
Sexual dysfunction and infertility in the male spina bifida patient
Introduction
Spina bifida is a congenital form of neural tube defect in which the fetus’ spinal cord and the surrounding sac fail to develop properly in-utero, resulting in potential lifelong neurological deficits. Per the Centers for Disease Control and Prevention, spina bifida affects 1,500 newborns a year with the highest prevalence of 3.8 per 10,000 live births in Hispanic mothers (1). A separate study by Shin et al. estimated that the prevalence of spina bifida in the United States is 3.1 per 10,000 or approximately 25,000 children and adolescents aged 0 to 19 years in 2002 (2).
While urologic manifestations of spina bifida such as neurogenic bladder have been extensively examined, few studies have focused on sexual dysfunction and infertility in this patient population. Recent medical advancements have contributed to the increasing life expectancy of spina bifida patients. Unlike the 1960s and 1970s, when the 5-year survival rate for infants with spina bifida was only 37%, nearly 50% of spina bifida patients surveyed in the past decade have survived to at least age 35 (3,4). Similarly, another study by Shin et al. estimated that from 1983 to 2003, over 85% of spina bifida patients in the United States survived to at least 20 years of age (5). In the 1960s, the combined inventions of artificial valves and silicone lead to improvements in ventriculoperitoneal shunting. In addition, the development of aggressive neonatal care and preventative bladder programs in the 1970s further improved survival (6). With more spina bifida patients surviving into adolescence and beyond, concerns regarding sexual function and fertility must be addressed.
Various genetic and pathologic defects can contribute to male infertility. In its simplest form, infertility is caused by defects in spermatogenesis and/or failure of sperm transport. Defined as a couple’s failure to achieve clinical pregnancy following more than 12 months of unprotected sexual intercourse, it is estimated that more than 72.4 million, or 9% of couples worldwide, suffer from infertility (7). Given these statistics on infertility in the general population, it is reasonable to expect that the rate of infertility in the spina bifida population would be at least equal if not much higher. Unfortunately, no study exists to directly estimate the prevalence of infertility among male spina bifida patients. Studies of sexual function in this population have indirectly yielded variable success rates of fertility based on small sample sizes. In a study by Cass et al. involving 12 spina bifida men, only one of the six men who engaged in sexual intercourse achieved fatherhood (8). In a separate study by Bomalaski et al. examining 18 men with spina bifida, 13% engaged in sexual intercourse, but only 1 patient (6%) achieved fatherhood (9). Likewise, a study by Decter et al. involving 57 men with myelodysplasia found that out of the 11 patients (19%) who attempted to achieve fatherhood, 8 (14%) were successful (10). Another study by Lassmann et al. showed that only two out of a cohort of 42 spina bifida men attempted to achieve paternity but both failed (11). A survey study of 52 spina bifida men by Cardenas et al. found that rates of paternity may be related to the presence of hydrocephalus associated with spina bifida as 14.8% of men without hydrocephalus reported having children while no men with hydrocephalus claimed to have children (12). These statistics shed light on the fact that infertility is prevalent among spina bifida men, yet few large-scale studies have examined its cause and treatment. In addition, all studies using surveys to assess health in the spina bifida population suffer from selection bias and thus may not be accurate.
Numerous studies have suggested that most spina bifida patients have intact libido, and a significant proportion of them wish to have children. Between 80–100% of spina bifida men had normal sexual desires (13-16). From video narratives on the lives of 14 spina bifida patients in one study, all of the participants expressed a desire for parenthood (17). Obstacles to achieving fatherhood among spina bifida men included erectile dysfunction, anejaculation, poor sperm quality, and physical or social limitations that interfere with sex (16). In this review, we examine the major causes of infertility experienced by spina bifida men, the severity of sexual dysfunction and infertility based on the level of neurological lesion, and the treatment strategies available to this patient population. We also explore the possibility of a genetic link between spina bifida and infertility and the need for improved patient education and research in this vulnerable patient population.
