Feasibility of using a novel non-invasive ambulatory tibial nerve stimulation device for the home-based treatment of overactive bladder symptoms
Introduction
Tibial nerve stimulation (TNS) has emerged as an effective alternative for the management of the overactive bladder (OAB). Consisting of intermittent electrical stimulation of the tibial nerve, the efficacy of this treatment has been demonstrated in several studies, including a multicentric, double-blind, randomised sham-controlled study of patients with idiopathic OAB (1,2). More recently, the treatment has appeared in National Institute for Health and Care Excellence (NICE) guidance as a second-line option for the management of female urinary incontinence (3,4).
In clinical practice, the tibial nerve is most often stimulated percutaneously (PTNS) by inserting a needle, however this entails regular visits to an outpatient clinic over 8 to 12 weeks. Being resource intense in terms of time, financial and staff commitments, this treatment is often not a feasible option from the point of view of health care delivery. Moreover, the treatment may not be an option for patients requiring to travel long distances, those having disabilities requiring special transport arrangements and those unable to commit to a 3-month block of treatment. Adverse effects such as pain, bruising, tingling or bleeding at the insertion site is reported in up to 8% of patients, and this may limit acceptability of this treatment (5). Perhaps reflecting these limitations, the results of a long-term follow up study of patients undergoing PTNS treatment showed poor compliance to PTNS over time (6).
Non-invasive alternatives, whereby the tibial nerve is stimulated transcutaneously (TTNS) at a home-based setting, have therefore been explored (7). Early results have been promising, demonstrating improvements in OAB symptom scores and urodynamic parameters (7,8). So far, these studies have been using a TENS (transcutaneous electrical nerve stimulation) machine for stimulating the tibial nerve at frequencies between 10 to 40 Hz, which can be administered by the patient at home using pre-determined stimulation settings (9). Using a TENS machine however restricts the mobility of patients during the time that the nerve is being stimulated. Recently, a self-applicating skin-adhering ambulatory device, known as geko™ (Firstkind Limited, Buckinghamshire, UK) (Figure 1) has been developed and has received a CE mark for the prevention of deep vein thrombosis through chronic popliteal fossa stimulation, with no reported safety concerns (10). This device has recently been piloted in a study exploring outcomes in a cohort of patients reporting faecal incontinence, with promising results (11). Whether this device has a role in managing urinary incontinence is uncertain, and therefore the aim of this study was to evaluate the safety, acceptability and pilot efficacy of the geko™ device for transcutaneous stimulation of the tibial nerve in patients with OAB.
Methods
Patients
Patients with OAB symptoms attending a tertiary centre teaching hospital who found conservative first-line management options either ineffective or intolerable were enrolled in this randomised open label parallel-arm 12-week pilot trial of once daily versus once weekly 30-minute stimulation of the tibial nerve.
All participants provided written informed consent prior to enrolment. Patient eligibility was based on meeting the criteria for an OAB, defined by the International Continence Society as an average urinary frequency ≥8 voids and ≥1 urgency episode (with or without incontinence) per 24 hours (12). Patients with neurological disease reporting OAB symptoms were enrolled if their Expanded Disability Status Scale (EDSS) score was ≤6.5. Exclusion criteria included use of botulinum toxin A treatment within the previous year or neuromodulation (TNS or sacral neuromodulation), patients with sensory loss in the gaiter region (cutaneous sensation to nociception was assessed in the lower limb), presence of urinary tract infection or any other documented LUT pathology. Participants on antimuscarinic medications for OAB went through a 2-week run-in washout period during which time medications were discontinued.
Intervention
Patients were assigned to one of two treatment arms using the sealed envelope stratified randomisation service (https://www.sealedenvelope.com/randomisation/internet/). A dedicated member of the study team recruited participants and collected baseline and follow-up data. It was not deemed possible to include a control group as TTNS relies upon supra-sensory threshold stimulation and often a motor response to confirm device placement and effect.
