
Epidemiology, aetiology and screening of bladder cancer
Epidemiology
Bladder cancer (BC) is the 9th most common cancer worldwide (1) and the 7th most common worldwide in men (2) (Figure 1). Worldwide data from GLOBOCAN [2012] revealed there are approximately 430,000 incident cases per year with 165,000 deaths (2). In 2018, there will be an estimated 81,190 new BC cases diagnosed in the USA with 17,240 deaths (3). Three-quarters of new cases occur in men (higher in some regions and reflects smoking and occupational differences and access to healthcare) (2), yet women have greater disease-specific mortality. Reasons for the disparity in gender incidence and mortality include differences in hormonal profiles (activity of the sex steroid hormone pathway) and systematic differences in the timeliness of female referrals to investigation from primary care (4). BC risk has been observed to be lower in women with older age at menarche, parity compared to nulliparous women), and the use of oestrogen and progestin therapy (4). Furthermore, women have been shown to have a higher stage at BC diagnosis, which is thought to be due to irritative lower urinary tract symptoms being more likely to receive a diagnosis of urinary tract infection. However, women have also been shown to have poorer survival outcomes when adjusted for all stages (5). This may represent differences in treatment efficacy and cancer biology and drug interactions.
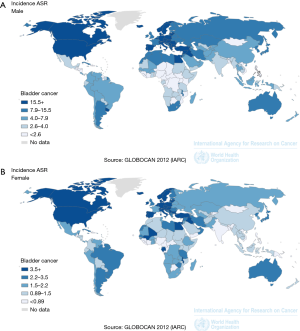
BC also increases with age and is more common in well-resourced countries (6-8). Partly this reflects tobacco smoking and environmental carcinogen prevalence. BC is the most expensive cancer to treat, with the cost of MIBC approaching $150,000 per capita (9).
BC is often considered as three disease entities owing to the difference in oncological natural histories; low grade non-muscle invasive (LG-NMIBC, the most indolent), high grade non-muscle invasive (HG-NMIBC, grade 3 pTis, PTa or pT1 cancers) and muscle invasive BC (MIBC, ≥T2) (10). Patients will then be started on a treatment pathway depending on the aggressiveness of the pathology found (as determined by histological grade and TNM staging). Approximately, 25–30% of patients will have MIBC at diagnosis, which is treated with cystectomy, radical radiotherapy or palliation.
Aetiology
Most BC’s arise secondary to exogenous exposure to carcinogens via the respiratory system, gastrointestinal tract or via skin contact. The most common risk factors for BC are tobacco smoke and occupational and environmental carcinogens (11). Tobacco smoke accounts for 50% of BCs, but the attributable risk varies with sex, smoking history (often heterogeneously reported in studies) and the type of tobacco consumed (blonde and black tobacco, which are cured by flue and air respectively). Black tobacco is more carcinogenic owing to a greater concentration of nitrosamines, biphenyls and arylamines (12-14). The smoking of opium has been shown in a meta-analysis of 17 studies to confer an increased BC risk (15) and cannabis has also been associated with BC through a large cohort study in the USA (16). However, tobacco is frequently a confounder is such studies. The legalisation of cannabis in some countries may increase the burden from this method of smoking.
Recently the use of electronic (‘e-’) cigarettes is on the rise in most high-income countries. E-cigarettes are battery-powered devices that work by heating a liquid to create and inhalable aerosol (vapour). The e-liquid commonly includes a propylene glycol/nicotine/flavouring mix. E-cigarettes have been in US and UK markets since 2007 and long-term data is yet to be published (17). They are perceived as offering the health benefit of not involving tar and harmful combustion by-products. At inception, the regulation of the constituents of e-cigarettes was relaxed and there were concerns regarding the inclusion of known bladder carcinogens such as arsenic in small quantities. However, the e-cigarette components are now more stringent. At present, there are no RCT or high evidence level studies to show harmful health effects from e-cigarettes and indeed they are endorsed as a healthier alternative to traditional cigarettes, and are considered a stepping-stone to smoking cessation (17).
Occupational carcinogen exposure accounts for approximately 6% (18). Historically, rubber and dye industries have been shown convincingly to be at risk of occupational BC. In 2015, we published a contemporary meta-analysis of 263 studies which showed that the pooled relative risk (pRR) for BC was greatest in tobacco workers [RR 1.72; 95% confidence interval (CI): 1.37–2.15] and dye industries (RR 13.4; 95% CI: 1.5–48.2). The highest pRR for mortality was in metal workers 10.2 (95% CI: 6.89–15.09) (11). The high mortality in metal workers might be part explained by the exposure to dye penetrants. These are usually a red azo dye (solvent red 164) or fluorescent dyes that are used to test metals for cracks and fatigue. In one prospective cohort study, patients’ exposed to these agents were diagnosed with a higher index stage of BC and the tumours were more likely to be multifocal (19).
