynaecological evaluation and andrological assessment. Baseline characteristics of participants included recipient age, male age, height and weight with body mass index (BMI) count, smoking and drinking status. Drug consumption, previous diseases or surgery, history of cryptorchidism, medication, and comorbidities were extrapolated from the medical records. All men underwent a preliminary physical examination to assess testicular size and to exclude anomalies in the seminal tract. The hormonal profile, including follicle stimulating hormone (FSH), luteinizing hormone (LH), total testosterone (TT), estradiol (E2) and prolactin (PRL) was checked in all patients. Genetic screening to detect karyotype anomalies was carried out in all patients. Urine analysis and urine culture was carried out to rule out the presence of urinary tract infections. A semen culture test was performed when leukocyte cells were detected in the seminal fluid. Infection from human immunodeficiency virus, hepatitis B virus, hepatitis C virus, Cytomegalovirus, Treponema pallidum and Syphilis was excluded in all patients.
On the day of ICSI, fresh semen was obtained by masturbation after 3–5 days of sexual abstinence. Abstinence time, volume, pH, viscosity, total sperm count/mL, total sperm motility (progressive motility and non-progressive motility) and normal sperm morphology were the seminal parameters evaluated. Prior to ICSI cycle, spermatozoa underwent in vitro capacitation in order to select spermatozoa with good morphology and motility and to remove the seminal plasma. After this process, count/mL and motility were collected.
The study was conducted in accordance with the declaration of Helsinki and after obtaining the institutional review board approval (2018-017 CINECA 10189). The analysis did not require any additional exam and had no impact on patients’ health during the study.
The main outcome measures included fertilization rate (FR), cleavage rate (CR), pregnancy rate (PR), miscarriage rate and live birth rate. In order to investigate the impact of paternal age on the outcomes of ICSI, cycles were arbitrarily subdivided according to paternal age in group 1 (≤45 years) and group 2 (>45 years). Moreover, we used 70% FR as cut-off for group distribution according to the international seminar (The Vienna Consensus: report of an expert meeting on the development of ART laboratory performance indicators—ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine), designed to establish consensus on definitions and recommended values for indicators for ART laboratory. Minimum performance values (“competence”) and aspirational values (“benchmarks”) were recommended for a total of 19 indicators, including 12 key performance indicators (KPIs), five performance indicators (PI) and two benchmarks.
Endometrial preparation of recipients, ICSI procedure and outcome measures
The programmed hormone replacement regimen consisted of oral contraceptive pill (OCP) with a single depot-dose of a GnRH agonist (triptorelin) (Decapeptyl® 3.75, Ipsen Spa, Milan, Italy) on days 20–21 of the cycle, followed by oral estradiol valerate (Progynova®, Bayer, Milan) 2 mg/day from day 2 through 6 of the menstrual cycle, 4 mg/day from days 7 to 10 and 6 mg/day on day 11 until embryo transfer. After endometrial preparation with estrogen, women received progesterone supplementation with 400 mg intravaginal capsules (Progeffik®/Proimetrium®) every 12 hours. Supplementation was started on the evening before the egg thawing and continued until the 10th week of gestation in case of pregnancy. All oocytes that had survived the thawing procedure were submitted for ICSI. Injected oocytes were incubated in 20 µL drops. All the zygotes were evaluated 16–18 hours after ICSI to confirm the presence of two distinct pronuclei. Subsequently, all the embryos were evaluated on days 2, 3, and 5 of the development using an inverted microscope with a Hoffmann modulation contrast system. The following parameters were recorded: number of blastomeres, percentage of fragmentation, variation in blastomere symmetry, presence of multinucleation, and defects in the zona pellucida and cytoplasm. High-quality cleavage-stage embryos were defined as those with all of the following characteristics: four cells on day 2 or 8–10 cells on day 3, <15% fragmentation, symmetric blastomeres, absence of multinucleation, colourless cytoplasm with moderate granulation and no inclusions, absence of perivitelline space granularity, and absence of zona