Patients were provided an antiseptic wipe to clean the area and a pad for simple skin exfoliation. They were trained to use the device and attach over the tibial nerve by a self-adhesive gel 1 cm behind the medial malleolus in a vertical position (Figure 1). The area of stimulation was up to 5 cm cephalad to the medial malleolus. Patients were asked to apply the device over the same ankle if possible.
The device has default stimulation parameters delivering a constant 27 mA current, at a frequency of 1 Hz. The pulse width was increased between a range of 7 settings (between 70 and 560 µs) and was sequentially increased depending upon the maximum tolerable sensory and best sensory-motor response (toe flexion and fanning, tingling sensation). Patients were encouraged to use the device at home and to carry on with daily activities with no restriction to ambulation, however bathing and driving with the device were discouraged.
Outcome measures
The primary objective of the study was to assess safety and acceptability of the device and this was measured through a customized compliance diary in which entries could be made by patients to record compliance as well as their experiences whilst using the device and any adverse effects. Additionally, a member of the research team made weekly phone calls to ensure patients were applying correctly and achieving adequate sensory-motor responses during stimulations.
Treatment response was assessed using the Global Response Assessment (GRA) at week 12 of treatment, and the International Consultation on Incontinence Questionnaires for the OAB and LUTS-related quality of life (ICIQ-OAB and ICIQ-LUTSqol) at baseline, week 4, 8 and 12. The GRA was based on a similar questionnaire used previously where patients were asked to assess their response to treatment using an ordinal scale of 0 to 3, referring to none, mild, moderate or marked improvement, respectively (1). Patients reporting moderate or marked improvement were considered to have responded to treatment (1). The ICIQ-OAB score is a 6-item questionnaire that assesses OAB symptom severity and bother and the ICIQ-LUTSqol score is a 20-item health related quality of life questionnaire. In both questionnaires, part A assesses symptom severity and part B assesses the accumulative bother to the patient. Higher scores in each suggests worse symptom profiles and negative impact on QoL, respectively. Patients were also asked to complete 3-day bladder diaries to capture the mean 24-hour urinary frequency episodes and number of urinary leakage episodes at the four time points.
Statistical analysis
A feasibility sample size of 48 patients was adopted and no formal power calculation was performed as is the convention for pilot studies with no prior data to base a sample size on (13). All data were presented as means with SDs. Paired student t-tests were used to provide an estimate of within group responses between baseline and 12 weeks.
The study received ethics approvals from the Surrey Borders NRES Committee London (Ref: 12/LO/1613) and the Medicines and Healthcare Products Regulatory Agency (MHRA).
Results
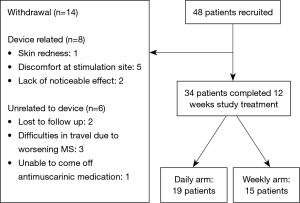
Forty-eight patients met the eligibility criteria [neurogenic OAB (multiple sclerosis, MS) (n=24) and idiopathic OAB (n=24)] and were 1:1 randomised to receive once daily (n=24) or once weekly (n=24) treatment. Participants randomized into the daily and weekly groups were comparable for age, sex, diagnosis and symptom severity. The mean age (range) for the daily and weekly arms were 46.4 [32–73] and 46.9 [20–81] years, female to male patients 18:6 and 20:4, and the number of patients with incontinence of 20 and 18 in the two respective arms. Thirty-four patients completed 12-week of treatment and the reasons for withdrawing are outlined in Figure 2.
Patient compliance and acceptability
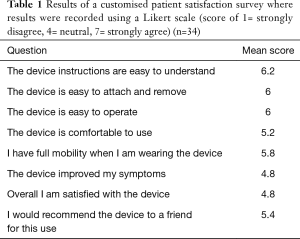
Compliance for using the device amongst participants randomized to receive daily treatment was 90.5% (76 out of the 84 daily applications) compared to 100% amongst patients randomized to receive weekly treatment (12 out of the 12 weekly applications). The participants throughout the course of treatment noted no significant concerns and the responses to the satisfaction survey are shown in Table 1. Five patients found the electrical stimulation uncomfortable and discontinued treatment (Figure 2). There were no significant safety concerns raised during the 12 weeks course of treatment. One patient developed mild skin redness at the site of stimulation, likely to be due to sensitivity to the adhesive, and was withdrawn from the study (Figure 2).
