
No survival difference between super extended and standard lymph node dissection at radical cystectomy: what can we learn from the first prospective randomized phase III trial?
The current situation
Radical cystectomy (RC) with pelvic lymph node dissection (LND) is the standard of care local treatment for patients with muscle-invasive bladder cancer (MIBC) (≥ pT2) (1,2). Current preoperative staging using computed tomography is suboptimal due to its low sensitivity of only 52.6% for the detection of positive lymph nodes (LNs), which leads to a significant understaging of nodal disease (3). This while approximately 25% of patients undergoing RC with LND for bladder cancer (BC) have positive LNs at pathological examination (4). Importantly, positive LNs are the most important driver of cancer specific mortality in organ confined MIBC (4-6). Ideally, the extent of the LND should be tailored to provide maximal oncological control and optimal staging, while preventing peri-operative complications and long-term morbidity.
Both European (EAU) and American (AUA) urological guidelines recommend the use of LND at the time of cystectomy for MIBC (1,2). However, there is great lack in uniformity concerning definitions and naming of the LND surgical template throughout literature. To minimize heterogeneity between studies, we recommend to implement the EAU Working Group on MIBC-definitions: limited LND (L-LND): obturator and/or perivesical fossa only, standard LND (S-LND): up to the common iliac arteries, extended LND (E-LND): up to the crossing of the ureters with the common iliac vessels or the aortic bifurcation, with or without the presacral LNs, and super extended LND (SE-LND): up to the inferior mesenteric artery (7). The EAU guidelines state that every RC should be accompanied with an LND [level of evidence (LE) 3]. Additionally, the EAU guidelines state that there is little evidence of a survival benefit of an E-LND over a S-LND or a L-LND (LE 3). The AUA guidelines state that a bilateral LND should be performed with every surgery with curative intent (grade B), and that the template should at least include the S-LND template with a minimum total LN count of 12 (1,2).
The rationale for performing a S-LND is that the standard template can identify 93–95% of node positive patients. Positive LNs rarely skip to a level above this template (0.6–5%). This means that almost all node positive patients will be accurately staged by the S-LND template (8-10). Justification for performing an E-LND or a SE-LND is that 6–43% of all positive LNs are found above the S-LND field in node positive patients, while they are left in place when only a S-LND is performed (8-11). These numbers are based on mapping studies of LNs in retrospective cohorts. However, the inherent bias of this type of studies lies in the fact that the quality of LND is highly dependent on the operating urologist and the examining pathologist. It is impossible to know how complete LN removal was within the template or whether all LNs have been assessed by the pathologist (12). In other types of cancer such as esophageal, gastric and pancreatic head cancer, comparative studies could not demonstrate a survival benefit by extending the template of the LND (13-16).
Another important point is the safety profile of a S-LND in comparison to a SE-LND. We have limited data which compare the extent of the LND and evaluate the long-term functional outcomes and perioperative complications but the result of a systematic review suggests that there are no differences between S-LND and SE-LND (7). A systematic review in prostate cancer could not demonstrate a survival benefit of extending the template of LND but demonstrated an increase in adverse outcomes in terms of operating time, blood loss, length of stay, and postoperative complications (17).
The extent of the LND in MIBC remains controversial as there is no level I evidence data from prospective randomized trials that support the use of either E-LND or SE-LND. Therefore, there is an urgent need for randomized trials evaluating the survival benefit of extending the LND template.
LEA trial
The recently published LEA trial (NCT01215071) is the first prospective randomized phase III trial comparing S-LND with SE-LND for which all contributors should be congratulated (18). The study has been long awaited, and examined whether an “extensive” LND yields a survival benefit over a “limited” LND. The definitions of the LND templates in the LEA trial differ slightly from the EAU Working Group on MIBC-definitions. A “limited” LND should be considered as a S-LND and an “extensive” LND as a SE-LND. Patients with pT1-4a BC were randomized 1:1 before RC to receive either a S-LND or a SE-LND. Neoadjuvant chemotherapy (NAC) was not given to these patients, as this is not considered standard of care in Germany. Postoperative adjuvant chemotherapy was given at the discretion of the treating physician in advanced (pT3–4) or node positive disease. The primary endpoint was recurrence free survival (RFS). Secondary endpoints were cancer-specific survival (CSS), overall survival (OS), complication rate, influence of adjuvant chemotherapy, influence on histopathologic N-stage, and localization of tumor recurrence.
