
Evaluating the impact of radiation therapy on patient quality of life following primary artificial urinary sphincter placement
Introduction
In the United States, the prevalence of urinary incontinence (UI) in men over 40 years of age is estimated at 45% and although definitions of incontinence vary, an increasing trend is seen with advancing age (1,2). UI can have a significant impact on mental health, in particular with regards to anxiety and depression, and has also been demonstrated to have a negative impact on health-related quality of life (QoL) (1). While there are multiple risk factors for UI, one of the most common iatrogenic causes is radical prostatectomy resulting in rates of stress UI estimated between 12–16% depending on surgical modality (3), with other iatrogenic causes including transurethral resection of the prostate and pelvic radiation therapy.
Originally introduced in 1972 (4), the artificial urinary sphincter (AUS) is considered the preferred surgical therapy for moderate to severe stress UI. While the AUS has demonstrated acceptable intermediate to long-term durability with 5-year revision-free survival rates of 60–75% (5-8), the impact on urinary continence reported has varied considerably with rates of social continence, generally defined as ≤1 pad per day (PPD), ranging between 61–100% and dry rates reported between 4–86% (9). Likewise rates of patient satisfaction vary considerably, with reports ranging from 73% to 95% patient satisfaction (10-13). Notably, data regarding preoperative factors that predict patient satisfaction are sparse.
Approximately 40% of men who undergo AUS placement after RP have received external beam radiation therapy (XRT) (14). Exposure to radiotherapy has been proposed as a risk factor for adverse AUS outcomes, though reports in the literature are conflicting (14-20). The impact of radiation therapy and AUS patient satisfaction and postoperative QoL has had minimal investigation, with no impact on long-term continence and patient satisfaction being reported in the one available report (19). Thus, we evaluated the impact of prior radiation therapy on patient satisfaction following primary AUS placement.
Methods
After Institutional Review Board approval, we identified 1,082 male patients who underwent primary AUS implantation at Mayo Clinic (Rochester, MN, USA) from 1983 to 2011 by three surgeons (William Furlow, David Barrett and Daniel S. Elliott). After excluding patients that died during follow-up and/or underwent device revisions or explantations, we identified 467 primary AUS devices in-situ at the time of mailing. Patients were excluded from analysis if they underwent primary AUS implantation for neurogenic bladder dysfunction, were less than 18 years old, or declined research consent. All implanted devices were American Medical Systems 800 (AMS 800; American Medical Systems, Inc., Minnetonka, MN, USA).
Patient clinical characteristics and details of the primary device placement were assessed. Given the retrospective study design, patients did not have standardized follow-up. Instead, following device placement, patients are evaluated 6 weeks post-operatively for device activation. Thereafter, patients are followed via office evaluation on an as needed basis, as determined by their continence or other device concerns. The Mayo Clinic AUS Registry monitors outcomes periodically by written patient correspondence and QoL questionnaires. Details regarding device survival were obtained from last office examination, operative reports, written or telephone correspondence.
All patients who underwent AUS device implantation between 1983 and 2011 were invited to participate in a mail-in survey (Figure 1). The questionnaire administered assessed current device status and any previous explanations during patient follow-up. Patient satisfaction was measured utilizing questions adapted from previous studies focusing on AUS and QoL. Urinary continence parameters were also captured on the questionnaire adopted from the validated Expanded Prostate Cancer Index Composite urinary domain (EPIC-UD) (21). Patient reported change in urinary control from pre-operative to post-AUS placement served as an indicator of overall AUS-QoL and satisfaction, and was assessed on a scale of 0 to 10. These answers were each multiplied by ten to convert to a 0–100 scale, with 100 representing the highest rate of satisfaction/continence. An AUS-QoL score ≥70 was considered to be associated with patient satisfaction and was utilized to include the highest quartile of urinary control.
Statistical analysis was performed using the SAS software package (SAS Institute, Inc.: Cary, NC, USA). Continuous features were summarized with medians and interquartile ranges (IQR); categorical features were summarized with frequency counts and percentages. After stratifying by overall, primary without radiation exposure and with radiation exposure, chi-square cross table analysis, and Spearman rank correlations, were used to investigate the relationship between overall satisfaction (defined by the AUS-related QoL score and likelihood to have AUS surgery again), as well as patient reported urinary continence outcomes. Survey outcomes were dichotomized to reflect the degree of satisfaction and urinary control. All statistical tests were 2-sided, with a P value <0.05 considered statistically significant.