Full table
Changes in OAB symptoms
Eighteen (53%) [daily treatment (n=9), weekly (n=9)] participants reported a moderate or significant improvement in symptoms on the GRA. No differences were noted between responders and non-responders with regards to age, gender, diagnosis, degree of disability (in the neurological group). Sixty-five percent (13/20) of neurological patients with OAB and 36% (5/14) of patients with idiopathic OAB responded to the intervention (Table 2).

Full table
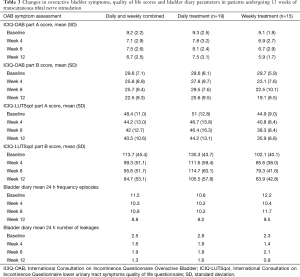
Improvements were observed in both ICIQ-OAB and ICIQ-LUTSqol scores between baseline and over the course of 12 weeks treatment in both the weekly and daily arms (Figure 3). In the daily arm, mean (SD) ICIQ-OAB part A subscores improved between baseline and week 12 from 9.3 (2.5) to 7.5 (3.1), and from 9.1 (1.9) to 5.9 (1.7) in the weekly arm. ICIQ-OAB part B subscores improved from 29.6 (8.1) to 25.6 (9.5) in the daily arm, and from 29.7 (5.9) to 19.1 (8.5) in the weekly arm (Figure 3).

ICIQ-LUTSqol part A subscores improved from 51 (12.8) to 44.2 (13.1) in the daily arm and from 44.9 (9.0) to 35.9 (8.8) in the weekly arm. ICIQ-LUTSqol part B subscores improved from 130.3 (43.7) to 105.5 (57.8) in the daily arm, and from 102.1 (40.1) to 63.9 (42.8) in the weekly arm (Table 3).
Full table
Improvements were also noted in the 3-day bladder diary mean. Twenty-four-hour urinary frequency improved from 11.5 at baseline to 8.8 at week 12 in the daily arm in both arms combined. And the mean number of leakages reduced from 2.5 to 1.3 at week 12 (Table 3).
Discussion
This study evaluates the feasibility of using a novel ambulatory device (geko™) for transcutaneously stimulating the tibial nerve in patients reporting OAB symptoms. Participants largely found the treatment satisfactory, tolerable and convenient to use. Compliance was assessed through the use of a diary which provided a written record of all the stimulation sessions. Patients receiving the treatment every day were compliant 90% of the time, whereas those receiving weekly treatment were 100% compliant. Being a new indication for the device, patients were contacted on a weekly basis to assess safety, and the device was found to be safe to use over the ankle with no significant adverse events reported. These results are in line with previous studies where the device was used to stimulate the tibial nerve for managing fecal incontinence (11), and over the popliteal fossa for preventing deep venous thrombosis (10).
Several studies have previously demonstrated the efficacy of TTNS for the management of OAB. In an earlier study, Amarenco et al. investigated TENS for 44 patients with demonstrated detrusor overactivity (DO), showing increased mean cystometric capacity and mean volume at involuntary detrusor contractions during stimulation (7). Schreiner et al. carried out a placebo controlled, randomized trial of 12 weeks of TTNS versus pelvic floor muscular training alone, to treat idiopathic urgency incontinence demonstrating significant efficacy of TTNS over placebo (14).
Satisfactory compliance was confirmed with the submission of a compliance diary for those patients who completed the therapy. The device was found to be safe as no significant adverse effects were noted, although 5 patients experienced some discomfort with the stimulation. This may be due to the slow frequency of the stimulation which is more noticeable than faster frequency stimulation as used with other devices, and also due to possible recruitment of cutaneous afferent nerves which does not occur with PTNS. Patients rated the device as easy to use and operate, and would recommend the treatment to a friend for use.