Occupational carcinogens known to cause BC include benzidine, ortho-toluidine, 2-naphylamine, 4-aminobiphenyl and 4,4'-methylene-bis (2-chloroaniline) (MBOCA) (11). A limitation of occupational risk studies is that often there is heterogeneity in the classification of occupations and that occupational-tasks rather than ‘umbrella categories’ are more salient as a description of the potential exposure risks (11).
There have been some studies that have assessed whether the stage at BC diagnosis differs depending on the occupational carcinogen burden. For example Noon et al. (20) showed that ‘miscellaneous construction workers’ and ‘male chemical workers’ were more likely to present with invasive > localized BC. Limitations to this study were the inability to control for smoking and to define treatment information. Furthermore data on the type of chemical handling and precautionary clothing used is not always available (20).
Other areas of interest have included dietary and environmental causes of BC as well as the relationship between medical conditions and treatments and BC, and the role of genetics. A few studies have shown that high alcohol consumption (21,22), low fruit and vegetable intake (23) and low hydration levels (24) can be linked to BC but to date, these links are only suspected. However, arsenic in drinking water is a recognized cause of BC with one systematic review citing a RR of 2.7 (95% CI: 1.2–4.1). Other contaminants of drinking water are disinfection by-products (chlorination) and trihalomethanes, which have been shown to increased BC risk (25,26). Radiotherapy [for pelvic malignancies including prostate cancer (PC)] has been shown to increase the risk of BC, the prognosis for patients who contract BC after radiotherapy is considered to be poorer (27). Other iatrogenic causes of BC include cyclophosphamide therapy and potentially pioglitazone treatment (an oral anti-diabetic medication) (28,29). Diabetes itself has been linked to BC but results are not conclusive. One meta-analysis showed a pRR of 1.35 (95% CI: 1.17–1.56) but there was a failure to adjust for many confounders (30). Schistosomiasis is a well-recognized cause of squamous cell BC. Other causes of chronic inflammation such as recurrent urinary tract infection and indwelling catheters have weaker associations (31,32).
Our awareness of the genetic basis of BC is growing increasingly. A recent breakthrough paper on somatic changes in MIBC recently characterised over 400 patients (33). Risks for BC include increased somatic copy numbers of FGFR3 and KRAS genes. These are generally acquired defects. The most recognized inherited genetic links to BC are polymorphisms of two carcinogen-detoxification genes NAT2 and GSTM1. Abnormalities in these genes lead to longer exposure to carcinogens (34). There are on-going studies to evaluate the relationship between environmental carcinogen exposure and gene-expression profiles to evaluate transient and permanent damages that can occur and cause BC.
BC screening
In 1968, Wilson and Jungner described a checklist for the World Health Organization of factors that should be considered for efficacious and ethical screening for disease. Their criteria suggested that the disease must be an important health problem, there should be an acceptable treatment of the disease, there should be a facility to recognize the disease at an early/latent/asymptomatic stage, and there should be a cost-effective acceptable test for detecting the disease (35). BC fulfills many of these factors; it is an expensive and morbid disease and LG-NMIBC confers a much greater survival outcome and much lower morbidity than MIBC. There is debate however about the nature of the test.
To the best knowledge of the authors, no country has adopted a screening programme for BC. But screening studies for BC in asymptomatic populations has been done previously and some benefits have been shown.
Two non-randomised studies have shown that haematuria testing in asymptomatic persons leads to down staging of cancers and may improve survival (36,37). Britton et al. (UK) screened 2,356 men and found 17 BC’s, of which all were NMIBC (36). At 7 years, 33% of the HG-NMIBC cohort had died from BC, reflecting potential under-management of this aggressive cancer (36,38). Messing et al. screened 1,575 American men and found a lower stage at diagnosis and a reduced BC mortality rate (0%) with screening, when compared to men from a matching state wide registry (16.4% mortality rate) (37). The benefit of screening appears greatest in high-risk populations. For example, Zlotta et al. screened persons known to have been exposed to a BC carcinogen aristolochic acid [a Chinese herb associated with BC and causing a so called aristolochic acid nephropathy (AAN)]. In this study, 48 persons who were experiencing AAN were enrolled in a screening programme of prospective cystoscopies biannually for 10 years. Zlotta found survival in patients exposed to aristolochic acid only occurred in those undergoing screening (100% mortality in non-screened patients) (39).
