Keeping up with the prostate-specific membrane antigens (PSMAs): an introduction to a new class of positron emission tomography (PET) imaging agents
Introduction
Prostate-specific membrane antigen (PSMA) is a transmembrane protein, which is present in the epithelium of surrounding prostatic ducts (1). Its coding domain is usually expressed in patients with clinically significant prostate cancer (PCa) and its over-expression on the cell membrane of prostatic epithelial cells has been shown to be a sensitive biomarker of aggressive disease. PSMA expression increases with both increasing stage and tumor grade and its radiolabeling has been investigated in a variety of PCa settings including local staging, metastasis, biochemical recurrence (BCR), and image-guided interventions (2-4).
PSMA ligands that are used in imaging bind to the surface receptor and are then internalized within cells. Due to their small size, unbound ligands are rapidly cleared by the body leading to high tumor to background ratios (5). PSMA is not specific to PCa, and its uptake is observed in normal organs including salivary/lacrimal glands, small bowel, proximal renal tubular cells, as well as the neovasculature of some tumors (transitional cell carcinomas, renal cell carcinomas, colon, esophageal, thyroid, lung and brain cancer) (6,7). Labeling the ligand with either 68-Gallium (68Ga) or 18-Fluoride (18F) results in an agent that is rapidly taken up in PCa and rapidly cleared. PSMA radioligands are administered as an intravenous bolus and scanning commences 1–2 hours post injection. Most PSMA agents are excreted via the urinary system except for PSMA-1007 which is excreted via the hepatobiliary system. The first PSMA agents to be reported in humans were 68Ga labeled tracers. 68Ga requires a generator and only a limited number of doses can be extracted per day; however, once obtained, labeling of the PSMA targeting ligand is straightforward (8). 68Ga has a 68-minute half-life, making it challenging to deliver from a centralized facility and its high positron energy leads to a slightly decreased spatial resolution. On the other hand, 18F can be produced in large batches at a centralized facility with a cyclotron, conjugated to its ligand and then shipped to local nuclear medicine departments making production simpler. Moreover, 18F has lower positron energies resulting in sharper imaging. Both agents are being currently commercialized.
Although thousands of patients have been scanned to date, none of the PSMA radioligands have been approved by the U.S. Food and Drug Administration or European Medicines Agency. Thus, PSMA positron emission tomography (PET) imaging lacks consensus regarding its indications. European guidelines suggest its use in the setting of staging and BCR (9). In the U.S., the only approved PSMA imaging agent is a radiolabeled anti-PSMA antibody, capromab pendetide (ProstaScint; EUSA Pharma) which has been available since the late 1990s. Unfortunately, this agent has multiple drawbacks including its very slow clearance and its targeting of the intracellular domain of PSMA causing low uptake and increased background signal (10,11). Moreover, capromab is labeled with 111-Indium (111In) which is a long-lived single photon emission computed tomography (SPECT) agent resulting in lower resolution images. For these reasons, this agent is not commonly employed in the clinic. The new generation of small molecule PSMA-based tracers show outstanding sensitivity and specificity for PCa detection, and are likely to become available in the clinic in the next few years.
A variety of other PET tracers have been proposed for imaging PCa. The most widely used oncologic PET/CT imaging agent,18F-fluorodeoxyglucose (FDG), is not useful for prostate imaging because of its low uptake in most PCa (12). European guidelines mention the use of 18F choline PET/CT in patients with recurrent PCa; however, its sensitivity is relatively poor and most patients must have PSA >2 ng/mL before 18F choline PET/CT turns positive (13). A recent study by Afshar et al. compared 68Ga-PSMA-11, to 18F-fluoromethylcholine PET/CT in a cohort of 37 men with biochemically recurrent PCa (13,14) and showed that 68Ga-PSMA-11 PET/CT was more sensitive than 18F-fluoromethylcholine PET/CT (87% vs. 70%). Moreover, the detection rate in 68Ga-PSMA-11 PET/CT group was significantly higher (78 lesions detected in 32 patients vs. 56 lesions in 26 patients, P=0.04). All 56 lesions observed in 18F-fluoromethylcholine PET/CT studies were also visualized in 68Ga-PSMA-11 PET/CT, indicating its superior performance in this small cohort. In line with this publication, more recent studies of PSMA tracers demonstrate superior performance consistent with this study over both 18F choline-based radiotracers (such as 18F-fluoromethylcholine) and fluciclovine (a recent FDA approved 18F radiotracer for detection of recurrent PCa by mechanisms of amino acid transport). 11C choline is approved in the U.S. for recurrent PCa but its use is limited by the short half-life of the agent (20 min) and the requirement for an onsite cyclotron and radiochemistry capabilities (15).