pellucida dysmorphism (classified with the score A). Embryos lacking any of these characteristics were considered to be of low quality (score B–C). To evaluate blastocyst formation, embryos were given a score (1 to 4) according to their degree of expansion and hatching status, as follows: 1, an early blastocyst with a blastocoel that was less than half of the volume of the embryo; 2, a blastocyst with a blastocoel that was greater than half of the volume of the embryo; 3, an expanded blastocyst with a blastocoel that completely filled the embryo; 4, a hatching blastocyst. Expanded and hatching blastocysts were classified as complete blastocysts. Thereafter, ICSI outcomes, as well as FR, CR and PR were evaluated. The total and normal oocyte FR was calculated by total number of fertilized oocytes and “two-pronuclear” (2PN) fertilized oocytes by the number of injected oocytes, respectively. The CR was calculated by the number of embryos obtained by the number of normal fertilized oocytes. Embryos were transferred into the uterine cavity 48 to 72 or 120 hours after ICSI procedure. At our centre, embryo transfers are performed under transabdominal ultrasound guidance using a Wallace catheter. Our laboratory policy on transfer of donor oocytes embryos is to transfer two embryos, either on day 3 or 5, based on morphology. Supernumerary embryos are frozen. After 14 days, HCG test was performed. Clinical pregnancy was defined by HCG levels above 50 mU/L and documented by transvaginal ultrasound visualization of an intrauterine gestational sac with a heartbeat at around 5–6 weeks of gestation. Pregnancy loss before 20 weeks of gestation and all biochemical pregnancies were considered as miscarriages. Live birth rate was defined as the percentage of all cycles that lead to live birth.
Statistical analysis
The association between male age and both sperm parameters and reproductive outcomes were analysed. Continuous variables are presented as median and interquartile range (IQR) and differences between groups were assessed by the Student independent t-test or the Mann-Whitney U test on the basis of their normal or not-normal distribution, respectively (normality of variables’ distribution was tested by the Kolmogorov-Smirnov test). Age-adjusted linear regression analysis was performed to determine the relationship between numeric dependent and independent parameters, and logistic regression analysis was employed to determine significant predictors of pregnancy, FR >70% and cleavage. The study was conducted at a confidence level of 95%. All statistical analyses were completed using Stata software, version 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP). For all statistical comparisons, significance was considered as P<0.05.
Results
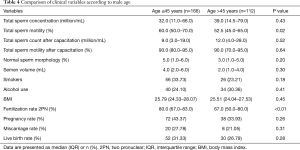
A total of 278 couples were included in the study. Overall, 1,724 frozen donor oocytes were obtained from Spain. After thawing, 1,642 oocytes survived, resulting viable and mature. Median overall oocyte survival rate was 100% (IQR, 85–100%). Baseline characteristics of the study cohort are depicted in Table 1. Median male age was 44±5.60 years (IQR, 31–70 years). Group 1 (≤45 years) included 166 men, group 2 (>45 years) 112 men. Recipients had a median age of 42±3.62 years (IQR, 29–50 years). No men suffered from medical conditions such as diabetes, cardiovascular disease and cancers. At baseline, all hormonal parameters collected were in the normal range. Mean right testicular volume, detected by Prader orchidometer scale was 18 mL (IQR, 10–25 mL), while mean left testicular volume was 17 mL (IQR, 6–25 mL). All patients had a normal urine analysis with a negative urine-culture and semen culture. Screening for sexual transmitted diseases was negative in all patients. Two hundred and seventy-eight fresh ICSI cycles were performed. “2PN” FR was 72.6%±0.20%. CR was 93.0%±0.16%. Clinical pregnancy per cycle was obtained in 110/278 cycles (overall PR, 39.6%). Miscarriage rate was 25.5%. Live birth rate per cycles was 29.5%. The great majority (89.2%) of the embryo transfers were performed on day 3. As shown in Table 2, comparison between group 1 and group 2 and FRs demonstrated a strong correlation with “2PN” FR, resulting 80.0% (IQR, 67.0–83.0%) and 67.0% (IQR, 50.0–80.0%) respectively (P<0.01). In addition, there were no significant differences between the two groups with respect to seminal parameters, except for a relationship between advanced male age and reduced total sperm motility (P=0.02).