Results
Of the 1,082 primary implantations during the study timeframe, 277 patients were deceased and 338 had undergone revision surgery prior to mailing. This left a total of 467 patients, living with their primary device-in situ. Of these patients, 64 patients with radiation and 165 patients without prior radiation therapy completed the survey, for a response rate of 229/467 (49%).
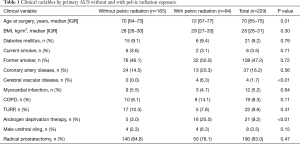
Clinical and demographic information for patients included in the study, stratified by prior pelvic radiation exposure are demonstrated in Table 1. Notably, men with prior radiation therapy were older (P=0.01) and more likely to have received androgen deprivation therapy (P<0.01). There was no significant difference between the cohorts in other clinical characteristics including body mass index (P=0.30) or coronary artery disease (P=0.56).
Full table
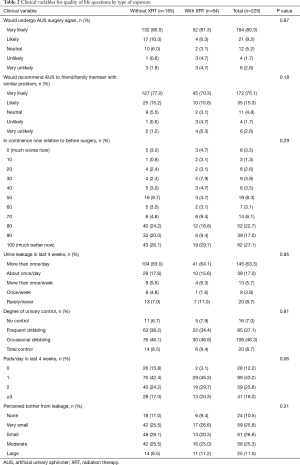
For all responders, at a median follow-up was 8.4 years (IQR 5.8–11.4), 73% reported an AUS-related QoL score ≥70/100, 90% would undergo surgery again, 56% wore ≤1 PPD, 82% reported leakage on a daily basis, and 64% reported minimal leakage-related bother. When stratified by exposure to prior radiation therapy (Table 2), there were no significant differences in patient reported AUS-related satisfaction (P=0.29) or the rate of those using ≤1 pad (P=0.06) between cohorts. Specifically, there were equivalent rates of perceived satisfaction between those with and without prior radiation therapy including willingness to undergo AUS surgery again (87% vs. 91%, P=0.87) and likelihood to recommend AUS surgery to a friend or family member (86% vs. 93%, P=0.18). Likewise, there was equivalence in patient reported AUS QoL following device implantation 70% vs. 77% (P=0.29). Of note, the median follow-up for those with prior radiation exposure was shorter than for patients without prior radiation therapy (median 7.1 vs. 8.9 years; P<0.01).
Full table
Discussion
Here, in a large single institution series of primary AUS placements with a median of 8.4 years of follow-up, we found a high-rate of AUS-related QoL and satisfaction, acceptable urinary control, and no significant differences in functional outcomes among men with primary AUS placement without or with radiation exposure. Overall, 73% of men reported an AUS-related QoL score ≥70 (scale 0–100) and 90% would elect to undergo AUS surgery again. Importantly, our findings demonstrate modest rates of complete urinary control with extended follow-up including 56% of patients reporting ≤1 PPD (social continence) and 82% with leakage on a daily basis. These findings highlight the need for appropriate patient counseling and expectations regarding long-term AUS functional outcomes.
There is limited data available in the literature regarding the impact of prior radiation therapy on QoL in patients undergoing AUS placement (19). In this series, Walsh et al. evaluated 98 patients, 22 having received radiation therapy, and 92% of patients without prior radiation therapy reported that they were “very or somewhat satisfied with surgery” versus 89% in irradiated patients (P value listed as not significant, no value provided) (19). However, they did report a significant difference between those with and without radiation regarding rates of infection and erosion, urethral atrophy and resolution of incontinence after AUS placement (P<0.05). Likewise, irradiated patients had significantly higher rates of patients with “little to no improvement” and fewer patients being completely dry. At a mean follow-up of 46 months they concluded that AUS placement in an irradiated patient did not affect long-term continence or overall satisfaction.
Our findings augment the existing literature in a larger cohort, with longer follow-up. We note similar results regarding patients satisfaction, specifically, there were equivalent rates of perceived satisfaction between those with and without prior radiation therapy including willingness to undergo AUS surgery again (87% vs. 91%) and likelihood to recommend AUS surgery to a friend or family member (86% vs. 93%). We noted no significant difference between these groups likewise with regards to continence with 56% of patients reporting ≤1 PPD, 82% leakage on a daily basis, and 36% significant leakage-related bother. Differences between our results and those previously published may be secondary to disparate populations, sample size, surgical technique and the longer follow-up available in our cohort (median 100 months, as compared to 46 months).
We recognize that our study is limited by its retrospective design, and as such, we were unable to objectively assess the degree of preoperative UI. Moreover, due to the inclusion of only primary implants and use of a survey there is the potential for selection bias. Furthermore, our survey response rate was 49% among those alive, which may introduce a response bias. Further investigations utilizing a standardized, validated patient follow-up are needed to further validate our data.