A variety of PSMA radioligands have been developed with slightly different properties. Almost all PSMA-based PET agents are urea-based molecules which bind with high affinity to the enzymatic portion of PSMA. Examples include 68Ga-PSMA-HBED-CC (PSMA-11), 68Ga-PSMA-617, 68Ga-PSMA-I&T, 18F-DCFBC, 18F-DCFPyL, and 18F-PSMA-1007, all of which will be discussed in this review. Additionally, new SPECT-based markers which are investigated in a few centers will be discussed as a feasible alternative for PSMA-PET imaging (16). Theranostic applications of PSMA are briefly discussed, but are beyond the scope of this article.
PSMA-PET tracers
68Ga-PSMA-11
68Ga-PSMA-11 (also known as 68Ga-HBED-CC-PSMA) was introduced in humans in 2012 and is the most widely used and studied PSMA-ligand (5,17,18) (Figure 1). 68Ga is eluted from a 68Ge/68Ga generator system and added to a binding kit containing the precursor (19). Many centers label the compound on-site because of the relatively short 68-minute half-life of 68Ga (20).
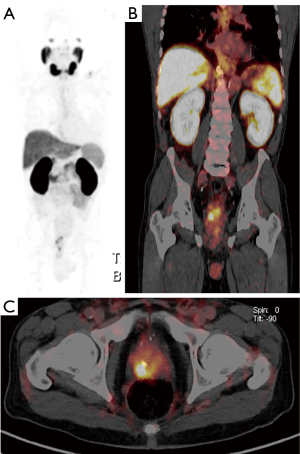
This agent has been investigated in all stages of PCa. It was histologically validated in a cohort with localized primary PCa by Eiber et al. which compared multiparametric magnetic resonance imaging (mpMRI), PET and combined 68Ga-PSMA-11 PET/MRI for localization of primary PCa in 53 men with biopsy-proven PCa (21). In this study, Eiber et al. showed that simultaneous 68Ga-PSMA-11 PET/MRI was superior to mpMRI or PET alone for PCa localization, with a sensitivity and specificity of 58% and 82% for mpMRI; 64% and 94% for PET; 76% and 97% for 68Ga-PSMA-11 PET/MRI, respectively. 68Ga-PSMA-11 PET images have been used to direct biopsy in patients with a high suspicion for localized PCa. This study additionally introduced the possibility of incorporating 68Ga-PSMA PET/MR into image-guided biopsy.
The role of 68Ga-PSMA-11 PET/CT is controversial for lymph node staging in primary diagnosed PCa. In the setting of primary PCa staging Herlemann et al. showed that 68Ga-PSMA-11 PET/CT scan provides high accuracy for preoperative lymph node staging in patients with intermediate to high risk PCa (22). This study was validated by nodal histopathology obtained at surgery in all 71 reported lymph node regions from 34 patients and it concluded that PSMA PET/CT was superior to CT for identifying lymph node metastases with a sensitivity of 84% vs. 65% and specificity of 82% vs. 76%, especially in nodes not fulfilling the size criteria on CT. A similar study performed PSMA-11 PET/CT imaging in 30 patients prior to radical prostatectomy and extended pelvic lymph node detection, found that 67% of patients were found to have false negative findings (23). The study images were not re-evaluated by imaging subspecialists, which raised a concern for under-reporting with considerable inhomogeneity in initial reports. Nevertheless, the use of PSMA in lymph node staging appears to have an added value to the previously available CT size criteria.