Full table

Full table
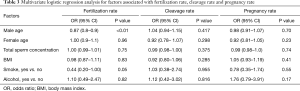
As depicted in Table 3, the multivariate logistic regression analysis confirmed that there is a strong correlation between male age and FR (OR 0.87; P<0.01). CR and PR were not found to be related significantly with paternal age (P=0.417, 0.70 respectively). It was highlighted just a mild correlation between smokers (29.5%) and FR (OR 0.44; P=0.05). Comparison of clinical variables according to male age confirmed the significant association between “2PN” FR and age groups (P<0.01) (Table 4). Overall, comparison of clinical variables according to FR revealed that median BMI, smoking status and alcohol consumption were not related with FR (P=0.88, 0.12, 0.71 respectively) (Table 2).
Full table
Full table
Discussion
Nowadays, the analysis of the association between male age and ART outcomes represents an important debated issue.
This retrospective study confirmed the impact of paternal age on reproductive outcomes of ICSI cycles with donor oocytes. Indeed, the results demonstrated a significant decline in FRs with advancing paternal age, in contrast with the evidences shown by the majority of previous studies.
Recently, various authors have focused on this topic (26-35). Overall, the majority did not identify a statistically significant association between paternal age and IVF outcomes. In particular, Bellver et al., in their study cohort including 1,412 IVF cycles, did not find any correlation between paternal age, implantation and FRs (26). Girsh et al. showed a tendency for decreased PR with advanced paternal age, but in this series no data about recipient’s age are available (33). A statistically significant correlation between advancing paternal age and FR was identified only in two studies. In particular, Duran et al. reported a statistically significantly reduced FR with advancing male age only in the ICSI case (12). Luna et al. instead found a statistically significantly lower rate of fertilization by conventional insemination in men older than 50 years, but no differences in the outcome of ICSI cycles (34).
In the current study, FRs were significantly lower in men older than 45 years (P<0.001). However, statistical association between paternal age and other IVF outcomes, including cleavage and PRs, was not detected. We arbitrarily divided men into two age groups (> or ≤45 years) in agreement with some previous data available in literature, suggesting an increased male-related infertility in men >40 of years (36).
Although the precise mechanism of how paternal age influences the IVF outcomes still remains unknown, previous research suggested that advancing age has several effects on DNA damage, chromatin integrity, gene mutations and aneuploidy in spermatozoa (37,38). Since it is generally accepted that part of the damaged sperm DNA can be repaired by the oocyte (39), the extent of DNA damage beyond the point of repair by the young oocyte could explain the impact of male age on severe male factor infertility. Moreover, an increased number of mutations in the sperm of older fathers may have subsequently increased adverse embryo development and IVF outcomes (37).
How shown by several studies, increasing male age could be associated with infertility (40), as well as decreased semen motility and volume (19,41). The largest of the studies demonstrated a significant relationship between paternal age and all sperm parameters analysed: for every additional 5 years of age, sperm volume decreased by 0.22 mL (P<0.001), concentration increased by 3.1 million sperm/mL (P=0.003), and percentage of motile spermatozoa decreased by 1.2% (P<0.001) (35). In our study, we found decreased semen volume and motility with increasing age, but we detected a statistically significant association between male age and total sperm motility (P=0.02).
We took as a reference the KPIs established in Vienna Consensus (ESHRE, 2017) and in the previous Alpha Consensus meeting (Alpha Scientists In Reproductive Medicine, 2012). Therefore, FR greater or equal to 70% was considered optimal, and the less than 70% was suboptimal.
In the current series, we selected cycles with overall oocyte survival rate greater than 85%, in order to reduce possible bias. The oocyte donation program represents a good method to reduce the confounding factors, since the quality of donor oocytes should be considered excellent. Nevertheless, other factors related to recipient’s age play a key role in ICSI success, such as the endometrial factor that could affect the outcome through implantation success (42,43). Since our recipients had a median age of 42 years, the endometrial receptivity could become a determinant of clinical pregnancy and explain the absence of correlation between male age and PR, how shown in our records.
In the current study the impact of BMI, smoking status and drinking habits on the outcomes of ICSI were analyzed (44). However, no significant correlation was found.
The current series has some strength and limitations. One strength was the inclusion of cycles with oocyte survival rate greater than 85%. As a result, the sample size of our study cohort was small. The standardization of donor stimulation protocols, recipients’ endometrial preparation and sperm sample evaluation strengthens our results.
