
Active surveillance for non-muscle invasive bladder cancer
Introduction
Carefully selected men with low-volume low-grade (well-differentiated) prostate cancers harbor less than 6% of risk of disease progression within 10 years (1). Therefore active surveillance (AS) has emerged as a validated therapeutic approach with the goal to avoid or at least postpone invasive radical treatment in these presumed low aggressive diseases. During AS, a radical treatment is offered in case of disease progression defined as an increased level of PSA, increased tumor volume (on MRI, number of positive biopsies) or in case of upgrading of the tumor (to Gleason score 7) (2,3). Currently AS is recommended by all international guidelines for low-risk prostate cancer management (4,5). Reducing morbidity for those patients is key, and some of them will undergo curative treatment.
A recent multicenter randomized trial showed non inferiority of MRI and targeted biopsies compare to standard biopsies (6). These results are a milestone for prostate cancer patients. In fact, in near future less indolent diseases will be diagnosed and more significant cancers will be treated which is exactly the goal of promoting AS in prostate cancer.
For kidney cancer and mostly for renal cell carcinoma (RCC), AS has also been evaluated. However, in this setting there is no level I evidence study supporting AS, exception made of the subgroup of patients harboring a von Hippel-Lindau disease (7-9). AS for kidney cancer is currently designed and used mostly in the elderly patients and those with important comorbidities harboring small renal masses with a low expected growth rate and a low risk of metastases development. In these patients, RCC specific mortality is therefore challenged by other causes-related mortality (i.e., competing risks) (10,11). AS for kidney cancer consists on an imaging-based surveillance with for some patients delayed intervention in order to reduce morbidity (12). One study aimed to compare radical nephrectomy, partial nephrectomy and AS for T1a renal tumors and find no significant difference in OS and CSS with a relatively short-term follow-up of 34 months (13). Results of this study were confirmed by a multicenter registry (14) even if patients enrolled in the AS group had worse ECOG scores and comorbidities.
With the growing experience of AS among other urologic cancers, some authors have tried to apply this treatment modality to the less harmful non-muscle invasive bladder cancer (NMIBC) (i.e., LG NMIBC). The rationale of AS in bladder cancer is based on NMIBC natural history. Most of LG tumors will experience disease recurrence and very few of them (<2%) will experience disease progression to a higher grade or stage (15,16). Furthermore, too many patients will undergo multiple TURBTs, without really impacting their overall risk of disease progression, metastases development and therefore cancer-specific mortality, while increasing the direct and indirect costs of their bladder cancer management. We also have to keep in mind that most of BC patients are frail and repeated TURBTs will face higher morbidity. Indeed TURBTs known adverse effects are bladder perforation, obturator nerve reflex and anesthesia-related adverse effects. Even if TURBT allows tumor resection it does not prevent disease recurrence. We acknowledge that some of urologists manage these patients with office-based fulguration, but this technique is not achievable in all outpatient clinical settings and has not reached daily practice in most of European centers.
Most of the available studies on AS enrolled patients with a history of LG NMIBC meaning that urologists shall predict tumor grade and stage based on cystoscopy findings only. The reliability of WLC to predict is not perfect but stay acceptable especially for LG (17,18). Thus AS is practicable for high volume bladder cancer surgeons with good and right confidence in their cystoscopies findings.
BC is the most expensive cancer to treat from diagnosis to death and required lifetime surveillance and repeated cystoscopies (19). Therefore AS could be an interesting treatment modality in order to reduce the cost burden by challenging the need of repeated TURBTs and intravesical instillation.
The goal of our study was to provide a literature review of AS for LG NMIBC including inclusion criteria, modalities and oncological outcomes.
Methods
This systematic review was registered in PROSPERO, the International prospective register of systematic reviews under the number CRD42018102935. We conducted a systematic review using the following electronic bibliographic databases: MEDLINE, EMBASE between June 2018 and August 2018. The search strategy included the following terms: LG, NMIBC, AS, urothelial neoplasm. The terms were combined with the Cochrane MEDLINE filter for controlled trials of interventions. The search strategy for MEDLINE is available in the published protocol and we followed PRISMA guidelines (20). The search terms were adapted for use with other bibliographic databases in combination with database-specific filters for controlled trials, where these are available. Only studies written in English were included. Studies published between January 1990 and the date the searches are run were sought. The searches were re-run just before the final analyses and no further studies were retrieved for inclusion. We included all trials to assess inclusion criteria, modalities and oncological outcomes of AS, and will supplement these with observational studies (including cohort and case-control studies). Titles and/or abstracts of studies retrieved using the search strategy and those from additional sources were screened independently by two review authors to identify studies that potentially meet the inclusion criteria outlined above. The full text of these potentially eligible studies were retrieved and independently assessed for eligibility by two review team members. Any disagreements between them over the eligibility of particular studies were resolved through discussion with a third reviewer. Extracted information included: study setting; study population and participant demographics and baseline characteristics; details of AS; study methodology; recruitment and study completion rates; outcomes; suggested mechanisms of intervention action; information for assessment of the risk of bias. Two review authors will extract data independently; discrepancies will be identified and resolved through discussion (with a third author where necessary). Two review authors independently assessed the risk of bias in included studies by considering the following characteristics: study type, patients selection methods, modalities used for AS and reasons to end AS.