It is estimated that BCR occurs in at least 20–30% of patients after definitive treatment of PCa (24). In this setting, PSMA PET/CT demonstrates the greatest advantage over other imaging methods, with multiple studies showing that 68Ga-PSMA-11 PET can detect the likely site of recurrence (local, nodal or distant) in most cases (25,26). 68Ga-PSMA-11 PET/CT can be effective even when PSA values are low (0.2–1.0 ng/mL) (27). At these prostate-specific antigen (PSA) levels, most other available tracers fail to detect metastasis. The patient gains the most from early diagnosis and the likelihood of cure decreases with advancing metastatic burden (17). The sensitivity of PSMA-11 depends on PSA doubling times and initial Gleason scores, indicating that even lower PSA values may still be possible to detect on imaging if the doubling time is rapid or the tumor grade is high (26). Afshar et al. analyzed data from 319 men with BCR, and found that 68Ga-PSMA-11 PET/CT was highly specific for PCa (25). Further analysis revealed an overall sensitivity of 88%, which was dependent on the PSA value. Eiber et al. correlated the efficacy of the 68Ga-PSMA-11 PET/CT scan and PSA concentration in 248 men (28). Among subjects with PSA values ≥2, 1–2, 0.5–1 or 0.2–0.5 ng/mL the efficacy was 97%, 93%, 73% and 58%, respectively. For instance, even when the PSA level was as low as <0.2 ng/mL or between 0.2 and <0.5 ng/mL the sensitivity was 44.4% and 72% respectively. Thus, 68Ga-PSMA-11 PET/MRI scan could be used for the detection of locally recurrent PCa. This result exceeds the sensitivity of other available non-PSMA PET tracers in the recurrence setting or for conventional imaging (29,30).
In the setting of metastatic disease, Pyka et al. compared standard plain 99mTc bone scintigraphy and 68Ga-PSMA-11 PET/CT scan for the detection of bone metastases in 126 patients with PCa (30). 68Ga-PSMA-11 PET/CT was shown to perform with a significantly higher diagnostic accuracy than bone scintigraphy for the assessment of overall bone involvement (PET/CT and scintigraphy sensitivity were 99–100% and 87–89%, respectively; specificity 88.2–100% and 61–96%, respectively).
68Ga-PSMA-617
The use of PSMA-targeted therapy has been revolutionized by modifying the most widely used Gallium-based ligand, PSMA-11, to PSMA-617. This altered PSMA-ligand, which has similar pharmacokinetic properties as PSMA-11, may be labelled with Actinium-255, 68Ga, 177Lu, 111In, or Yttrium-99, and has gained considerable interest in the scientific community (31).
The compound has been evaluated in phase I studies for its dosimetry, safety, and efficacy as a diagnostic agent, as well as response and tolerability in its therapeutic form (32,33). Its excretory characteristics, which show rapid renal clearance in preclinical studies has thus far shown to be slower than PSMA-11 (34,35). For this reason, it is currently hypothesized that 18F-PSMA-1007, a compound which is structurally similar to PSMA-617 may serve as a better surrogate marker for imaging and therapy monitoring than PSMA-11 (36). A more recent study analyzed lesion-based parameters (i.e., SUVmax, metabolic tumor volume) which were shown to correlate well with Gleason score and PSA levels indicating that it may be used to predict metastatic PCa, but has not been compared head-to-head to PSMA-11 or other compounds (37).
The use of 177Lu-PSMA-617 for radionuclide treatment in metastatic castration-resistant PCa has been an area of interest, as this population had most therapeutic options exhausted. A study by Hofman et al. on a group of 30 patients, as part of a prospective phase II trial showed its potential benefit in this cohort (38). The results showed that the treatment was well tolerated, with 37% of patients experiencing a minimum of a ten-point improvement in global health score, including a decrease in pain severity. The most commonly experienced toxic effects included dry mouth (87%), low-grade nausea and fatigue (50%), all of which were attributed to the treatment. Similarly, favorable results were shown from a study that retrospectively analyzed 145 patients previously treated with multiple cycles of 177Lu-PSMA-617 (39). This study also identified that patients with very advanced disease with a high alkaline phosphatase (>220 IU/L) and visceral metastasis poorly responded to the treatment, and were more likely to relapse.