Causes of infertility in spina bifida men
Spermatogenic defects
Causes of infertility can be broadly classified as defects in spermatogenesis and/or sperm transport. Spermatogenic defects often lead to poor sperm quality and may be related to a marked decrease in spermatogenic cells as seen in testicular biopsies. Hultling et al. examined semen analyses obtained from either unassisted or assisted ejaculates of nine spina bifida men 22 to 39 years old and found that only 5 of 9 men had enough sperm in their ejaculates for fertilization via assisted reproductive technology (ART); four men produced no spermatozoa in their ejaculates (18). Four out of the 5 men with enough sperm for fertilization had sperm counts that were described as “extremely low”, and only 1 of the 5 men had more than 10,000 live sperm on semen analysis. Among the five men with enough isolated sperm, very few showed normal morphology. These results, albeit from a small study, indicate that neurological deficits associated with spina bifida may exert a downstream effect on spermatogenesis resulting in both oligo- and/or azoospermia with abnormal sperm morphology.
This finding may be explained by the lack of testicular neurological innervation that is observed in men with spina bifida. This may potentially alter normal testis development, which is supported by testicular biopsies from infertile men with spina bifida. A preliminary study by Reilly and Oates on ten spina bifida males with higher spinal cord lesions causing impotence revealed that all men in the study were azoospermic following assisted ejaculation, and all ten testicular biopsies revealed Sertoli cell only histology (19). Furthermore, a comparison of sperm motility and morphology between men with spina bifida and men who suffered spinal cord injuries showed that men with spina bifida had much poorer sperm quality, further suggesting that normal gonadal development and spermatogenesis are dependent on adequate gonadal innervation from an early age (18,20). Cryptorchidism, the failure of a testis to fully descend into the scrotum during development, occurs at a higher rate in spina bifida males. Incidence has been reported between 15% to 58%, whereas it occurs only at 1–3% in full term males without spina bifida (21-24). Biopsies at time of orchidopexy in these patients showed germ cell aplasia, delayed maturation, and severely reduced quantity of germ cells in some (25).
Failure of sperm transport
While proper spermatogenesis is a key component of fertility, delivery of sperm into the female reproductive tract is equally important for natural conception. Major impediments to this delivery of sperm include issues surrounding erectile function and ejaculation.
Erectile dysfunction
The prevalence of erectile dysfunction in this patient population varies from 12% to 75%. Of note, the prevalence in young, neurotypical men is only 15% in men younger than 45 years (26-28). When stratified by level of neurological lesion, approximately 36% of men with lesions at or below T10 and 86% of men with lesions above T10 suffered from erectile dysfunction, suggesting that higher lesions correlate with worse erectile function (27,29,30). Diamond et al. found that out of 52 post-pubertal males with spina bifida, 70% could have erections (29). Other smaller studies have observed different rates of erectile function in men with spina bifida. For instance, out of 12 men in a study by Cass et al., 92% (11 of 12) of spina bifida men had erections (8). In a study by Bomalaski et al., 56% (9 of 16) of spina bifida men reported normal erections (9). In contrast, Shurtleff et al. observed only one of 28 spina bifida men had normal erections (31). These data illustrate how the exact prevalence of erectile dysfunction in spina bifida men has yet to be clearly defined. Lee et al. attempted to evaluate the severity of erectile dysfunction in 17 spina bifida men in their 20s using the Sexual Health Inventory for Men (SHIM) questionnaire and found that 59% (10 of 17) suffered from severe erectile dysfunction; 12% (2 of 17) had moderate erectile dysfunction, 18% (3 of 17) had mild to moderate erectile dysfunction, 6% (1 of 17) had mild erectile dysfunction, and 6% (1 of 17) had no dysfunction (32).
In a study by Decter et al. of 57 spina bifida men over 18 years old, 72% had erections and 35% successfully engaged in sexual intercourse (10). In a separate questionnaire study of 22 spina bifida men with a mean age of 24 years who were not stratified by neurological lesion level, 95% of the men claimed to have achieved erections by visual stimulation only, while 86% achieved erections by tactile stimulation (15). However, when asked about their satisfaction with erections, only 27% of those who achieved erections were satisfied with their penile rigidity, suggesting that the majority of perceived erections may be inadequate for intercourse and thus sperm delivery.
Besides neurological lesion level, other comorbidities associated with spina bifida may also contribute to the degree of erectile dysfunction. Decter et al. found that the presence of a ventriculo-peritoneal (VP) shunt was associated with more erectile dysfunction. Out of 57 spina bifida men, only 61% of those with VP shunts experienced erections while 88% of those without VP shunts reported having erections (10). These results may be explained by the fact that spina bifida patients that require VP shunts have more severe hydrocephalus and extensive neurological deficits.
