
Intravesical bacillus Calmette-Guérin for bladder cancer: are all the strains equal?
Background
Trans-urethral resection of the bladder (TURB) followed by adjuvant intravesical instillation with bacillus Calmette-Guérin (BCG) is the most effective conservative therapy for the treatment of high-risk non-muscle-invasive bladder cancer (NMIBC) (1-3). It can significantly reduce the probability of both disease recurrence and progression, thereby potentially improving survival (4,5). However, treatment schedules vary widely in clinical practice and there is a significant lack of adherence to international guideline recommendations for its optimal use (6,7). While current guidelines do not make a clear recommendation regarding preferences for a BCG strain over the other, there is mounting data underlying clinically significant differences (1,8,9). Passaging and subculturing through different distributors over the last decades may have, indeed, selected mycobacteria with differential biological activity profiles, virulences and reactogenicities (10,11). Various retrospective and prospective have aimed to compare the efficacy of different strains. Unfortunately, most with variable study designs, assertion bias, lead time bias, heterogeneity bias, lack of statistical power and study design issues such as maintenance cycles (12).
In this review, we sought to summarize the current evidence on the efficacy of different BCG strains in the treatment of NMIBC.
The BCG family tree
The first BCG strain was developed at the Pasteur institute in 1921 from an attenuated strain of Mycobacterium bovis. Over the course of decades several laboratories developed their own daughter BCG strain and its primary use was in the prevention of tuberculosis. In 1976, Morales et al. used BCG for the first time in the treatment of bladder cancer. Using the vaccine manufactured by the Institute Armand Frappier (Montreal), these investigators demonstrated a favorable impact of the compound on bladder cancer recurrence rates (13). Subsequent studies confirmed the efficacy of BCG in reducing disease recurrence and, even, progression rates in appropriately selected patients with NMIBC. Federal Drug Administration approval for the treatment of high-grade NMIBC was finally obtained in 1990.
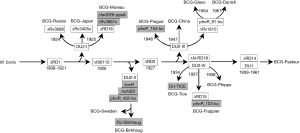
During decades the subculturing process of BCG, also known as passaging, caused a genetic evolution of the original strain (14,15). Therefore, it would be more appropriate referring to BCG strains in the plural, as they differ from a biologic, genetic point and probably clinical point of view. Studies on DNA sequences and their correlation with genetic and phenotypic differences revealed that BCG daughter strains have extensive differences in the level of expression of surface proteins and immunodominant protein antigens that influence virulence and reactogenicity (Figure 1) (10,11). Comparative genomics identified region of difference (RD) such as deletions and insertions, which are used as genetic markers. These critical gene mutations ultimately define the branches in the phylogenetic three of BCG. The loss of RD1, for example, is the fundamental difference between M. bovis and BCG, and is present in all subsequent cultured strains (Figure 1).
Despite the long clinical experience with this compound, its mechanism of action is incompletely understood. The main antitumor effect relies on immune system stimulation with CD4+ and CD8+ lymphocytes, natural killer cells and granulocytes recruitment (16,17). To allow this process, attachment of BCG to the urothelium and internalization by cancer cells is required (18-20).
The ability of BCG to bind the urothelium is primary mediated by fibronectin (21). The genetic evolution of BCG and the changes in protein expression could generate the hypothesis that there might be a clinical difference between strains. In an in vitro study, Hudson et al. could not corroborate this hypothesis. Authors found similar binding activities between three commercially available strains (BCG Tice, BCG Connaught, and BCG Armand Frappier) (22). The caveat being that these three strains are genetically closely related, which may have led to this lack of difference.
Ikeda et al. compared the characteristics of BCG Tokyo 172 with BCG Connaught in a mouse model. The mean percent of cancer cells with attached bacilli was 60.98% with BCG Tokyo-172 compared to only 13.13% with BCG Connaught (23). These findings suggest that BCG substrains differ also in their biological activity as well as their interaction with the host. Whether this translates in different response rates and, consequently, clinical outcomes have still to be elucidated.