The marker’s robust capabilities to be conjugated with either diagnostic (e.g., 68Ga) or therapeutic (e.g., 177Lu) radioisotopes is currently being investigated in a large multi-center trial in patients with metastatic castrate-resistant PCa as part of the VISION trial (40), and its adoption in Europe is rapidly growing. Larger, multi-center studies will help elucidate its role in imaging and therapy, especially in patients with metastatic castrate-resistant disease.
68Ga-PSMA-I&T
68Ga-PSMA-I&T demonstrates similar biodistribution and imaging properties as 68Ga-PSMA-11, with I &T referring to “imaging and therapy” (41). Thus, this compound can be used both for imaging and, when labeled with a therapeutic radioisotope, for treatment. Both tissue distribution pattern and time of accumulation are comparable to PSMA-11. However, biodistribution studies showed slightly lower physiologic tracer uptake in the liver, spleen and intestine and a slightly higher uptake in the proximal tubules of the kidneys and salivary glands. The standard injected activity has been determined to be about the same when compared to 68Ga-PSMA-11 (150 MBq) (42). These changes have an impact on the effective dose coefficients for specific organs (1.71E-02 vs. 1.99E-02 mSv/MBq, for total body, 1.73E-01 vs. 6.74E-02 mSv/MBq for urinary bladder wall, and 1.22E-01 vs. 2.20E-01 mSv/MBq for kidney, respectively) (42,43). Similar to 68Ga-PSMA-11, 68Ga-PSMA-I&T demonstrates predominantly renal excretion. To overcome this problem, an intravenous diuretic injection just after imaging at 60 min was shown to lower mean urine activity at 180 min, especially for prostatic bed assessment, as well as improve detection of pelvic lymph nodes by ureteric wash-out (44).
Similar to 68Ga-PSMA-11 PET/CT, 68Ga-PSMA-I&T PET/CT has been used for assessment of primary PCa before prostatectomy (45) and BCR (46). In a study published by Schmuck et al., 240 men with BCR were scanned with 68Ga-PSMA-I&T. Cancer detection rates were 94.2% for PSA value ≥2 ng/mL; 72% for 1 to <2 ng/mL; 59% for 0.5 to <1 ng/mL; 56% for >0.2 to <0.5 ng/mL and 39% for 0.01 to 0.2 ng/mL. In their retrospective analysis of 83 men, Berliner et al. demonstrated, that 68Ga-PSMA-I&T PET/CT detection rate also positively correlated with PSA serum levels, and were 100% for PSA ≥10.0 ng/mL, 100% for 5.0 to <10.0 ng/mL, 93% for 2.0 to <5.0 ng/mL, 70% for 1.0 to <2.0 ng/mL, 55% for 0.5 to <1.0 ng/mL, and 52% for <0.5 ng/mL, thus both findings are comparable to the performance of 68Ga-PSMA-11 (47).
68Ga-PSMA-I&T differs from 68Ga-PSMA-11 in its chemical structure which results in slightly higher receptor affinity leading to a slightly higher diagnostic accuracy when compared 68Ga-PSMA-11 (48). PSMA-I&T can be labelled with 177Lu or Actinium-255 without significant changes in affinity and thus, can be used in PCa therapy (41). Nevertheless, most research on PCa theranostic ligands is performed with a compound closely related to PSMA-11; PSMA-617 (41).
18F-DCFBC and 18F-DCFPyL
These two 18F-labeled PSMA agents arose from the same laboratory. 18F-DCFBC was the first-generation agent and was also a small-molecule urea-derivative PSMA-targeted inhibitor of PSMA. The second generation agent is known as 18F-DCFPyL {2-(3-(1-carboxy-5-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid} (49) and was developed in part to overcome the limitation of 18F-DCFBC which bound serum proteins. Thus, it had a long clearance time from the blood pool which interfered with lymph-node detection in the retroperitoneum and pelvis adjacent to large blood vessels. The next generation radiotracer, 18F-DCFPyL, not only exhibits substantially greater binding affinity for PSMA but also had much less blood pool activity than its predecessor. Because of these enhanced characteristics, the focus of development is now shifted to 18F-DCFPyL which is in the process of commercialization and approval.