The authors could not show a significant benefit of a SE-LND over S-LND in RFS, CSS, and OS, which led to the question of whether we should stop offering SE-LNDs to our patients. The authors suggest several explanations for these somewhat unexpected outcomes. Their study demonstrated a non-significant RFS difference of 5.5% while it was powered to detect an RFS difference of 15%. First, the power analysis was based on a historical series of 447 patients undergoing LND in which CSS and RFS were correlated with the total and positive LN count (19). However, no formal LN mapping based on templates of LNDs was performed, only a total and positive LN count, and a cut-off of 15 LNs was used to calculate survival differences. Therefore, the results of this study in term of survival outcome are hard to extrapolate to an LND template, which could have led to an overestimation of the RFS difference in the power calculation of the LEA trial. Second, the high median LN count of 19 [interquartile range (IQR): 12–26] in the S-LND and 31 (IQR: 22–47) in the SE-LND could have contributed to the smaller difference in RFS. LN count is only known postoperatively and is an independent prognostic factor for survival in retrospective series (12). Therefore, anatomical templates are more generalizable and thus more favorable to be used in studies, mostly because the LN count is dependent on variation of urologist and pathologist as stated before (12). Third, 14% of patients had T1G3 disease. Previous research has shown that patients with T1 disease have a limited risk (2–10%) for pathologically node positive (pN+) disease, which could lead to skewing of the data (8-10,19). The authors did not include information regarding the contribution of pN+ disease in the T1G3-population compared to MIBC. Fourth, 56% of the included patients were ≤ pT2. This is important as historically only 3% of patients with ≤ pT2 disease are reported to have positive LNs outside the S-LND template (9). Lastly, patients did not receive NAC, but adjuvant chemotherapy at discretion of the treating physician. Adjuvant chemotherapy is not standard of care in most countries and is infrequently used (1). The use of adjuvant chemotherapy in patients with advanced disease without nodal involvement (pT3N0) could have influenced the outcome of the study and could have led to a selection bias. Furthermore, we have to take into account that survival of patients diagnosed with pN+ disease is influenced by subsequent therapy. In the LEA trial a total of 58 patients (28%) with pT3/4 and/or pN+ were treated with adjuvant chemotherapy. Usage of adjuvant chemotherapy was evenly distributed between both treatment groups and improved RFS significantly. Therefore, the therapeutic effect of the LND template itself is difficult to interpret due to the confounding effect of adjuvant chemotherapy in patients with pT3/4 and/or pN+ disease. To decrease bias of subsequent therapy, an ideal trial design would in theory compare no LND with a SE-LND. However, such a trial would not be in line with current evidence and guidelines, and should thus be considered as unethical. NAC on the other hand is being increasingly used and advocated by both EAU and AUA guidelines, with an OS benefit of 8% (1,2). This heterogeneity in perioperative chemotherapy render the results of the LEA trail difficult to interpret and translate into clinical practice, where patients with MIBC tend to receive NAC and no adjuvant chemotherapy.
The LEA trial confirms that there are limited-to-no differences in complication rates between S-LND and SE-LND. The authors only describe a higher incidence of lymphoceles requiring drainage at 90 days postoperatively in the SE-LND group. Long term follow-up functional data regarding e.g., lymphedema and erectile function are currently lacking.
Even though the overall results were negative, the LEA trial has taught us some valuable lessons. In the SE-LND group, positive LNs were located outside the S-LND template in 11% of patients. These positive LNs would have led to a false diagnosis of node negative disease in 2% of patients if a S-LND template was used, due to directly skipping outside the limited S-LND template. Furthermore, 35% of all identified positive LNs were located solely in the extended SE-LND template and would not have been resected using the S-LND template. In conclusion, by using a SE-LND we identify 2% more patients as node positive, which would have been falsely diagnosed as node negative using the S-LND template, and resect 35% more positive LNs, which would have been left behind using a S-LND, with a limited increase in morbidity.