Conclusions
In a large cohort of primary AUS implants, we noted a high-level of satisfaction and modest urinary control at a median follow-up of over 8 years. Importantly, we found no differences in QoL outcomes in among patients with versus without prior radiation therapy among those with the primary device in place. This information can be used when counselling potential AUS patients who have undergone previous XRT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Mayo Clinic Institutional Review Board (No. 13-001920).
References
- Coyne KS, Kvasz M, Ireland AM, et al. Urinary incontinence and its relationship to mental health and health-related quality of life in men and women in Sweden, the United Kingdom, and the United States. Eur Urol 2012;61:88-95. [Crossref] [PubMed]
- Markland AD, Goode PS, Redden DT, et al. Prevalence of urinary incontinence in men: results from the national health and nutrition examination survey. J Urol 2010;184:1022-7. [Crossref] [PubMed]
- Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 2009;302:1557-64. [Crossref] [PubMed]
- Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J Urol 1974;112:75-80. [Crossref] [PubMed]
- Kim SP, Sarmast Z, Daignault S, et al. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of Michigan. J Urol 2008;179:1912-6. [Crossref] [PubMed]
- Lai HH, Hsu EI, Teh BS, et al. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol 2007;177:1021-5. [Crossref] [PubMed]
- Linder BJ, de Cogain M, Elliott DS. Long-term device outcomes of artificial urinary sphincter reimplantation following prior explantation for erosion or infection. J Urol 2014;191:734-8. [Crossref] [PubMed]
- Linder BJ, Rivera ME, Ziegelmann MJ, et al. Long-term Outcomes Following Artificial Urinary Sphincter Placement: An Analysis of 1082 Cases at Mayo Clinic. Urology 2015;86:602-7. [Crossref] [PubMed]
- Van der Aa F, Drake MJ, Kasyan GR, et al. The artificial urinary sphincter after a quarter of a century: a critical systematic review of its use in male non-neurogenic incontinence. Eur Urol 2013;63:681-9. [Crossref] [PubMed]
- Litwiller SE, Kim KB, Fone PD, et al. Post-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. J Urol 1996;156:1975-80. [Crossref] [PubMed]
- Haab F, Trockman BA, Zimmern PE, et al. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of followup. J Urol 1997;158:435-9. [Crossref] [PubMed]
- Gousse AE, Madjar S, Lambert MM, et al. Artificial urinary sphincter for post-radical prostatectomy urinary incontinence: long-term subjective results. J Urol 2001;166:1755-8. [Crossref] [PubMed]
- Montague DK, Angermeier KW, Paolone DR. Long-term continence and patient satisfaction after artificial sphincter implantation for urinary incontinence after prostatectomy. J Urol 2001;166:547-9. [Crossref] [PubMed]
- Rivera ME, Linder BJ, Ziegelmann MJ, et al. The Impact of Prior Radiation Therapy on Artificial Urinary Sphincter Device Survival. J Urol 2016;195:1033-7. [Crossref] [PubMed]
- Aaronson DS, Elliott SP, McAninch JW. Transcorporal artificial urinary sphincter placement for incontinence in high-risk patients after treatment of prostate cancer. Urology 2008;72:825-7. [Crossref] [PubMed]
- Bates AS, Martin RM, Terry TR. Complications following artificial urinary sphincter placement after radical prostatectomy and radiotherapy: a meta-analysis. BJU Int 2015;116:623-33. [Crossref] [PubMed]
- Wang Y, Hadley HR. Experiences with the artificial urinary sphincter in the irradiated patient. J Urol 1992;147:612-3. [Crossref] [PubMed]
- Pérez LM, Webster GD. Successful outcome of artificial urinary sphincters in men with post-prostatectomy urinary incontinence despite adverse implantation features. J Urol 1992;148:1166-70. [Crossref] [PubMed]
- Walsh IK, Williams SG, Mahendra V, et al. Artificial urinary sphincter implantation in the irradiated patient: safety, efficacy and satisfaction. BJU Int 2002;89:364-8. [Crossref] [PubMed]
- Gundian JC, Barrett DM, Parulkar BG. Mayo Clinic experience with use of the AMS800 artificial urinary sphincter for urinary incontinence following radical prostatectomy. J Urol 1989;142:1459-61. [Crossref] [PubMed]
- Bukavina L, Chaparala H, Kartha G, et al. Public Restroom Habits in Patients After Artificial Urinary Sphincter Implantation. Urology 2015;86:171-5. [Crossref] [PubMed]