BCG concentrations
The guiding principle in drug administration is to maximize efficacy while minimizing toxicity. In the case of BCG, there has been no phase I trial, and the first administered dose of 120 mg [106–108 colony forming units (cfu)] by Morales et al. was partly arbitrary and partly based on animal studies (13). What the lowest dose of BCG is to maintain current efficacy is question of importance, especially in the current shortage of BCG availability. The Club Urológico Español de Tratamiento Oncológico (CUETO) 95011 trial compared 27 mg of BCG Connaught vs. 13.5 mg BCG Connaught vs. 30 mg Mitomycin. The authors conclude that 13.5 mg BCG Connaught has the same efficacy as 30 mg Mitomycin at the cost of higher toxicity and that 27 mg BCG Connaught seems to be the minimum effective dose for adjuvant treatment (24). Nevertheless, the short follow-up of 5 months in that study does not allow definitive conclusions.
Retrospective studies deliver controversial evidence. Single arm series evaluating dose reduction (i.e., 75 mg BCG Pasteur) showed similar outcomes compared to other series at the time (25-29).
In contrast, series directly comparing the administration of full with reduced dose of BCG showed that the full dose could achieve a better tumor eradication as well as longer recurrence-free survival. This was true independently of the investigated strain (30,31).
A small series evaluated the efficacy of 75 mg BCG Pasteur. During the study, the strain production was interrupted and had to be replaced by 27 mg BCG Connaught. Both compounds achieved comparable response rates, but numbers were small in both groups (n=32 and n=25) (32).
What these studies have in common, is the superior effect of full dose BCG to reduced dose in patients with multiple risk factors such as tumor multifocality and higher recurrence rates, suggesting a potential for a risk adapted dosage. This issue was addressed by the randomized EORTC 30962 study which compared a full-dose and one-third dose of intravesical BCG TICE, both with a 1- or 3-year maintenance course. Full-dose BCG was not superior to one-third dose BCG (5-year DFS: 62% vs. 59%; P=0.092). However, subgroup analyses revealed that in high-risk patients, 3 years of maintenance with full dose BCG significantly reduced recurrence rates compared to one third dose (P=0.009). There were no significant differences in side effects based on dose or duration of maintenance schedule given (33).
In a CUETO randomized trial including 500 patients showed no significant difference in disease-free survival, progression-free survival or overall survival between patients treated with a full dose of BCG (81 mg) compared to a one third reduced dose of BCG (27 mg) (34). Nevertheless, one should be aware of the lacking statistical power of the study to prove equivalence or non-inferiority beyond clinically acceptable margins. Therefore, results should be interpreted with caution.
Ikeda et al. performed DNA measurements on lyophilized preparations of BCG Tokyo127 and BCG Connaught and showed that BCG Tokyo172 contained (48.8±5.43)×108 cfu per dose and BCG Connaught contained (3.8±1.4)×108 cfu per dose but there were no differences in antitumor efficacy in a mice model (23). These results are corroborated by the findings of Rentsch et al. comparing BCG Connaught and BCG TICE. Differences in bacterial titer or formulations seem to have no influence on the ability to induce priming of BCG-specific T cells (35). The inferiority in efficacy of dose reduction seems to be independent of BCG strain. One retrospective single-arm study reported on the efficacy of 80 mg (6.5×106 cfu/mg) BCG Moreau (36). Patients were treated with maintenance cycles for up to 3 years. The reported 5 years RFS and PFS rates were 65% (95% CI, 52–74%) and 81% (95% CI, 65–90%), respectively. Heterogeneity in treatment schedules, analyses and definition of treatment failure do not allow a direct comparison between studies. Finally, the heterogeneity in distribution of colonies in the vials makes an aliquot distribution in the reduced dose impossible, so that the expected/calculated concentration of colonies forming units of BCG in the reduced dose is probably not accurate.
Treatment schedules
Despite general acceptance of BCG for the treatment of high-risk NMIBC, there are still some controversies regarding the optimal number of instillations and treatment duration.
Animal models showed that repeated instillations with BCG are required to stimulate a robust immune response (37). In the first series by Morales et al., a 6-week protocol was proposed. Six instillations were given because the strain came packaged in six separate vials and at least 3 weeks of treatment were thought to be necessary to achieve an adequate immune stimulation. Moreover, the weekly schedule was chosen to minimize side effects (13). In order to optimize treatment, maintenance instillations beyond the 6-week induction were adopted. First randomized trials on maintenance schedules failed to demonstrate a significant difference in treatment arms but these studies were underpowered (38,39).