However, further larger prospective studies are needed to investigate the relationship between sperm DNA damage, aneuploidy, gene mutations, chromatin integrity and reproductive outcomes in older couples.
Conclusions
This study showed the significant role of paternal age in determining the outcomes of ICSI when the oocytes are young and healthy. FR seemed to be the major determinant of morphological embryo quality in IVF cycles. Accordingly, it would be recommended to pay more emphasis on the advancing male age when counselling older couples who undergo egg donation program.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the declaration of Helsinki and after obtaining the institutional review board approval (2018-017 CINECA 10189). Written informed consent was obtained from all the patients for publication of this manuscript and any accompanying images.
References
- Mathews TJ, Hamilton BE. Mean age of mother, 1970-2000. Natl Vital Stat Rep 2002;51:1-13. [PubMed]
- Mills M, Rindfuss RR, McDonald P, et al. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update 2011;17:848-60. [Crossref] [PubMed]
- Dain L, Auslander R, Dirnfeld M. The effect of paternal age on assisted reproduction outcome. Fertil Steril 2011;95:1-8. [Crossref] [PubMed]
- Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update 2010;16:65-79. [Crossref] [PubMed]
- Angell RR. Aneuploidy in older women. Higher rates of aneuploidy in oocytes from older women. Hum Reprod 1994;9:1199-200. [Crossref] [PubMed]
- Meldrum DR. Female reproductive aging - ovarian and uterine factors. Fertil Steril 1993;59:1-5. [Crossref] [PubMed]
- Munné S, Alikani M, Tomkin G, et al. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil. Steril 1995;64:382-91. [PubMed]
- Wood C, Calderon I, Crombie A. Age and fertility: results of assisted reproductive technology in women over 40 years. J Assist Reprod Genet 1992;9:482-4. [Crossref] [PubMed]
- Maheshwari A, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Hum Reprod 2008;23:538-42. [Crossref] [PubMed]
- Heffner LJ. Advanced maternal age—how old is too old? N Engl J Med 2004;351:1927-9. [Crossref] [PubMed]
- Brahem S, Mehdi M, Elghezal H, et al. The effects of male aging on semen quality, sperm DNA fragmentation and chromo- somal abnormalities in an infertile population. J Assist Reprod Genet 2011;28:425-32. [Crossref] [PubMed]
- Duran EH, Dowling-Lacey D, Bocca S, et al. Impact of male age on the outcome of assisted reproductive technology cycles using donor oocytes. Reprod BioMed Online 2010;20:848-56. [Crossref] [PubMed]
- Schwartz D, Mayaux MJ, Spira A, et al. Semen characteristics as a function of age in 833 fertile men. Fertil Steril 1983;39:530-5. [Crossref] [PubMed]
- Spandorfer SD, Avrech OM, Colombero LT, et al. Effect of paternal age on fertilization and pregnancy characteristics in couples treated by intracytoplasmic sperm injection. Hum Reprod 1998;13:334-8. [Crossref] [PubMed]
- Moskovtsev SI, Willis J, Mullen JB. Age-related decline in sperm deoxyribonucleic acid integrity in patients evaluated for male infertility. Fertil Steril 2006;85:496-9. [Crossref] [PubMed]
- Flanagan JM, Popendikyte V, Pozdniakovaite N, et al. Intra- and interindividual epigenetic variation in human germ cells. Am J Hum Genet 2006;79:67-84. [Crossref] [PubMed]
- Cocuzza M, Athayde KS, Agarwal A, et al. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology 2008;71:490-4. [Crossref] [PubMed]
- Ford WC, North K, Taylor H, et al. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. The ALSPAC Study Team (Avon Longitudinal Study of Pregnancy and Childhood). Hum Reprod 2000;15:1703-8. [Crossref] [PubMed]
- Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril 2001;75:237-48. [Crossref] [PubMed]
- Schmid TE, Eskenazi B, Baumgartner A, et al. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod 2007;22:180-7. [Crossref] [PubMed]