Disagreements between the review authors over the risk of bias in particular studies will be resolved by discussion, with involvement of a third review author where necessary. We anticipate that there will be limited scope for this review because of the range of different outcomes measured across the small number of existing trials. Aggregate participant data will be used and a narrative (descriptive) synthesis is planned.
Results
Overall, six studies that reached our scope of review were included cumulating 403 patients (21-26). Only 2 of them were prospective (22,23). Inclusion criteria, AS modalities and reported outcomes are shown in Table 1.
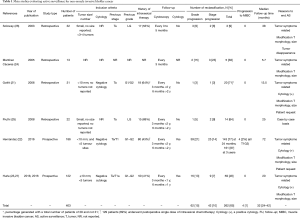
Full table
Inclusion criteria
All of the available studies enrolled patients with a known history of LG (G1 and G2) NMIBC. Some of them included only Ta patients (21,25,26) while 2 studies included also T1 patients (22,23). A negative cytology was mandatory for 3 of them (21-23). All of the studies enrolled patients with low volume and low number of tumors. Only two studies clearly specified that patients needed to harbor less than 10 mm size tumors and less than five different tumors sites (22,23). But none of them provided any technique of tumor measurement and even the need of a bladder diagram as requested by the EAU guidelines (28).
AS modalities
Most of the studies advocated a follow-up cystoscopy every 3 months during the first two years and every 6 months afterwards. Only two of them required a follow-up cytology with the same schedule as cystoscopies (22,23). Criteria for active treatment and therefore AS dropout were presence of tumor-related symptoms, a positive cytology, a modification of tumor morphology or size or finally in case of patient’s request.
AS oncological outcomes
Pooled data showed an overall 65% reclassification rate where 15% of patients were reclassified based on grade and 10% on stage with a median follow-up of 32 months (IQR, 24–42 months) (Table 1).
However, we found that reported outcomes of AS were heterogeneous among the published studies. The reclassification rates ranged from 9% to 87%. This wide range narrows when looking only on grade or stage progression. Grade-based reclassification occurred from 2% to 21% and stage-based progression varied from 2% to 14%. Only one study reported on progression to MIBC in 4 patients out of 186 (2%) (22). In this study they included patients with history of T1 tumors and had the longest median follow-up of 72 months.
Discussion
We provided oncological outcomes of AS for LG NMIBC as well as inclusion criteria and AS modalities. Nearly 2/3 of patients involved in AS for recurrent LG NMIBC will end AS, 15% patients will progress on grade and 10% on stage. One percent of patients progressed to an invasive stage of bladder cancer (at least pT2 disease). Patients who did not experience grade or stage progression during the follow-up period but experienced reclassification are not known. We hypothesized that they were reclassified either on tumor volume, tumor number or they decided to end AS which the rate cannot be calculate on these data. However one group recently published the oncological outcomes of patients who failed AS (29): of 181 AS events (167 patients), 61 (33.7%) required treatment because of positive cytology (n=10), visible haematuria (n=11), and increased tumor number (n=15), increased tumor size (n=17), or both (n=8) and none of them withdrawn from the study. Interestingly among AS failures, 20 cases did not show any malignant lesion on TURBT pathology report.
While AS is part of treatment modalities for both RCC and PCa, AS for LG NMIBC has been criticized by some authors (30) stating that TURBTs can misdiagnose some flat lesions and/or be incomplete. Therefore AS for bladder cancer is highly controversial.
According to the previously cited literature there are some shortcomings that need to be addressed. First of all, inclusion criteria were not consistent among studies. Authors’ tumors description remained vague and there is no clear definition of what a small tumor is or even how tumors are measured. Even if urologists can recognize recurrent LG-lookalike tumors, the accuracy is not perfect. Only high volume BC surgeons can be reliable enough to perform trial on AS based only on white light cystoscopy.