Like the gallium-labeled compounds, the family of fluorinated compounds are small, urea-based molecules, that binds to the extracellular domain of PSMA. Unlike the gallium compounds which have a chelate attached to the PSMA-binding moiety, the fluorinated compounds are directly labeled resulting in a slightly smaller molecular weight. The absence of a chelate, however, means that the interchangeability of radioisotopes, including therapeutic radioisotopes, is more limited. As small molecules, these PSMA agents are predominantly excreted through the urinary tract, consequently the bladder shows the highest activity, followed by the stomach, heart and kidneys, but like the gallium compounds, it also accumulates in the salivary glands, kidneys, urinary bladder, liver, spleen, and small intestines (50). The effective dose is 0.0165 mSv/MBq for a 370 MBq dose, with the kidneys receiving the highest estimated radiation dose (0.0945 mGy/MBq) followed by the urinary bladder wall (0.0864 mGy/MBq), submandibular glands (0.0387 mGy/MBq), and liver (0.0380 mGy/MBq) (50). These dose-limiting organs should be taken into consideration for the potential use of this compound in the PSMA treatment setting (51).
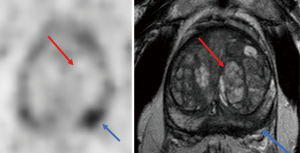
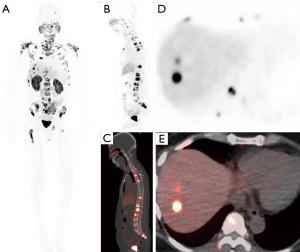
Currently, more studies have been published with 18F-DCFBC than 18F-DCFPyL. Turkbey et al. (4), showed that localized PCas were detected by 18F-DCFBC and mpMRI and these lesions were subsequently validated with MRI/transrectal ultrasound guided biopsy or radical prostatectomy. Tumor uptake was noted to be very high with maximum standardized uptake values (SUVmax) greater than 100 in some lesions 1 and 2 h post-injection (50). The method was highly sensitive for intermediate and high-grade primary cancers (4) (Figure 2). 18F-DCFPyL PET uptake, was contingent on higher Gleason pattern 4 within tumors, whereas lower-grade PCa with Gleason pattern 3 tended to have much lower radiotracer uptake (52) (Figure 3) (2).
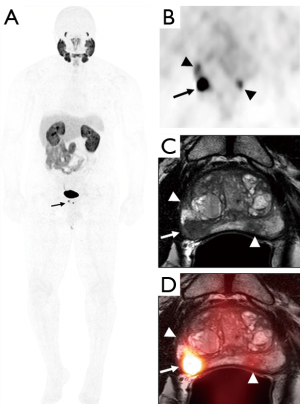
These agents have also been used in PCa staging. Gorin et al. (3) prospectively evaluated the diagnostic performance of 18F-DCFPyL PET/CT in staging high-risk PCa in 25 men (53). In the study, 18F-DCFPyL-PET correctly identified 5 of the 7 cases with otherwise clinically occult positive lymph nodes when compared with surgical pathology, while over staging the remaining two cases. In these two cases, this may have been caused by attributing ureteric uptake as focal lymph node uptake, and thus assigning it to a false-positive finding. In the metastatic setting, this series of 18F-DCFPyL-PET identified sites of distant disease in 12% of the patients (53).