Ejaculatory dysfunction
Similar to erections, male ejaculation is under specific neurological control. As a result, many patients with spina bifida suffer from ejaculatory dysfunction including anejaculation and retrograde ejaculation. Studies have estimated that up to 75% of spina bifida men are capable of ejaculation, with most experiencing this in a weak, drip-like emission pattern (8,10,15). These estimates are confounded by the fact that approximately 20% of spina bifida men fail to perceive ejaculation due to loss of sensory innervation (10). Among 41 spina bifida men who experienced penile erections in study by Decter et al., 66% [27] experienced ejaculation while 3 of the 16 men without erections ejaculated, with an overall ejaculation success rate of 53% (30 out of 57 men) (10). Similar to erectile dysfunction, the presence of a VP shunt was associated with more ejaculatory dysfunction as 70% (23 out of 33 patients) of spina bifida men with VP shunts suffered from anejaculation while 83% (20 out of 24 patients) of men without VP shunts ejaculated normally (10). Additionally, spina bifida men with tethered cord may also suffer from retrograde ejaculation into the bladder due to bladder neck incompetence even though their semen emission mechanism may be intact (33).
Psychosocial and physical restrictions on sexual intercourse
Despite having intact neurological control over erection and ejaculation, other physical limitations and social barriers may hinder sexual intercourse and contribute to infertility in spina bifida men. In Decter et al.’s study, all eight men who achieved paternity were ambulatory (10). Through a video narrative study of seven spina bifida males aged 13 to 28 years old, all subjects viewed their physical conditions (i.e., crutches, leg braces, and wheel-chair bound) as a barrier to maintaining romantic relationships (17). In a cross-sectional interview study from the Netherlands, more than 60% of wheelchair-dependent spina bifida men perceived their impaired ambulatory status as an obstacle to starting a relationship (34). However, when Lee et al. correlated scores on SHIM, a validated measure of sexual dysfunction, from 17 spina bifida men with their ambulatory status, ranging from completely bed bound to ambulating without assistance, there was no correlation (P=0.15) (32). Additionally, there was no correlation between ambulatory status and erectile dysfunction as measured by the SHIM (P=0.26) (32). Lack of self-confidence and dependence on others for activities of daily living were mentioned as impediments to exploring romantic relationships (17). A study by Game et al. found that spina bifida men who engaged in sexual intercourse tended to be older (31.9±5.7 years vs. 27.7±5.5 years, P=0.027) and no longer living with parents (27). Furthermore, Gatti et al. found that spina bifida patients more than 26 years old were 2.5 times more likely to have a partner compared to those between 18 and 25 years old, and that the odds ratio for sexual activity increased by 1.1 for each year increase in age (35).
Urinary incontinence is another source of embarrassment that may contribute to social and performance anxiety when it comes to sexual interactions. Cardenas et al. reported that patients with urinary incontinence were 0.77 times less likely to be sexually active (12). A cross-sectional interview study conducted by Verhoef et al. revealed that close to 50% of incontinent spina bifida men perceived their lack of sphincter control as an obstacle to starting a relationship (34). Furthermore, continent patients were 2 times more likely to have a partner and 2.2 times more likely to have engaged in sexual contact compared to incontinent counterparts (34). A separate study conducted by Gatti et al. found that continent spina bifida patients were 3.5 times more likely to have a partner and 2.4 times more likely to be sexually active compared to incontinent patients (35). Interestingly, sexual function in spina bifida males did not appear to be related to social and economic status (27). While these data show that the presence of incontinence and physical disability may discourage sexual activity, Lassmann et al. found that sexual activity was not related to the severity of incontinence or extent of ambulatory status as a measure of physical disability and dependence (11). This underscores the significant effects of disability and incontinence on sexual function as the mere presence of these comorbidities, regardless of their severity, can negatively impact a spina bifida male’s ability to engage in intercourse and therefore reach his fertility potential (Table 1).