Most patients are referred for BC diagnostics after presenting with either visible haematuria (VH) or urinary symptoms +/− non-visible haematuria (NVH) that is dipstick detected (irritative lower urinary tract symptoms or recurrent urinary infections). Urine dipstick is often used in primary care settings and NVH will detect BC in about 4% of cases (40).
Cystoscopy is the gold standard diagnostic tool but this is invasive. Most urology units combine cystoscopy with upper tract radiological imaging (ultrasound or computed tomography) as well as clinical examination as part of a ‘one-stop shop’ for diagnosis of BC (and kidney cancer). No urinary biomarker has been shown to be better than urinary cytology and cystoscopy and neither the European Association of Urologists (EAU) (41) or National Institute for Health and Care Excellence (NICE) (42) recommend urinary biomarkers.
Furthermore, one of the reasons cited for not pursuing a nationwide screening study in the UK or USA has been the relatively low prevalence of BC (which has cost-effectiveness implications) and the absence of an ideal screening tool. The background prevalence of BC can hopefully be increased by screening high-risk populations (older males, smokers). For example, currently in the UK there is a screening trial for lung cancer, which entails community based chest computed tomography (CT) scanning for current or previous smokers 55–80 years of age. This follows work in the USA, which has shown a survival benefit from screening hard to reach at risk populations (43,44). This is encouraging as the two diseases share aetiological factors.
Screening for urological cancers has in general been a challenge and an area of debate. No more so than in PC. The issue has been that screening using a PSA blood test confers only a mild PC-specific survival whilst exposing many to over-diagnosis and over-treatment (45). One attempt to lower this overtreatment has been to find a tool able to discern between clinically significant (csPC) and clinically non-significant PC [clinically significant cancer defined as Gleason score ≥4+3 or a maximum cancer core length 6 mm or longer (46)]. Multiparametric MRI is making a bid to become one such tool. Ahmed et al. showed in the PROMIS study, a prospective cohort study of 740 screened men, that mpMRI has a 93% sensitivity and 41% specificity at detecting csPC compared to standard-of-care transrectal ultrasound guided (TRUSS) biopsy (46). Ultimately, this will reduce biopsy rates for men. There have been steps made to develop an mpMRI protocol for BC (47) using the VI-RADS scoring system (Vesical Imaging-Reporting And Data System) but unlike the prostate, the bladder is a “moving-target” due to filling and it will take time to optimize such a strategy. Non-invasive strategies will hopefully improve the acceptance of screening for BC but may be issues with capacity.
It is fairly clear that developing an improved screening tool is a difficult challenge. In BC, there have been huge bodies of work over recent years to identify a biomarker for BC but none have been superior to the combination of urine dipstick testing and cystoscopy (48). Promising candidates have included NMP22 (nuclear matrix protein-22, expression of which reflects mitotic activity) (49), UroVysion (detects aneuploidy for chromosomes 3, 7, 17, and the loss of 9p21 locus (tumour suppressor) using fluorescence in situ hybridization) and ImmunocCyst (uses fluorescence-labelled antibodies to exfoliated antigens from BC cells) (50), but none have gained widespread uptake owing to the wide sensitivity and specificity ranges [30–100% (EAU)]. In the UK, it remains that most patients are diagnosed with BC after having been investigated for either VH or NVH. The prevalence of BC with VH is approximately 20%, whereas it its 4% in NVH (40,51). However, haematuria can be caused by numerous other medical conditions including infection, calculi, recent instrumentation, chronic renal disease and some medications.
Despite these difficulties there is ongoing work in this field. Most excitingly the DETECT 1 and 2 studies in the UK, which will investigate the utility of the UroMark assay, which interrogates 150 loci and provides a biomarker panel rather than a standalone single test (52). DETECT 1 and 2 are prospective observational studies recruiting from 40 UK hospitals. DETECT 1 focuses on the negative predictive value of the biomarker assay. DETECT 2 will be focusing on the ability of the assay to detect low, intermediate and high-risk BCs, and will determine the sensitivity of the test. DETECT 2 will importantly, also provide quality of life data for patients undergoing BC investigations through semi-structured questionnaires.
Conclusions
BC confers a significant disease burden, particularly in industrialized nations. Tobacco smoking remains the primary risk factor. Emerging evidence for environmental, dietary, medical and genetic risk factors is occurring. Tobacco- and environmental-gene interactions form a large part of current research practice. Incidence patterns are dependent on the shifting patterns of tobacco smoking, occupational landscapes and access to healthcare. With the advent of more intricate urinary and serum tests for BC, screening studies (particularly of high-risk populations) will become more commonplace and hopefully more accurate for prevention and monitoring of this disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- Ferlay J. GLOBOCAN 2012. Estimated cancer incidence, mortality and prevalence worldwide in 2012 (online).