Fifty-three percent of our patients completing 12 weeks of treatment reported a moderate to significant improvement using the GRA scale. This is comparable to the improvements seen in a phase 3 multicentric randomized study comparing PTNS treatment with sham stimulation (SUmiT study) where 54.5% of the patients in the treatment arm reported moderate or marked improvements in bladder symptoms using the same GRA scale (1).
The preliminary results of this study seem to suggest a benefit in bladder diary parameters, OAB scores and LUT symptom related quality of life scores, however this exploratory phase 2 study was not designed to evaluate efficacy and therefore conclusions cannot be drawn. The improvements observed in both arms of the study would help inform the design of a properly powered study to evaluate efficacy of this device. Unexpectedly, benefits were observed as early as 4 weeks into the treatment, however continued to improve at week 8 and at week 12 of treatment. Greater improvements noted in the weekly treatment arm was surprising, and a future study should include in the design an evaluation of different frequencies of treatment sessions.
It appeared in our study that neurological patients more often responded to treatment (65%) compared to patients with idiopathic OAB (36%). There were no significant differences in tolerability between groups. This supports previous observations of the benefits of TNS in patients with neurological disease. Several studies have already demonstrated the efficacy of PTNS in different patient groups with neurological disease (15-20). de Seze et al. performed a multicentric study of 70 patients with MS reporting symptoms of OAB using a TENS machine which was applied for 20 minutes daily and noted by day 30 significant improvements in urgency and frequency (8). Likewise, benefits of TTNS have been demonstrated in patients with Parkinson’s disease (20).
Our study was limited by the high attrition rate. There were several reasons for patients dropping out including device-related (n=8), perceived lack of improvement in OAB symptoms (n=2) and local discomfort (n=5) where the nerve stimulation was felt to be unpleasant or too intense. Participants were permitted to adjust the strength of stimulation to achieve the balance of a device setting high enough for sufficient sensory-motor response and yet not too high where it may become unpleasant.
There are several advantages of transcutaneous TTNS over PTNS because of the convenience of home-based neuromodulation, without the need for regular outpatient visits. Using a self-contained skin-adhering device such as the device used in this study has the added advantage of permitting ambulation during the treatment, without restricting activity of the patient. As the study lacked a sham arm, a placebo effect could not be entirely excluded, especially considering the early benefits in OAB and quality of life scores noted in patients receiving weekly treatment. Whereas a sham arm may be possible when studying PTNS (1), it is a challenging prospect in a randomized double blinded TTNS study and therefore future studies would need to be designed comparing TTNS against established treatments. Furthermore, a future study should be designed to compare different stimulation parameters such as pulse intensity and frequency, for optimization of stimulation settings (21).
The prospects of a portable, non-intrusive, cost-effective, transcutaneous mode of stimulation delivery has clear advantages and warrants further investigation for this morbid and prevalent condition.
Conclusions
This study demonstrates that the use of a novel non-invasive ambulatory TNS device was both safe and acceptable for patients with high levels of compliance. Low frequency stimulation of the tibial nerve at 1 Hz was shown, for the first time in a clinical study, to improve storage symptom severity from both quality of life questionnaire and 3-day bladder diary data. Further studies are however required to evaluate the efficacy and optimal treatment frequency of transcutaneous TNS using this novel device for the management of OAB.
Acknowledgements
The work was undertaken at UCLH/UCL Institute of Neurology and is supported in part by funding from the United Kingdom’s Department of Health NIHR Biomedical Research Centres funding scheme. JH Seth was awarded research scholarship funding from The Urology Foundation. However, the study was investigator-led, including design and execution; the data was controlled and analyzed by the investigators.
Footnote
Conflicts of Interest: Firstkind Ltd. provided financial support for conduct of the study through the Trust’s R&D Department, and provided the devices used in the study.
Ethical statement: The study received ethics approvals from the Surrey Borders NRES Committee London (Ref: 12/LO/1613) and the Medicines and Healthcare Products Regulatory Agency (MHRA).