Full table
Relationship between level of neurological lesion, hydrocephalus, and infertility
Level of neurological lesion
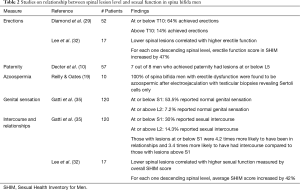
In patients with spina bifida, level of spinal cord lesion determines the degree of deficits, sexual function and fertility. Therefore, it is expected that the degree of sexual dysfunction may be related to the level of lesion and other comorbidities. Numerous studies have associated lower spinal cord lesions with less severe neurological deficits and more intact sexual functions. Out of 52 post-pubertal males with spina bifida, Diamond et al. found that 64% of men with lesions at T10 or lower had erections while only 14% of men with lesions above T10 obtained erections (29). Additionally, the likelihood of paternity was observed to be higher in those with lower lesions as 7 out of 8 men who achieved paternity in Decter et al.’s study had L5 or sacral lesions (10). Studies have also suggested that infertility in spina bifida men with higher lesions may be attributed to more than just erectile or ejaculatory dysfunction as these men were also at an increased risk of azoospermia. In a preliminary study by Reilly and Oates that included ten spina bifida males with erectile dysfunction, all 10 were found to be azoospermic following electroejaculation, and their testicular biopsies revealed Sertoli cell only histology (19). However, Hultling et al. found no association between level of spinal cord lesion and semen quality based on an examination of nine spina bifida men 22 to 39 years old (18). In a study of 120 spina bifida men by Gatti et al., those with lesions at or below S1 reported more normal genital sensation (53.5% vs. 7.2% with intact sensation, P<0.05) and more frequent intercourse (30% vs. 14.3% with sexual contact, P<0.05) compared to those with lesions at or above L2 (35). It is important to note that the decreased frequency of sexual contact seen in men with higher lesions was found to be independent of their ability to form relationships (35). However, patients with lesions at or below S1 were 4.2 times more likely to have been in a relationship and 3.4 times more likely to have had experience with intercourse compared to those with higher lesions (35). Similarly, Lassmann et al. found that significantly more patients with higher lesions at thoracic and lumbar levels were not sexually active (11). Using the SHIM to quantitatively evaluate overall sexual function, Lee et al. found that men with lower spinal lesions had higher sexual function as reflected by overall SHIM score (P=0.02), increased satisfaction (P=0.046), and higher erectile function score (P=0.02) (32). In fact, for each descending lesion level down the spinal column, the average SHIM score increased by 42% while the average erectile function score increased by 47% (32).
Game et al. observed that erectile dysfunction was more common in those with impaired sacral reflexes, as seen on electromyography, associated with sacral nerve root lesions (27). Similarly, Diamond et al. noted that in cases where the lesion occurred above the sympathetic outflow (T10), those with anocutaneous (sacral) reflexes were more likely to have erections compared to those without this reflex (29). In a study of 12 spina bifida males, Cass et al. found that all males with intact sacral reflexes and urinary continence had potency (8). In those without sacral reflexes, 64% of men with lesions below T10 were potent while rates of potency decreased to only 14% for those with higher lesions above T10 (8). These studies convincingly show that spina bifida men with lower lesions and intact sacral reflexes have the best chance at preserved sexual function and fertility potential. This data is summarized in Table 2.
Full table
Hydrocephalus
Hydrocephalus affects more than 85% of infants with spina bifida and compromises development, learning and cognitive function (36). Additionally, the presence of hydrocephalus is associated with worse sexual dysfunction and infertility. Verhoef et al. reported that patients with hydrocephalus were less likely to have a relationship, engage in sexual activity, and have normal sexual function compared to their counterparts without hydrocephalus (34). Compared to those with hydrocephalus, patients without hydrocephalus were 3.2 times more likely to have a partner and 9 times more likely to have had sexual contact (34). Males with hydrocephalus reported significantly more issues with sexual excitement, erection, orgasm, and ejaculation than those without hydrocephalus (34). In Decter et al.’s series, all eight men who achieved paternity did not have VP shunts at time of conception (10). Among the 33 patients with VP shunts, 61% obtained erections and 30% ejaculated (10). On the other hand, out of 24 patients without VP shunts, 88% obtained erections and 83% ejaculated (10). In a questionnaire study by Cardenas et al. on 121 spina bifida patients, no men with hydrocephalus fathered children while 15% of men without hydrocephalus achieved paternity (12). In a questionnaire study of 76 spina bifida patients, 90% of those who were not sexually active had VP shunts (11).