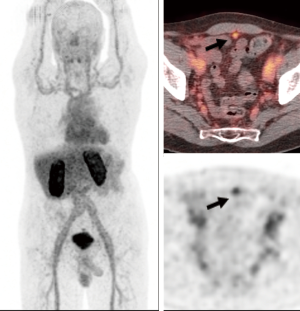
In the BCR setting, Turkbey et al. (4) demonstrated that 18F-DCFBC could detect recurrences (either local or lymph node) in 60.3% of patients without evidence of disease on conventional imaging (Figure 4). Imaging detection rate correlated to PSA values with a threshold PSA of 0.78 ng/mL or above, highly predictive of a positive scan. Interestingly, focal 18F-DCFBC results changed clinical management in 51.2% of patients due to the detection of more-than-expected disease. This compelling application for non-invasive detection of recurrent disease by radiolabeled PSMA binding agents is promising in advancing therapeutic strategies. Subsequently, it was shown that the sensitivity of both 18F-DCFPyL (n=62) and 68Ga-PSMA-11 (n=129) was significantly associated with absolute PSA levels (54). In this study, Dietlein et al. showed that for a PSA range of 0.5–3.5 µg/L, the PSA-stratified sensitivity of 18F-DCFPyL significantly exceeded that of 68Ga-PSMA-11 (88% vs. 66%). Outside of this range, sensitivity was comparably low (for PSA <0.5 µg/L) or high (for PSA >3.5 µg/L). After radiotherapy, tracer sensitivity was largely PSA-independent. In a subsample of 25 patients examined with both tracers, the distribution patterns of 18F-DCFPyL and 68Ga-PSMA-11 were comparable, but 18F-DCFPyL scans detected additional lesions in 36% of the patients (54). Since both radiotracers target the same epitope on the PSMA molecule, the improved lesion detection efficiency of 18F-DCFPyL relative to the 68Ga-labelled PSMA agent may reflect the intrinsic advantages of 18F as a PET radionuclide or subtle pharmacokinetic differences. 18F-DCFPyL has the advantages of being cyclotron-based and has a longer half-life and more favorable energy levels, resulting in improved spatial resolution, owing to lower background activity in non-target tissues (54).
The fluorinated compounds have also been used in the metastatic setting. 18F-DCFBC outperformed conventional imaging and detected a larger number of lesions in lymph nodes, bone and soft tissue with a sensitivity of 92% compared to 71% for traditional imaging modalities (55). In the metastatic setting, Rowe et al. (56) also showed that 18F-DCFPyL PET/CT outperformed conventional imaging by detecting an overall larger number of positive sites of either local recurrence, lymph nodes, or bones (138 definitive sites and 1 equivocal versus 30 definitive sites with 15 equivocal) (56). Several prospective and multicenter trials are currently under way to better determine the diagnostic performance of 18F-DCFPyL PET for distant sites of PCa (Figures 5,6).
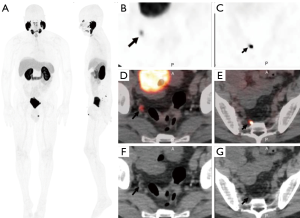
Imaging of metastatic bone disease has yielded intriguing findings. In comparison to the highly sensitive but nonspecific 18F-NaF, the detection of metastatic bone lesions by 18F-DCFBC or 18F-DCFPyL depends on the treatment status and disease stage (57) (Figure 7). In patients on androgen deprivation therapy, 18F-NaF identifies more bone lesions than 18F-DCFBC in the early, castrate sensitive phase but as disease advances to castration resistance, detection rates are similar in both tracers. This is thought to occur due to hormonal suppression of PCa cells leading to senescence and reduced PSMA expression which is reactivated when resistance develops. The secondary effects of bone remodeling appear to persist throughout and are consequently visible with 18F-NaF but not PSMA PET/CT (58). The implication is that PSMA radiotracers may be able to distinguish castration sensitive and castration resistant disease of the bones when combined with a cross sectional bone scan method. This may provide insights into the origins of castration resistance.