References
- Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 2010;183:1438-43. [Crossref] [PubMed]
- Vandoninck V, Van Balken MR, Finazzi Agro E, et al. Posterior tibial nerve stimulation in the treatment of urge incontinence. Neurourol Urodyn 2003;22:17-23. [Crossref] [PubMed]
- NICE. Percutaneous posterior tibial nerve stimulation for overactive bladder syndrome. In: NICE interventional procedure guidance. 2010. Available online: https://www.nice.org.uk/guidance/ipg362
- NICE. Urinary incontinence in women: management. 2013. Available online: https://www.nice.org.uk/guidance/cg171
- Burton C, Sajja A, Latthe PM. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Neurourol Urodyn 2012;31:1206-16. [Crossref] [PubMed]
- Peters KM, Carrico DJ, Wooldridge LS, et al. Percutaneous tibial nerve stimulation for the long-term treatment of overactive bladder: 3-year results of the STEP study. J Urol 2013;189:2194-201. [Crossref] [PubMed]
- Amarenco G, Ismael SS, Even-Schneider A, et al. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol 2003;169:2210-5. [Crossref] [PubMed]
- de Seze M, Raibaut P, Gallien P, et al. Transcutaneous posterior tibial nerve stimulation for treatment of the overactive bladder syndrome in multiple sclerosis: results of a multicenter prospective study. Neurourol Urodyn 2011;30:306-11. [Crossref] [PubMed]
- Slovak M, Chapple CR, Barker AT. Non-invasive transcutaneous electrical stimulation in the treatment of overactive bladder. Asian J Urol 2015;2:92-101. [Crossref] [PubMed]
- Tucker A, Maass A, Bain D, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol 2010;19:e31-7. [Crossref] [PubMed]
- Rimmer CJ, Knowles CH, Lamparelli M, et al. Short-term Outcomes of a Randomized Pilot Trial of 2 Treatment Regimens of Transcutaneous Tibial Nerve Stimulation for Fecal Incontinence. Dis Colon Rectum 2015;58:974-82. [Crossref] [PubMed]
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 2002;21:167-78. [Crossref] [PubMed]
- Julious S. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceutical Statistics 2005;4:287-91. [Crossref]
- Schreiner L, dos Santos TG, Knorst MR, et al. Randomized trial of transcutaneous tibial nerve stimulation to treat urge urinary incontinence in older women. Int Urogynecol J 2010;21:1065-70. [Crossref] [PubMed]
- Zecca C, Digesu GA, Robshaw P, et al. Motor and sensory responses after percutaneous tibial nerve stimulation in multiple sclerosis patients with lower urinary tract symptoms treated in daily practice. Eur J Neurol 2014;21:506-11. [Crossref] [PubMed]
- Kabay S, Kabay SC, Yucel M, et al. The clinical and urodynamic results of a 3-month percutaneous posterior tibial nerve stimulation treatment in patients with multiple sclerosis-related neurogenic bladder dysfunction. Neurourol Urodyn 2009;28:964-8. [Crossref] [PubMed]
- Kabay SC, Kabay S, Yucel M, et al. Acute urodynamic effects of percutaneous posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with Parkinson's disease. Neurourol Urodyn 2009;28:62-7. [Crossref] [PubMed]
- Gobbi C, Digesu GA, Khullar V, et al. Percutaneous posterior tibial nerve stimulation as an effective treatment of refractory lower urinary tract symptoms in patients with multiple sclerosis: preliminary data from a multicentre, prospective, open label trial. Mult Scler 2011;17:1514-9. [Crossref] [PubMed]
- Fjorback MV, van Rey FS, van der Pal F, et al. Acute urodynamic effects of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with MS. Eur Urol 2007;51:464-70; discussion 471-2. [Crossref] [PubMed]
- Ohannessian A, Kabore FA, Agostini A, et al. Transcutaneous tibial nerve stimulation in the overactive bladder syndrome in patients with Parkinson's syndromes. Prog Urol 2013;23:936-9. [Crossref] [PubMed]
- Slovak M, Barker AT, Chapple CR. The assessment of a novel electrical stimulation waveform recently introduced for the treatment of overactive bladder. Physiol Meas 2013;34:479-86. [Crossref] [PubMed]