Treatment strategies
Infertility treatments for men with spina bifida include pharmacological and surgical interventions each aimed at targeting a specific component of infertility. Here, we review the treatment options for spina bifida men whose infertility may have resulted from solely testicular dysfunction or a combination of erectile dysfunction, ejaculatory dysfunction, and decreased spermatogenesis.
Erectile dysfunction
Men with spina bifida and erectile dysfunction typically have intact penile architecture and vasculature. Up to 75% of spina bifida men suffer from erectile dysfunction, primarily the ability to maintain erections (27). The PDE5i sildenafil can improve erections in this patient population. In a randomized placebo-controlled study of 15 spina bifida men with erectile dysfunction, sildenafil 25 and 50 mg taken one hour before planned sexual activity led to significant improvements in erectile function sub-scores of the International Index of Erectile Function (IIEF) by 50% and 88%, respectively, when compared to a placebo (37,38). Furthermore, of the 80% of men who responded to this therapy, mean erection duration while on 25 and 50 mg of the drug increased by 192% and 266%, respectively (37). The mean frequency of erections while on 25 and 50 mg of the drug also improved by 61% and 96%, respectively (37). With the improvements in erectile function while on sildenafil, the authors also observed increased sexual confidence in these men. Other PDE5is have not been studied specifically in this population, but no known contraindication exists to any PDE5i in this spina bifida patients. However, it is important to note that improving erectile dysfunction with pharmacological agents may not solve infertility as impotence in spina bifida men may also be associated with azoospermia (19).
Ejaculatory dysfunction, including retrograde ejaculation
Ejaculatory dysfunction is a significant cause of infertility among spina bifida men. In instances of complete neurogenic failure of seminal emission, rectal probe electroejaculation under general anesthesia appears to be an effective method of collecting semen from which viable sperm can be isolated (33). In a preliminary study by Reilly and Oates involving ten spina bifida men with ejaculatory dysfunction, rectal probe electroejaculation produced ejaculates in all the patients (19). In a study by Hultling et al. evaluating the success of electroejaculation, the authors found that this method allowed 4 out of 7 men who were not able to achieve unassisted ejaculation to produce enough spermatozoa for assisted fertilization (18). In addition, surgical retrieval of sperm from the male genitourinary tract, including the bladder, vas deferens, epididymis, and testicle, is also a valid option to collect sperm for assisted fertilization in those men suffering from retrograde ejaculation (33).
Additionally, surgical interventions have been proposed to increase penile sensation aimed at facilitating erection and ejaculation in spina bifida patients. In cases of pudendal nerve dysfunction, Jacobs et al. performed ilioinguinal-to-dorsal-penile neurorrhaphy on two men with penile anesthesia and found that both men reported improved sensation to the penis and glans during intercourse at 24 months post-operation (39). A similar procedure performed by Overgoor et al. on 30 spinal lesion patients with no baseline penile sensation, including 18 with spina bifida, found that 24 patients gained glans penis sensation following the TOMAX (TO MAX-imize sensation, sexuality and quality of life) procedure (40,41). Compared to their pre-operative evaluations, these men reported better overall sexual function (P=0.022) and satisfaction (P=0.004) after the TOMAX procedure (40).
ART
ART involves manipulation of gametes or embryos to maximize the chances of successful fertilization or implantation. Today, various methods of ARTs are available to treat infertility, and they all require some level of baseline spermatogenesis, as sperm must be collected and isolated for fertilization from either the ejaculate or anywhere along the reproductive tract. With the advent of in vitro fertilization (IVF) in the 1970s, an embryo could be derived from two gametes in a controlled laboratory setting. Furthermore, intrauterine insemination (IUI) is another form of ART that has the potential to increase successful fertilization rates (42).
The development of intracytoplasmic sperm injection (ICSI) in 1992 dramatically reduced the number and even quality of viable sperm required for successful fertilization (33). Though outcomes after ART yield live births for males with significant spermatogenic defects, their outcomes specifically in spina bifida fathers have not been reported. In addition, ICSI is associated with increased incidence of congenital defects, multiple gestations leading to low birth weight and preterm infants, sex chromosome defects, and various epigenetic syndromes (43). Children of fathers with spina bifida are also at increased risk of neural tube defects, making prenatal care imperative (16). Whether ART specifically in the spina bifida male has any effect on the offspring is unknown.