Second, no enhanced cystoscopy methods were used in these studies. AS surveillance methodology could have requested BLC or NBI technology for office-based cystoscopy (31). There is no doubt about the efficacy to increase BC detection and decrease recurrences with the use of enhanced cystoscopies such as NBI or PDD (28,31). The use of these technologies may lead to a better detection but they will not serve as reliable technologies to diagnose grade or stage.
Third, some authors included cytology as inclusion criteria. Cytology performs poorly in LG patients and has a low sensitivity even for HG disease (32,33). Therefore cytology is not suitable to eradicate a possible HG disease missed during previous TURBTs or follow-up cystoscopies.
Fourth, for patients with recurrent LG NMIBC the rate of single postoperative bladder instillation is low in studies reported here. According to international guidelines all of the patients included in these studies should have benefit from a postoperative instillation but nearly a 2/3 of them got at least one.
Finally, there are only two prospective trials published with relatively low level of evidence.
AS results presented here varies deeply, unlighted the need for stronger level of evidence. As a matter of a fact, some authors offered office-based tumors fulguration with acceptable oncological outcomes (28). This method of recurrent LG NMIBC is acceptable and most suitable for patients eligible to AS nowadays. All the arguments exposed here and the lack of sufficient level of evidence, AS should not be offer in daily practice and need further investigation.
Next generations of biomarkers may help patients selection or even AS modalities. For example, UROseek (34) or bladderEpicheck (Nucleix, Rehovot, Israel) (35) are currently available biomarkers and perform better than cytology currently. But if such surveillance modalities are chosen they are not reliable enough to outperformed cystoscopy.
Moreover, last decade was the stage of molecular subtypes for bladder cancer. Even if this knowledge has helped to better understand genetic pathways the correlation between grade and molecular subtypes is unclear and highly controversial. Therefore, even a submolecular classification will not help AS for LG NMIBC.
Some authors described office-based tumors fulguration (36). This method may be a better method of choice in order to reduce the need of TURBTs for patients. Going further, others described the feasibility of chemoresection of bladder tumors with an impressive 50% of complete response for small recurrent bladder tumors (37).
We acknowledge that current data is not supporting the use of AS for LG NMIBC but with others technologies emerging AS may play a role in the years to come. There is an unmet need for a RCT with well design protocols including the systematic use of intravesical agents and enhanced cystoscopy methods currently use to decrease disease recurrence, including direct and indirect cost analyses.
Conclusions
Most of patients enrolled in an AS protocol for recurrent LG NMIBC will undergo a TURBT eventually reducing the need and comorbidity of TURBTs. Many patients may be eligible to this therapeutic approach but current knowledge does not support its use in daily practice outside of a clinical trial.
Acknowledgements
G Marcq was funded by Lille University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Albertsen PC. Observational studies and the natural history of screen-detected prostate cancer. Curr Opin Urol 2015;25:232-7. [Crossref] [PubMed]
- Bruinsma SM, Roobol MJ, Carroll PR, et al. Expert consensus document: Semantics in active surveillance for men with localized prostate cancer - results of a modified Delphi consensus procedure. Nat Rev Urol 2017;14:312-22. [Crossref] [PubMed]
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272-7. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J Urol 2018;199:990-7. [Crossref] [PubMed]
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med 2018;378:1767-77. [Crossref] [PubMed]
- Campbell S, Uzzo RG, Allaf ME, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol 2017;198:520-9. [Crossref] [PubMed]
- Duffey BG, Choyke PL, Glenn G, et al. The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. J Urol 2004;172:63-5. [Crossref] [PubMed]
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913-24. [Crossref] [PubMed]
- Hollingsworth JM, Miller DC, Daignault S, et al. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer 2007;109:1763-8. [Crossref] [PubMed]