Subsequent RCTs have shown controversial results. Palou et al., for example, randomized patients who were free of disease at six months to observation versus maintenance with 6-week instillations every 6 months for 2 years; BCG Connaught was used. No difference in recurrence could be observed (P=0.07) (40). Similarly, maintenance cycles using BCG Tokyo-172 did not improve RFS (41,42).
Interestingly, in the RCT by Rentsch et al., patients treated with BCG Connaught had a significant longer 5-year RFS compared to patients treated with BCG TICE (74% vs. 48%; P=0.001). However, patients were not treated with maintenance cycles (35).
These findings were corroborated in large observational study by Witjes et al., who showed that when no maintenance treatment was given, BCG Connaught was more effective than BCG TICE with regards to the time to first recurrence (HR 1.48; 95% CI, 1.20–1.82; P<0.001). However, when maintenance was given, BCG TICE was more effective than BCG Connaught regarding time to first recurrence (HR 0.66; 95% CI, 0.47–0.93; P=0.019) (43).
The Southwest Oncology Group (SWOG) 8507 trial delivers the most convincing evidence on the optimal instillation protocol (44). In this prospective trial patients, after a 6-week induction course of intravesical and percutaneous BCG Connaught, were randomized to a 36-month maintenance or observation. Patients allocated to the intervention arm had significant longer 5-year RFS (60%, 95% CI, 53–67% vs. 41%, 95% CI, 35–49%, P<0.001) and PFS (76%, 95% CI, 70–83%, vs. 70%, 95% CI, 63–76%, P=0.04) compared to those who underwent observation. This was true even if only 16% of the 243 maintenance cases received all 8 scheduled maintenance courses over the 3 years. Moreover, the proposed treatment schedule in the SWOG 8507 showed a significant improvement in RFS when retrospectively compared to a single maintenance, every 3 months for 2 years (SWOG 8216) (45) and a monthly maintenance for 11 months (SWOG 8795) (46).
Based on current evidence, we can conclude that, independently of the BCG strain used for induction, it is critical to adhere to treatment schedules with maintenance cycles in patients with high-risk disease, in order to improve treatment efficacy. Whether this is true in all patients with intermediate risk disease is still un-adjudicated. It appears that induction with BCG Connaught can achieve a stronger immune response, translating in better oncological outcomes and generating the hypothesis that the effect of specific BCG strains can be influenced by maintenance therapy. Discrepancy between study findings could be explained by small patient number and the selection bias of a more favorable disease. Indeed, in in some studies the prevalence of low-grade disease reached 77% (41), other included patients who were free of disease at 6 months (40).
Differences in strains efficacy
Interestingly, despite decades of administration and widespread use, there are only few clinical trials directly comparing the efficacy of different trains. We could identify seven RCTs and one observational study (Table 1). Two studies showed a statistically significant difference in terms of RFS in favor of BCG Connaught when compared to BCG TICE, but only if no maintenance cycle was given (35,43). An explanation could be found in the mice model proposed by Rentsch et al. Indeed, authors found that intravesical BCG Connaught had a stronger immune system stimulation and greater cellular recruitment compared to BCG TICE (P<0.002) (35). The observation of Witjes et al. that maintenance cycles of BCG TICE can achieve a better RFS compared to maintenance with BCG Connaught (HR 0.66; 95% CI, 0.47–0.93; P=0.019) generates the hypothesis that the optimal efficacy of different strains may be dependent of different maintenance protocols (43).
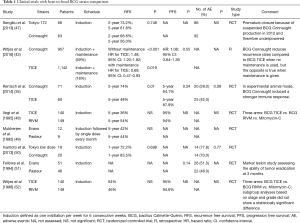
Full table
Only in one of all RCTs, patients were treated with maintenance therapy (49). Since maintenance has shown to achieve better outcomes when compared to induction alone and is, therefore, the standard of care (33,44), results from trials in which maintenance was not administered should be interpreted with caution and are only hypothesis generating.
A recent meta-analysis addressed the efficacy of different BCG strains against other intravesical therapies. They found that BCG Tokyo-172 was associated with a nonsignificant better RFS compared to all other BCG strains, while BCG Connaught was associated with a nonsignificant increase in disease recurrence compared to BCG Pasteur, BCG TICE and BCG Tokyo-172. Authors concluded that BCG is superior to chemotherapy in preventing recurrence, but no BCG strain was significantly superiority when compared to the others (53). A direct comparison of BCG Tokyo-172 with BCG Connaught did not show a significant difference in RFS between the two arms (47). In this trial, complete response rates were 90.3% and 85.0% for patients treated with BCG Tokyo-172 and BCG Connaught, respectively (P=0.9) with 2-year RFS rates of 73.2% and 68.8%, respectively. This trial had to be closed prematurely due to suspended BCG Connaught production in 2012 and is therefore underpowered.