The genetics of spina bifida and infertility
While certain genetic predispositions for spina bifida and genes causing infertility are known, genes associated with both spina bifida and male infertility have yet to be identified. Prior to the widespread use of prenatal folic acid, the incidence of neural tube defects among children born to men with spina bifida was 3.7%, significantly higher than the incidence in the general population (16). In fact, the recurrence of neural tube defects in newborns increases from 1–5% if one parent or sibling has spina bifida to 15% if both parents have spina bifida (36). Polymorphisms in homocysteine metabolism involving the enzyme methylenetetrahydrofolate reductase (MTHFR 677C→T) have been implicated in the heritability of neural tube defects and male infertility (44,45). The reduced activity of MTHFR leads to low plasma folate, a vitamin essential to the development of fetal nervous system which controls sexual function, such as erection, ejaculation, testis development, and spermatogenesis, later in life (18,20,45).
In mouse studies, knockouts in the apoB gene, a component of lipoprotein particles, have been implicated in both neural tube defects and male infertility (46). In humans, significant differences in the genotype distributions of apoB signal peptide polymorphism were seen in men with oligoasthenoteratozoospermia and fertile men (47). Other candidate genes implicated in male infertility also affect lipid metabolism, such as apoE receptor-2 gene (apoER2), acid sphingomyelinase gene (ASM), and ATP-binding cassette transporter 1 gene (ABCA1) (48-50). Despite the many candidate genes that have been linked to either infertility or spina bifida, none have been definitively found to cause spina bifida and its associated testis failure. Perhaps the least studied aspect of infertility in spina bifida men, the genetic basis of this association represents a significant direction for future research and an exciting avenue for future treatment of infertility among this unique patient population.
Summary and conclusions
Spina bifida is a congenital defect in neural tube closure with potential to affect various aspects of life, including sexual function and fertility. Infertility in this population can be caused by problems of sperm transport or defects in spermatogenesis. Reproductive potential is favored by lower lesions with intact sacral reflexes and absence of hydrocephalus as a comorbidity. Treatment strategies vary depending on the cause of infertility. Sildenafil citrate is an effective pharmacotherapy for maintaining erections while assisted reproductive technologies, such as sperm retrieval followed by ICSI, represent promising treatment options for infertility in those with ejaculatory dysfunction or decreased spermatogenesis. Genetics of infertility as it relates to spina bifida is still an area of growing research and represent an avenue for future studies. Finally, a large majority of adolescents and young adults suffering from spina bifida lack the essential knowledge regarding how spina bifida may affect their sexual health and reproductive potential. Failure of healthcare professionals to address this important matter poses significant challenges to men with spina bifida who wish to achieve fatherhood.
Acknowledgements
Funding: AW Pastuszak is a National Institutes of Health K08 Scholar supported by a Mentored Career Development Award (K08DK115835-01) from the National Institute of Diabetes and Digestive and Kidney Diseases. This work is also supported in part through a Urology Care Foundation Rising Stars in Urology Award (to AW Pastuszak), and NIH grants K12 DK0083014, the Multidisciplinary K12Urologic Research (KURe) Career Development Program awarded to DJL (N Thirumavalavan is a K12 Scholar) from the National Institute of Kidney and Digestive Diseases to Dolores J Lamb.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclosure: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Spina Bifida Data and Statistics. Centers for Disease Control and Prevention. 2016. Available online: https://www.cdc.gov/ncbddd/spinabifida/data.html. Accessed June 4 2017.