SWOG-1011 trial
Parallel to the LEA trial, the results of SWOG-1011 (NCT01224665) are eagerly awaited as well. The design can be considered similar as the authors also compare oncological outcomes between S-LND and SE-LND. The SWOG-1011 trial is a prospective randomized controlled phase III trial comparing S-LND with SE-LND, but in patients with MIBC only. A total of 620 patients have been randomized. A comparison of the characteristics of the LEA trial and the SWOG-1011 trial can be found in Table 1.
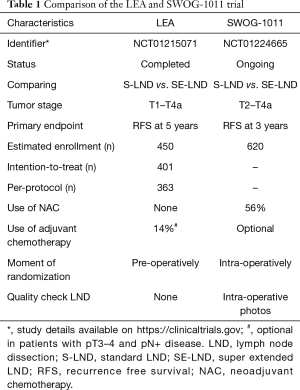
Full table
One of the most notable differences is the exclusion of patients with pT1 disease in the SWOG-1011 trial. Another prominent distinction is that NAC was allowed and was used in 56% of patients, which will render results more representative. Patients are randomized intra-operatively and intra-operative photos are required to assess the quality of the LND, decreasing possible bias. The SWOG-1011 trial is powered to detect a RFS difference of 10%. The estimated study completion date is August 2022. In order to determine whether more extended LNDs render a survival benefit, the results of this study need to be awaited.
The most important question for treating physicians remains: should we continue to perform SE-LNDs while the first prospective trial did not show a survival benefit? With no level 1 evidence favoring SE-LND, our only option is to patiently await the SWOG-1011 trial, and until then adhere to current guidelines.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- Chang SS, Bochner BH, Chou R, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol 2017;198:552-9. [Crossref] [PubMed]
- Horn T, Zahel T, Adt N, et al. Evaluation of Computed Tomography for Lymph Node Staging in Bladder Cancer Prior to Radical Cystectomy. Urol Int 2016;96:51-6. [Crossref] [PubMed]
- Hautmann RE, Gschwend JE, de Petriconi RC, et al. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol 2006;176:486-92; discussion 491-2. [Crossref] [PubMed]
- Gschwend JE, Dahm P, Fair WR. Disease specific survival as endpoint of outcome for bladder cancer patients following radical cystectomy. Eur Urol 2002;41:440-8. [Crossref] [PubMed]
- Hautmann RE, de Petriconi RC, Pfeiffer C, et al. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol 2012;61:1039-47. [Crossref] [PubMed]
- Bruins HM, Veskimae E, Hernandez V, et al. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: a systematic review. Eur Urol 2014;66:1065-77. [Crossref] [PubMed]
- Dorin RP, Daneshmand S, Eisenberg MS, et al. Lymph node dissection technique is more important than lymph node count in identifying nodal metastases in radical cystectomy patients: a comparative mapping study. Eur Urol 2011;60:946-52. [Crossref] [PubMed]
- Vazina A, Dugi D, Shariat SF, et al. Stage specific lymph node metastasis mapping in radical cystectomy specimens. J Urol 2004;171:1830-4. [Crossref] [PubMed]
- Leissner J, Ghoneim MA, Abol-Enein H, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J Urol 2004;171:139-44. [Crossref] [PubMed]
- Seiler R, von Gunten M, Thalmann GN, et al. Pelvic lymph nodes: distribution and nodal tumour burden of urothelial bladder cancer. J Clin Pathol 2010;63:504-7. [Crossref] [PubMed]
- Perera M, McGrath S, Sengupta S, et al. Pelvic lymph node dissection during radical cystectomy for muscle-invasive bladder cancer. Nat Rev Urol 2018;15:686-92. [Crossref] [PubMed]
- Farnell MB, Pearson RK, Sarr MG, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 2005;138:618-28; discussion 628-30. [Crossref] [PubMed]
- Lagergren J, Mattsson F, Zylstra J, et al. Extent of Lymphadenectomy and Prognosis After Esophageal Cancer Surgery. JAMA Surg 2016;151:32-9. [Crossref] [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. [Crossref] [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. [Crossref] [PubMed]
- Fossati N, Willemse PM, Van den Broeck T, et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur Urol 2017;72:84-109. [Crossref] [PubMed]
- Gschwend JE, Heck MM, Lehmann J, et al. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur Urol 2019;75:604-11. [Crossref] [PubMed]
- Leissner J, Hohenfellner R, Thüroff JW, et al. Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosis. BJU Int 2000;85:817-23. [Crossref] [PubMed]