The absence of well-designed head-to-head trials, utilizing homogeneous contemporaneous cohorts and therapeutic strategies in accordance with the standard of care, limits definitive conclusions on the superiority of one over the other BCG strains. Moreover, there is significant heterogeneity in oncologic outcomes between studies using the same BCG strain (Table 2) which makes comparisons even more challenging.
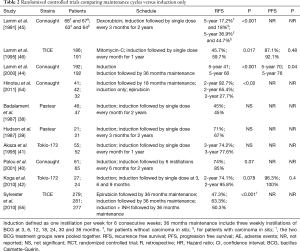
Full table
Conclusions and further perspectives
During decades BCG passaging caused genetic alterations of the original strain and generated a wide phylogenetic tree. However, due to study design and substantial differences in treatment schedules, it is still unclear whether this evolution process translates in different potency of strains with differential induction of host response.
Further randomized trials are needed, taking into account the genetic profile for strain selection. Moreover, the horizon of intravesical therapy is expanding through studies involving novel immunomodulatory drugs. Nevertheless, the heterogeneity in BCG strains may make the results of the combination studies more heterogeneous, undermining therapeutic counselling when translated for patient counselling and differential clinical decision making relative to alternative therapeutic strategies. Combinations therapies are the most promising to improve upon the current treatment efficacy achieved by optimal BCG therapy. With the first immunotherapy, BCG, urologists have gained much experience and understanding of the immune system and its richness in helping prevent, treat and cure bladder cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Babjuk M, Böhle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol 2009;56:247-56. [Crossref] [PubMed]
- Shelley MD, Kynaston H, Court J, et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int 2001;88:209-16. [Crossref] [PubMed]
- Sylvester RJ. van der MEIJDEN AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 2002;168:1964-70. [Crossref] [PubMed]
- Spencer BA, McBride RB, Hershman DL, et al. Adjuvant intravesical bacillus calmette-guérin therapy and survival among elderly patients with non-muscle-invasive bladder cancer. J Oncol Pract 2013;9:92-8. [Crossref] [PubMed]
- Witjes JA, Palou J, Soloway M, et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guérin (BCG): results of an international individual patient data survey (IPDS). BJU Int 2013;112:742-50. [Crossref] [PubMed]
- Chamie K, Saigal CS, Lai J, et al. Quality of care in patients with bladder cancer: a case report? Cancer 2012;118:1412-21. [Crossref] [PubMed]
- Kamat AM, Hegarty PK, Gee JR, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Screening, diagnosis, and molecular markers. Eur Urol 2013;63:4-15. [Crossref] [PubMed]
- Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007;178:2314-30. [Crossref] [PubMed]
- Brosch R, Gordon SV, Garnier T, et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A 2007;104:5596-601. [Crossref] [PubMed]
- Leung AS, Tran V, Wu Z, et al. Novel genome polymorphisms in BCG vaccine strains and impact on efficacy. BMC Genomics 2008;9:413. [Crossref] [PubMed]
- Shariat SF, Chade DC, Karakiewicz PI, et al. Update on intravesical agents for non-muscle-invasive bladder cancer. Immunotherapy 2010;2:381-92. Erratum in: Immunotherapy 2014;6:1238. [Crossref] [PubMed]
- Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 1976;116:180-3. [Crossref] [PubMed]
- Behr MA. BCG--different strains, different vaccines? Lancet Infect Dis 2002;2:86-92. [Crossref] [PubMed]
- Gan C, Mostafid H, Khan MS, et al. BCG immunotherapy for bladder cancer--the effects of substrain differences. Nat Rev Urol 2013;10:580-8. Erratum in: Nat Rev Urol 2015;12:360. [Crossref] [PubMed]
- Old LJ, Clarke DA, Benacerraf B. Effect of Bacillus Calmette-Guerin infection on transplanted tumours in the mouse. Nature 1959;184:291-2. [Crossref] [PubMed]
- Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol 2014;11:153-62. [Crossref] [PubMed]
- Becich MJ, Carroll S, Ratliff TL. Internalization of bacille Calmette-Guerin by bladder tumor cells. J Urol 1991;145:1316-24. [Crossref] [PubMed]
- Coplen DE, Brown EJ, McGarr J, et al. Characterization of fibronectin attachment by a human transitional cell carcinoma line, T24. J Urol 1991;145:1312-5. [Crossref] [PubMed]
- Durek C, Brandau S, Ulmer AJ, et al. Bacillus-Calmette-Guérin (BCG) and 3D tumors: an in vitro model for the study of adhesion and invasion. J Urol 1999;162:600-5. [Crossref] [PubMed]
- Schorey JS, Holsti MA, Ratliff TL, et al. Characterization of the fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Mol Microbiol 1996;21:321-9. [Crossref] [PubMed]
- Hudson MA, Ritchey JK, Catalona WJ, et al. Comparison of the fibronectin-binding ability and antitumor efficacy of various mycobacteria. Cancer Res 1990;50:3843-7. [PubMed]
- Ikeda N, Honda I, Yano I, et al. Bacillus calmette-guerin Tokyo172 substrain for superficial bladder cancer: characterization and antitumor effect. J Urol 2005;173:1507-12. [Crossref] [PubMed]
- Ojea A, Nogueira JL, Solsona E, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. Eur Urol 2007;52:1398-406. [Crossref] [PubMed]
- Mack D, Frick J. Five-year results of a phase II study with low-dose bacille Calmette-Guerin therapy in high-risk superficial bladder cancer. Urology 1995;45:958-61. [Crossref] [PubMed]
- Lebret T, Gaudez F, Hervé JM, et al. Low-dose BCG instillations in the treatment of stage T1 grade 3 bladder tumours: recurrence, progression and success. Eur Urol 1998;34:67-72. [Crossref] [PubMed]
- Lebret T, Bohin D, Kassardjian Z, et al. Recurrence, progression and success in stage Ta grade 3 bladder tumors treated with low dose bacillus Calmette-Guerin instillations. J Urol 2000;163:63-7. [Crossref] [PubMed]
- Hurle R, Losa A, Ranieri A, et al. Low dose Pasteur bacillus Calmette-Guerin regimen in stage T1, grade 3 bladder cancer therapy. J Urol 1996;156:1602-5. [Crossref] [PubMed]
- Losa A, Hurle R, Lembo A. Low dose bacillus Calmette-Guerin for carcinoma in situ of the bladder: long-term results. J Urol 2000;163:68-71; discussion 71-2. [Crossref] [PubMed]
- Morales A, Nickel JC, Wilson JW. Dose-response of bacillus Calmette-Guerin in the treatment of superficial bladder cancer. J Urol 1992;147:1256-8. [Crossref] [PubMed]
- Takashi M, Wakai K, Ohno Y, et al. Evaluation of a low-dose intravesical bacillus Calmette-Guérin (Tokyo strain) therapy for superficial bladder cancer. Int Urol Nephrol 1995;27:723-33. [Crossref] [PubMed]
- Mack D, Frick J. Low-dose BCG in superficial bladder cancer with strain Connaught Canada--as effective as strain Pasteur Paris? Eur J Cancer 1994;30A:1728-9. [Crossref] [PubMed]
- Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 2013;63:462-72. [Crossref] [PubMed]
- Martínez-Piñeiro JA, Flores N, Isorna S, et al. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guérin with a reduced dose of 27 mg in superficial bladder cancer. BJU Int 2002;89:671-80. [Crossref] [PubMed]
- Rentsch CA, Birkhäuser FD, Biot C, et al. Bacillus Calmette-Guérin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol 2014;66:677-88. [Crossref] [PubMed]
- Hofbauer SL, Shariat SF, Chade DC, et al. The Moreau Strain of Bacillus Calmette-Guerin (BCG) for High-Risk Non-Muscle Invasive Bladder Cancer: An Alternative during Worldwide BCG Shortage? Urol Int 2016;96:46-50. [Crossref] [PubMed]
- Bisiaux A, Thiounn N, Timsit MO, et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J Urol 2009;181:1571-80. [Crossref] [PubMed]
- Badalament RA, Herr HW, Wong GY, et al. A prospective randomized trial of maintenance versus nonmaintenance intravesical bacillus Calmette-Guérin therapy of superficial bladder cancer. J Clin Oncol 1987;5:441-9. [Crossref] [PubMed]