- Shin M, Besser LM, Siffel C, et al. Prevalence of spina bifida among children and adolescents in 10 regions in the United States. Pediatrics 2010;126:274-9. [Crossref] [PubMed]
- Althouse R, Wald N. Survival and handicap of infants with spina bifida. Arch Dis Child 1980;55:845-50. [Crossref] [PubMed]
- Woodhouse CR. Myelomeningocele: neglected aspects. Pediatr Nephrol 2008;23:1223-31. [Crossref] [PubMed]
- Shin M, Kucik JE, Siffel C, et al. Improved survival among children with spina bifida in the United States. J Pediatr 2012;161:1132-7. [Crossref] [PubMed]
- Webb TS. Optimizing health care for adults with spina bifida. Dev Disabil Res Rev 2010;16:76-81. [Crossref] [PubMed]
- Boivin J, Bunting L, Collins JA, et al. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 2007;22:1506-12. [Crossref] [PubMed]
- Cass AS, Bloom BA, Luxenberg M. Sexual function in adults with myelomeningocele. J Urol 1986;136:425-6. [Crossref] [PubMed]
- Bomalaski MD, Teague JL, Brooks B. The long-term impact of urological management on the quality of life of children with spina bifida. J Urol 1995;154:778-81. [Crossref] [PubMed]
- Decter RM, Furness PD 3rd, Nguyen TA, et al. Reproductive understanding, sexual functioning and testosterone levels in men with spina bifida. J Urol 1997;157:1466-8. [Crossref] [PubMed]
- Lassmann J, Garibay Gonzalez F, Melchionni JB, et al. Sexual function in adult patients with spina bifida and its impact on quality of life. J Urol 2007;178:1611-4. [Crossref] [PubMed]
- Cardenas DD, Topolski TD, White CJ, et al. Sexual functioning in adolescents and young adults with spina bifida. Arch Phys Med Rehabil 2008;89:31-5. [Crossref] [PubMed]
- Sandler AD, Worley G, Leroy EC, et al. Sexual function and erection capability among young men with spina bifida. Dev Med Child Neurol 1996;38:823-9. [Crossref] [PubMed]
- Dorner S. Sexual interest and activity in adolescents with spina bifida. J Child Psychol Psychiatry 1977;18:229-37. [Crossref] [PubMed]
- Hirayama A, Yamada K, Tanaka Y, et al. Evaluation of sexual function in adults with myelomeningocele. Hinyokika Kiyo 1995;41:985-9. [PubMed]
- Bong GW, Rovner ES. Sexual health in adult men with spina bifida. ScientificWorldJournal 2007;7:1466-9. [Crossref] [PubMed]
- Akre C, Light A, Sherman L, et al. What young people with spina bifida want to know about sex and are not being told. Child Care Health Dev 2015;41:963-9. [Crossref] [PubMed]
- Hultling C, Levi R, Amark SP, et al. Semen retrieval and analysis in men with myelomeningocele. Dev Med Child Neurol 2000;42:681-4. [Crossref] [PubMed]
- Reilly JM, Oates RD. Preliminary investigation of the potential fertility status of postpubertal males with myelodysplasia. J Urol 1992;151:114-9.
- Brackett NL, Lynne CM, Weizman MS, et al. Endocrine profiles and semen quality of spinal cord injured men. J Urol 1994;151:114-9. [Crossref] [PubMed]
- Kolon TF, Herndon CD, Baker LA, et al. Evaluation and treatment of cryptorchidism: AUA guideline. J Urol 2014;192:337-45. [Crossref] [PubMed]
- Kropp KA, Voeller KK. Cryptorchidism in meningomyelocele. J Pediatr 1981;99:110-3. [Crossref] [PubMed]
- Hutson JM, Beasley SW, Bryan AD. Cryptorchidism in spina bifida and spinal cord transection: a clue to the mechanism of transinguinal descent of the testis. J Pediatr Surg 1988;23:275-7. [Crossref] [PubMed]
- Ferrara P, Rossodivita A, Ruggiero A, et al. Cryptorchidism associated with meningomyelocele. J Paediatr Child Health 1998;34:44-6. [Crossref] [PubMed]
- Patel RP, Kolon TF, Huff DS, et al. Cryptorchid testis histopathology in myelomeningocele patients. J Pediatr Urol 2008;4:434-7. [Crossref] [PubMed]
- Sawyer SM, Roberts KV. Sexual and reproductive health in young people with spina bifida. Dev Med Child Neurol 1999;41:671-5. [Crossref] [PubMed]
- Game X, Moscovici J, Game L, et al. Evaluation of sexual function in young men with spina bifida and myelomeningocele using the International Index of Erectile Function. Urology 2006;67:566-70. [Crossref] [PubMed]