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Dobruch J, Daneshmand S, Fisch M, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol 2016;69:300-10. [Crossref] [PubMed]
- Mungan NA, Kiemeney LA, van Dijck JA, et al. Gender differences in stage distribution of bladder cancer. Urology 2000;55:368-71. [Crossref] [PubMed]
- Bray F, Colombet M, Mery L, et al. Cancer Incidence in Five Continents, Vol. XI (electronic version). Lyon: International Agency for Research on Cancer, 2017.
- Greiman AK, Rosoff JS, Prasad SM. Association of Human Development Index with global bladder, kidney, prostate and testis cancer incidence and mortality. BJU Int 2017;120:799-807. [Crossref] [PubMed]
- Mahdavifar N, Ghoncheh M, Pakzad R, et al. Epidemiology, Incidence and Mortality of Bladder Cancer and their Relationship with the Development Index in the World. Asian Pac J Cancer Prev 2016;17:381-6. [Crossref] [PubMed]
- Svatek RS, Hollenbeck BK, Holmang S, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol 2014;66:253-62. [Crossref] [PubMed]
- Noon AP, Albertsen PC, Thomas F, et al. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer 2013;108:1534-40. [Crossref] [PubMed]
- Cumberbatch MG, Cox A, Teare D, et al. Contemporary Occupational Carcinogen Exposure and Bladder Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2015;1:1282-90. [Crossref] [PubMed]
- Samanic C, Kogevinas M, Dosemeci M, et al. Smoking and bladder cancer in Spain: effects of tobacco type, timing, enviromental tobacco smoker, and gender. Cancer Epidemiol Biomarkers Prev 2006;15:1348-54. [Crossref] [PubMed]
- De Stefani E, Correa P, Fierro L, et al. Black tobacco, mate, and bladder cancer. A case-control study from Uruguay. Cancer 1991;67:536-40. [Crossref] [PubMed]
- Momas I, Daures JP, Festy B, et al. Bladder cancer and black tobacco cigarett smoking. Some results from a French case-control study. Eur J EPidemiol 1994;10:599-604. [Crossref] [PubMed]
- Afshari M, Janbabaei G, Bahrami MA, et al. Opium and bladder cancer: A systematic review and meta-analysis of the odds ratios for opium use and the risk of bladder cancer. PloS One 2017;12:e0178527. [Crossref] [PubMed]
- Thomas AA, Wallner LP, Quinn VP, et al. Association between cannabis use and the risk of bladder cancer: results from the California Men's Health Study. Urology 2015;85:388-92. [Crossref] [PubMed]
- Bourke L, Bauld L, Bullen C, et al. E-cigarettes and Urologic Health: A Collaborative Review of Toxicology, Epidemiology, and Potential Risks. Eur Urol 2017;71:915-23. [Crossref] [PubMed]
- Rushton L, Bagga S, Bevan R, et al. Occupation and cancer in Britain. Br J Cancer 2010;102:1428-37. [Crossref] [PubMed]
- Noon AP, Pickvance SM, Catto JW. Occupational exposure to crack detection dye penetrants and the potential for bladder cancer. Occup Environ Med 2012;69:300-1. [Crossref] [PubMed]
- Noon AP, Martinsen JI, Catto JWF, et al. Occupation and Bladder Cancer Phenotype: Identification of Workplace Patterns That Increase the Risk of Advanced Disease Beyond Overall Incidence. Eur Urol Focus 2018;4:725-30. [Crossref] [PubMed]
- Zaitsu M, Nakamura F, Toyokawa S, et al. Risk of Alcohol Consumption in Bladder Cancer: Case-Control Study from a Nationwide Inpatient Database in Japan. Tohoku J Exp Med 2016;239:9-15. [Crossref] [PubMed]
- Botteri E, Ferrari P, Roswall N, et al. Alcohol consumption and risk of urothelial cell bladder cancer in the European prospective investigation into cancer and nutrition cohort. Int J Cancer 2017;141:1963-70. [Crossref] [PubMed]
- Yao B, Yan Y, Ye X, et al. Intake of fruit and vegetables and risk of bladder cancer: a dose-response meta-analysis of observational studies. Cancer Causes Control 2014;25:1645-58. [Crossref] [PubMed]
- Di Maso M, Bosetti C, Taborelli M, et al. Dietary water intake and bladder cancer risk: An Italian case-control study. Cancer Epidemiol 2016;45:151-6. [Crossref] [PubMed]
- Silverman D. Schottenfeld and Fraumeni cancer epidemiology and prevention. New York: Oxford University Press, 2018.