- Hudson MA, Ratliff TL, Gillen DP, et al. Single course versus maintenance bacillus Calmette-Guerin therapy for superficial bladder tumors: a prospective, randomized trial. J Urol 1987;138:295-8. [Crossref] [PubMed]
- Palou J, Laguna P, Millán-Rodríguez F, et al. Control group and maintenance treatment with bacillus Calmette-Guerin for carcinoma in situ and/or high grade bladder tumors. J Urol 2001;165:1488-91. [Crossref] [PubMed]
- Akaza H, Hinotsu S, Aso Y, et al. Bacillus Calmette-Guérin treatment of existing papillary bladder cancer and carcinoma in situ of the bladder. Four-year results. The Bladder Cancer BCG Study Group. Cancer 1995;75:552-9. [Crossref] [PubMed]
- Koga H, Ozono S, Tsushima T, et al. Maintenance intravesical bacillus Calmette-Guérin instillation for Ta, T1 cancer and carcinoma in situ of the bladder: randomized controlled trial by the BCG Tokyo Strain Study Group. Int J Urol 2010;17:759-66. [Crossref] [PubMed]
- Witjes JA, Dalbagni G, Karnes RJ, et al. The efficacy of BCG TICE and BCG Connaught in a cohort of 2,099 patients with T1G3 non-muscle-invasive bladder cancer. Urol Oncol 2016;34:484.e19-e25. [Crossref] [PubMed]
- Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124-9. [Crossref] [PubMed]
- Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N Engl J Med 1991;325:1205-9. [Crossref] [PubMed]
- Lamm DL, Blumenstein BA, David Crawford E, et al. Randomized intergroup comparison of bacillus calmette-guerin immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder a southwest oncology group study. Urol Oncol 1995;1:119-26. [Crossref] [PubMed]
- Sengiku A, Ito M, Miyazaki Y, et al. A prospective comparative study of intravesical bacillus Calmette-Guérin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. J Urol 2013;190:50-4. [Crossref] [PubMed]
- Vegt PD, Witjes JA, Witjes WP, et al. A randomized study of intravesical mitomycin C, bacillus Calmette-Guerin Tice and bacillus Calmette-Guerin RIVM treatment in pTa-pT1 papillary carcinoma and carcinoma in situ of the bladder. J Urol 1995;153:929-33. [Crossref] [PubMed]
- Mukherjee A, Persad R, Smith PJ. Intravesical BCG treatment for superficial bladder cancer: long-term results using two different strains of BCG. Br J Urol 1992;69:147-50. [Crossref] [PubMed]
- Inamoto T, Ubai T, Nishida T, et al. Comparable effect with minimal morbidity of low-dose Tokyo 172 strain compared with regular dose Connaught strain as an intravesical bacillus Calmette-Guérin prophylaxis in nonmuscle invasive bladder cancer: Results of a randomized prospective comparison. Urol Ann 2013;5:7-12. [Crossref] [PubMed]
- Fellows GJ, Parmar MK, Grigor KM, et al. Marker tumour response to Evans and Pasteur bacille Calmette-Guérin in multiple recurrent pTa/pT1 bladder tumours: report from the Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party). Br J Urol 1994;73:639-44. [Crossref] [PubMed]
- Witjes WP, Witjes JA, Oosterhof GO, et al. Update on the Dutch Cooperative Trial: mitomycin versus bacillus Calmette-Guérin-Tice versus bacillus Calmette-Guérin RIVM in the treatment of patients with pTA-pT1 papillary carcinoma and carcinoma in situ of the urinary bladder. Dutch South East Cooperative Urological Group. Semin Urol Oncol 1996;14:10-6. [PubMed]
- Boehm BE, Cornell JE, Wang H, et al. Efficacy of bacillus Calmette-Guérin Strains for Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Network Meta-Analysis. J Urol 2017;198:503-10. [Crossref] [PubMed]
- Hinotsu S, Akaza H, Naito S, et al. Maintenance therapy with bacillus Calmette-Guérin Connaught strain clearly prolongs recurrence-free survival following transurethral resection of bladder tumour for non-muscle-invasive bladder cancer. BJU Int 2011;108:187-95. [Crossref] [PubMed]
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guérin, and bacillus Calmette-Guérin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 2010;57:766-73. [Crossref] [PubMed]