- Costa P, Avances C, Wagner L. Erectile dysfunction: knowledge, wishes and attitudes. Results of a French study of 5.099 men aged 17 to 70. Prog Urol 2003;13:85-91. [PubMed]
- Diamond DA, Rickwood AM, Thomas DG. Penile erections in myelomeningocele patients. Br J Urol 1986;58:434-5. [Crossref] [PubMed]
- Shridharani AN, Brant WO. The treatment of erectile dysfunction in patients with neurogenic disease. Transl Androl Urol 2016;5:88-101. [PubMed]
- Shurtleff DB, Hayden PW, Chapman WH, et al. Myelodysplasia. Problems of long-term survival and social function. West J Med 1975;122:199-205. [PubMed]
- Lee NG, Andrews E, Rosoklija I, et al. The effect of spinal cord level on sexual function in the spina bifida population. J Pediatr Urol 2015;11:142.e1-6. [Crossref] [PubMed]
- Hsieh MH, Hollander A, Lamb DJ, et al. The genetic and phenotypic basis of infertility in men with pediatric urologic disorders. Urology 2010;76:25-31. [Crossref] [PubMed]
- Verhoef M, Barf HA, Vroege JA, et al. Sex education, relationships, and sexuality in young adults with spina bifida. Arch Phys Med Rehabil 2005;86:979-87. [Crossref] [PubMed]
- Gatti C, Del Rossi C, Ferrari A, et al. Predictors of successful sexual partnering of adults with spina bifida. J Urol 2009;182:1911-6. [Crossref] [PubMed]
- Visconti D, Noia G, Triarico S, et al. Sexuality, pre-conception counseling and urological management of pregnancy for young women with spina bifida. Eur J Obstet Gynecol Reprod Biol 2012;163:129-33. [Crossref] [PubMed]
- Palmer JS, Kaplan WE, Firlit CF. Erectile dysfunction in patients with spina bifida is a treatable condition. J Urol 2000;164:958-61. [Crossref] [PubMed]
- Palmer JS, Kaplan WE, Firlit CF. Erectile dysfunction in spina bifida is treatable. Lancet 1999;354:125-6. [Crossref] [PubMed]
- Jacobs MA, Avellino AM, Shurtleff D, et al. Reinnervating the penis in spina bifida patients in the United States: ilioinguinal-to-dorsal-penile neurorrhaphy in two cases. J Sex Med 2013;10:2593-7. [Crossref] [PubMed]
- Overgoor ML, de Jong TP, Cohen-Kettenis PT, et al. Increased sexual health after restored genital sensation in male patients with spina bifida or a spinal cord injury: the TOMAX procedure. J Urol 2013;189:626-32. [Crossref] [PubMed]
- Overgoor ML, Braakhekke JP, Kon M, et al. Restoring penis sensation in patients with low spinal cord lesions: the role of the remaining function of the dorsal nerve in a unilateral or bilateral TOMAX procedure. Neurourol Urodyn 2015;34:343-8. [Crossref] [PubMed]
- Shibahara H, Toji H, Shigeta M, et al. Successful pregnancies in a case of retrograde ejaculation associated with tethered spinal cord syndrome. J Assist Reprod Genet 2000;17:233-7. [Crossref] [PubMed]
- Alukal JP, Lamb DJ. Intracytoplasmic sperm injection (ICSI)--what are the risks? Urol Clin North Am 2008;35:277-88. ix-x. [Crossref] [PubMed]
- van der Put NM, Steegers-Theunissen RP, Frosst P, et al. Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet 1995;346:1070-1. [Crossref] [PubMed]
- Tuttelmann F, Rajpert-De Meyts E, Nieschlag E, et al. Gene polymorphisms and male infertility--a meta-analysis and literature review. Reprod Biomed Online 2007;15:643-58. [Crossref] [PubMed]
- Huang LS, Voyiaziakis E, Markenson DF, et al. apo B gene knockout in mice results in embryonic lethality in homozygotes and neural tube defects, male infertility, and reduced HDL cholesterol ester and apo A-I transport rates in heterozygotes. J Clin Invest 1995;96:2152-61. [Crossref] [PubMed]
- Peterlin B, Zorn B, Volk M, et al. Association between the apolipoprotein B signal peptide gene insertion/deletion polymorphism and male infertility. Mol Hum Reprod 2006;12:777-9. [Crossref] [PubMed]
- Andersen OM, Yeung CH, Vorum H, et al. Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem 2003;278:23989-95. [Crossref] [PubMed]
- Butler A, He X, Gordon RE, et al. Reproductive pathology and sperm physiology in acid sphingomyelinase-deficient mice. Am J Pathol 2002;161:1061-75. [Crossref] [PubMed]
- Selva DM, Hirsch-Reinshagen V, Burgess B, et al. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J Lipid Res 2004;45:1040-50. [Crossref] [PubMed]