- Villanueva CM, Cantor KP, Cordier S, et al. Disinfection byproducts and bladder cancer: a pooled analysis. Epidemiology 2004;15:357-67. [Crossref] [PubMed]
- Abern MR, Dude AM, Tsivian M, et al. The characteristics of bladder cancer after radiotherapy for prostate cancer. Urol Oncol 2013;31:1628-34. [Crossref] [PubMed]
- Turner RM, Kwok CS, Chen-Turner C, et al. Thiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysis. Br J Clin Pharmacol 2014;78:258-73. [Crossref] [PubMed]
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone Use and Risk of Bladder Cancer and Other Common Cancers in Persons With Diabetes. JAMA 2015;314:265-77. [Crossref] [PubMed]
- Zhu Z, Wang X, Shen Z, et al. Risk of bladder cancer in patients with diabetes mellitus: an updated meta-analysis of 36 observational studies. BMC Cancer 2013;13:310. [Crossref] [PubMed]
- Silverman DT, Koutros S, Figueroa J, et al. Bladder Cancer. Schottenfeld and Fraumeni cancer epidemiology and prevention. New York: Oxford University Press, 2018.
- Lee WY, Sun LM, Lin CL, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol 2014;32:51.e1-7. [Crossref] [PubMed]
- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017;171:540-556.e25. [PubMed]
- Figueroa JD, Koutros S, Colt JS, et al. Modification of Occupational Exposures on Bladder Cancer Risk by Common Genetic Polymorphisms. J Natl Cancer Inst 2015.107. [PubMed]
- Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. Available from: http://www.who.int/bulletin/volumes/86/4/07-050112BP.pdf
- Britton JP, Dowell AC, Whelan P. Dipstick haematuria and bladder cancer in men over 60: results of a community study. BMJ 1989;299:1010-2. [Crossref] [PubMed]
- Messing EM, Young TB, Hunt VB, et al. Comparison of bladder cancer outcome in men undergoing hematuria home screening versus those with standard clinical presentations. Urology 1995;45:387-96; discussion 396-7. [Crossref] [PubMed]
- Thomas F, Rosario DJ, Rubin N, et al. The long-term outcome of treated high-risk nonmuscle-invasive bladder cancer: time to change treatment paradigm? Cancer 2012;118:5525-34. [Crossref] [PubMed]
- Zlotta AR, Roumeguere T, Kuk C, et al. Select screening in a specific high-risk population of patients suggests a stage migration toward detection of non-muscle-invasive bladder cancer. Eur Urol 2011;59:1026-31. [Crossref] [PubMed]
- Sutton JM. Evaluation of hematuria in adults. JAMA 1990;263:2475-80. [Crossref] [PubMed]
- Babjuk M, Burger M, Comperat E, et al. Non-muscle invasive bladder cancer. European Association of Urology guidelines 2017. Online. Available online: http://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/
- Bladder cancer diagnosis and management. National Institute for Health and Care Excellence. Online (cited July 2018). Available online: https://www.nice.org.uk/guidance/ng2
- Yorkshire Lung Cancer Screening Trial (online). Available online: https://yorkshirecancerresearch.org.uk/news/yorkshire-cancer-research-announces-uks-largest-lung-cancer-screening-trial/
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Ilic D, Neuberger MM, Djulbegovic M, et al. Screening for prostate cancer. Cochrane Database Syst Rev 2013.CD004720. [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Panebianco V, Narumi Y, Altun E, et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur Urol 2018;74:294-306. [Crossref] [PubMed]
- Friedrich MG, Toma MI, Hellstem KP, et al. Comparison of multitarget fluorescence in situ hybridization in urine with other noninvasive tests for detecting bladder cancer. BJU Int 2003;92:911-4. [Crossref] [PubMed]
- Lotan Y, Svatek RS, Krabbe LM, et al. Prospective external validation of a bladder cancer detection model. J Urol 2014;192:1343-8. [Crossref] [PubMed]
- Greene KL, Berry A, Konety BR. Diagnostic Utility of the ImmunoCyt/uCyt+ Test in Bladder Cancer. Rev Urol 2006;8:190-7. [PubMed]
- Ritchie CD, Bevan EA, Collier SJ. Importance of occult hematuria found at screening. Br Med J (Clin Res Ed) 1986;292:681-3. [Crossref] [PubMed]
- Tan WS, Feber A, Dong L, et al. DETECT I & DETECT II: a study protocol for a prospective multicentre observational study to validate the UroMark assay for the detection of bladder cancer from urinary cells. BMC Cancer 2017;17:767. [Crossref] [PubMed]