- Slama R, Bouyer J, Windham G, et al. Influence of paternal age on the risk of spontaneous abortion. Am J Epidemiol 2005;161:816-23. [Crossref] [PubMed]
- Astolfi P, De Pasquale A, Zonta LA. Late paternity and stillbirth risk. Hum Reprod 2004;19:2497-501. [Crossref] [PubMed]
- Andersen AM, Vastrup P, Wohlfahrt J, et al. Fever in pregnancy and risk of fetal death. Results from the better health for mother and child-project. Ugeskr Laeger 2004;166:53-6. [PubMed]
- Yang Q, Wen SW, Leader A, et al. Paternal age and birth defects: how strong is the association? Hum Reprod 2007;22:696-701. [Crossref] [PubMed]
- Choi JY, Lee KM, Park SK, et al. Association of paternal age at birth and the risk of breast cancer in offspring: a case control study. BMC Cancer 2005;5:143. [Crossref] [PubMed]
- Bellver J, Garrido N, Remohi J, et al. Influence of paternal age on assisted reproduction outcome. Reprod. Biomed. Online 2008;17:595-604. [Crossref] [PubMed]
- Campos I, Gomez E, Fernandez-Valencia AL, et al. Effects of men and recipients’ age on the reproductive outcome of an oocyte donation program. J Assist Reprod Genet 2008;25:445-52. [Crossref] [PubMed]
- Garcia-Velasco JA, Isaza V, Caligara C, et al. Factors that determine discordant outcome from shared oocytes. Fertil Steril 2003;80:54-60. [Crossref] [PubMed]
- Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril 2009;91:1215-23. [Crossref] [PubMed]
- Gallardo E, Simon C, Levy M, et al. Effect of age on sperm fertility potential: oocyte donation as a model. Fertil Steril 1996;66:260-4. [Crossref] [PubMed]
- Paulson RJ, Milligan RC, Sokol RZ. The lack of influence of age on male fertility. Am J Obstet Gynecol 2001;184:818-22. [Crossref] [PubMed]
- Frattarelli JL, Miller KA, Miller BT, et al. Male age nega- tively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil Steril 2008;90:97-103. [Crossref] [PubMed]
- Girsh E, Katz N, Genkin L, et al. Male age influences oocyte-donor program results. J Assist Reprod Genet 2008;25:137-43. [Crossref] [PubMed]
- Luna M, Finkler E, Barritt J, et al. Paternal age and assisted reproductive technology outcome in ovum recipients. Fertil Steril 2009;92:1772-5. [Crossref] [PubMed]
- Beguería R, Garcia D, Obradors A, et al. Paternal age and assisted reproductive outcomes in ICSI donor oocytes: is there an effect of older fathers? Hum Reprod 2014;29:2114-22. [Crossref] [PubMed]
- Hassan MA, Killick SR. Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertil Steril 2003;79 Suppl 3:1520-7. [Crossref] [PubMed]
- Sartorelli EM, Mazzucatto LF, de Pina-Neto JM. Effect of paternal age on human sperm chromosomes. Fertil Steril 2001;76:1119-23. [Crossref] [PubMed]
- Sloter E, Nath J, Eskenazi B, et al. Effects of male age on the frequencies of germinal and heritable chromosomal abnormalities in humans and rodents. Fertil Steril 2004;81:925-43. [Crossref] [PubMed]
- Aitken RJ, De Iuliis GN. Origins and consequences of DNA damage in male germ cells. Reprod Biomed Online 2007;14:727-33. [Crossref] [PubMed]
- de La Rochebrochard E, Thonneau P. Paternal age >or=40 years: an important risk factor for infertility. Am J Obstet Gynecol 2003;189:901-5. [Crossref] [PubMed]
- Eskenazi B, Wyrobek AJ, Sloter E, et al. The association of age and semen quality in healthy men. Hum Reprod 2003;18:447-54. [Crossref] [PubMed]
- Soares SR, Velasco JA, Fernandez M, et al. Clinical factors affecting endometrial receptiveness in oocyte donation cycles. Fertil. Steril 2008;89:491-501. [Crossref] [PubMed]
- Toner JP, Brzyski RG, Oehninger S, et al. Combined impact of the number of pre- ovulatory oocytes and cryopreservation on IVF outcome. Hum Reprod 1991;6:284-9. [Crossref] [PubMed]
- Rubio C, Vassena R, García D, et al. Influence of Donor, Recipient, and Male Partner Body Mass index on Pregnancy Rates in Oocyte Donation Cycles. JBRA Assist Reprod 2015;19:53-8. [PubMed]