- Lane BR, Abouassaly R, Gao T, et al. Active treatment of localized renal tumors may not impact overall survival in patients aged 75 years or older. Cancer 2010;116:3119-26. [Crossref] [PubMed]
- Volpe A, Panzarella T, Rendon RA, et al. The natural history of incidentally detected small renal masses. Cancer 2004;100:738-45. [Crossref] [PubMed]
- Patel N, Cranston D, Akhtar MZ, et al. Active surveillance of small renal masses offers short-term oncological efficacy equivalent to radical and partial nephrectomy. BJU Int 2012;110:1270-5. [Crossref] [PubMed]
- Pierorazio PM, Johnson MH, Ball MW, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol 2015;68:408-15. [Crossref] [PubMed]
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466-75; discussion 475-7. [Crossref] [PubMed]
- Fernandez-Gomez J, Madero R, Solsona E, et al. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin: external validation of the EORTC risk tables. Eur Urol 2011;60:423-30. [Crossref] [PubMed]
- Herr HW. Does cystoscopy correlate with the histology of recurrent papillary tumours of the bladder? BJU Int 2001;88:683-5. [Crossref] [PubMed]
- Satoh E, Miyao N, Tachiki H, et al. Prediction of muscle invasion of bladder cancer by cystoscopy. Eur Urol 2002;41:178-81. [Crossref] [PubMed]
- Leal J, Luengo-Fernandez R, Sullivan R, et al. Economic Burden of Bladder Cancer Across the European Union. Eur Urol 2016;69:438-47. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Gofrit ON, Pode D, Lazar A, et al. Watchful waiting policy in recurrent Ta G1 bladder tumors. Eur Urol 2006;49:303-6; discussion 306-7. [Crossref] [PubMed]
- Hernández V, Llorente C, de la Peña E, et al. Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer. Urol Oncol 2016;34:165.e19-23. [Crossref] [PubMed]
- Hurle R, Lazzeri M, Vanni E, et al. Active Surveillance for Low Risk Nonmuscle Invasive Bladder Cancer: A Confirmatory and Resource Consumption Study from the BIAS Project. J Urol 2018;199:401-6. [Crossref] [PubMed]
- Martínez Cáceres P, Hidalgo Arroyo JG, Chéchile Toniolo GE. Is it necessary to always treat the relapses of superficial bladder tumour at the moment of diagnosis? Preliminary communication. Actas Urol Esp 2005;29:567-71. [PubMed]
- Pruthi RS, Baldwin N, Bhalani V, et al. Conservative management of low risk superficial bladder tumors. J Urol 2008;179:87-90; discussion 90. [Crossref] [PubMed]
- Soloway MS, Bruck DS, Kim SS. Expectant management of small, recurrent, noninvasive papillary bladder tumors. J Urol 2003;170:438-41. [Crossref] [PubMed]
- Hurle R, Pasini L, Lazzeri M, et al. Active surveillance for low-risk non-muscle-invasive bladder cancer: mid-term results from the Bladder cancer Italian Active Surveillance (BIAS) project. BJU Int 2016;118:935-9. [Crossref] [PubMed]
- Babjuk M, Bohle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Hurle R, Colombo P, Lazzeri M, et al. Pathological Outcomes for Patients Who Failed To Remain Under Active Surveillance for Low-risk Non-muscle-invasive Bladder Cancer: Update and Results from the Bladder Cancer Italian Active Surveillance Project. Eur Urol Oncol 2018;1:437-42. [Crossref]
- Cotte J, Rouprêt M. Re: Long-term Oncological Outcomes of an Active Surveillance Program in Recurrent Low Grade Ta Bladder Cancer. Eur Urol 2017;72:152. [Crossref] [PubMed]
- Chang TC, Marcq G, Kiss B, et al. Image-Guided Transurethral Resection of Bladder Tumors - Current Practice and Future Outlooks. Bladder Cancer 2017;3:149-59. [Crossref] [PubMed]
- Têtu B. Diagnosis of urothelial carcinoma from urine. Mod Pathol 2009;22 Suppl 2:S53-59. [Crossref] [PubMed]
- Yafi FA, Brimo F, Steinberg J, et al. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol 2015;33:66.e25-31. [Crossref] [PubMed]
- Springer SU, Chen CH, Rodriguez Pena MDC, et al. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. Elife 2018.7.
- Witjes JA, Morote J, Cornel EB, et al. Performance of the Bladder EpiCheckTM Methylation Test for Patients Under Surveillance for Non-muscle-invasive Bladder Cancer: Results of a Multicenter, Prospective, Blinded Clinical Trial. Eur Urol Oncol 2018;1:307-13. [Crossref]
- Al Hussein Al Awamlh B, Lee R, Chughtai B, et al. A cost-effectiveness analysis of management of low-risk non-muscle-invasive bladder cancer using office-based fulguration. Urology 2015;85:381-6. [Crossref] [PubMed]
- Decaestecker K, Lumen N, Ringoir A, et al. Ablative Intravesical Chemotherapy for Small Recurrent Non-Muscle-Invasive Bladder Cancer: A Prospective Study. Urol Int 2016;96:14-9. [Crossref] [PubMed]