18F-PSMA-1007
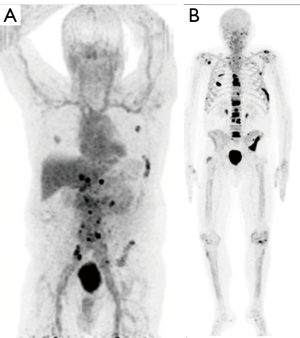
Recently, a compound based on 68Ga-PSMA-617, which is 18F-labeled has been introduced with a similar diagnostic and therapeutic potential to 68Ga-PSMA-11 or 68Ga-PSMA-I&T (36). PSMA-1007 presents a unique biodistribution compared to the other known PSMA-ligands as excretion follows the hepatobiliary pathway, instead of the more common urinary route (59). This provides several advantages with regard to primary staging and local recurrence, as there is less uptake in the anatomical area of the prostate and surrounding tissue. Also, the radiation dose is comparable to other PSMA-ligands, but due to hepatobiliary excretion there is a slightly different organ dose distribution with a higher dose to the liver parenchyma and lower radiation dose to the urinary bladder (Figure 8). In preliminary studies, this excretory pathway is beneficial in the visualization of prostate and ureter region as well as pelvis for metastatic lymph nodes (59-61), but comparative studies have not been done. When PSMA-1007 was compared to mpMRI for local staging, both modalities exhibited similar accuracy (60). Paddubny et al. presented its use in the setting of BCR and equivocal findings on MRI (Figure 9). In this study, the 18F-PSMA-1007 PET/CT scan allowed identification of local PCa recurrence, which was otherwise not seen on mpMRI (62).
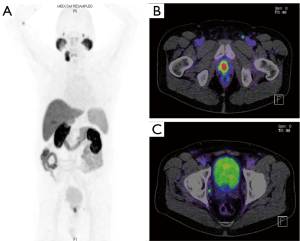
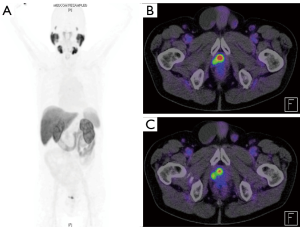
A recent publication with PSMA-1007 showed a high sensitivity in BCR patients with low (0.5–1 ng/mL) and very low (0.2–0.5 ng/mL) PSA of 74% and 62%, respectively. This study demonstrated an improved detection rate in over 150 BCR patients with a PSA-level between 0.2–0.5 ng/mL of over 60%, which was most likely due to the different energy profile using fluorine vs. gallium (63).
PSMA-1007 has shown high sensitivity in specific cases. Nodes as small as 1mm were detected with a very high sensitivity reaching 95% (59). Since PSMA-1007 has a similar structure to PSMA-617, it can also be used as a theranostic with 177Lu-PSMA-617 and future studies will define the clinical importance of this compound (36,64).
SPECT PSMA
SPECT is a broadly available imaging technique. SPECT is widely used in myocardial perfusion and functional brain imaging (65). The modality uses a conventional gamma camera, which has a relatively lower unit price making SPECT more economical. Instead of planar views, which are used in scintigraphy, a set of several 2D projections are acquired and used to create 3D images using tomographic reconstruction. The additional use of CT, as in PET imaging, may then be applied for improved spatial colocalization.
The emergence of PSMA as a prostate-specific marker, and its recent developments in 68Ga and 18F-labelled PET imaging have been encouraging for possible developments of Technetium-based PSMA radiotracers which could be used in SPECT. One PSMA radiotracer, 99mTc-MIP-1404 has demonstrated low blood pool and hepatobiliary excretion in a phase I clinical trial (66). A more recent phase II clinical trial of 105 patients with intermediate and high-risk PCa prior to radical prostatectomy was found to perform with an overall detection rate of 94%, and showed tumor-to-background ratios of lesions correlating with Gleason score. Lymph node invasion (LNI) was detected with a sensitivity of 50% and specificity of 87% (67). Another study by Reinfelder et al., had slightly lower overall detection of 77% for cancer, but this cohort was restricted to patients with BCR (68,69).
With the widespread availability of SPECT scanners, and the relatively low cost compared to PET, there is a possibility that the current clinical practice of using 99mTc bone scintigraphy (bone scan) may be replaced with a PSMA-specific 99mTc marker for PCa staging. 68Ga-PSMA PET has previously shown to perform better than bone scan in the detection of bone metastasis (70). The use of PSMA-PET for bone staging, though was superior with the use of the anatomical colocalization with CT, which essentially doubles the radiation dose compared to the classic planar bone scan. SPECT/CT with PSMA ligand 99mTc-MIP-1427 was compared to conventional bone scans by Rathke et al. in 21 patients with metastatic disease. The study showed an advantage for PSMA SPECT/CT, mainly by decreasing the number of equivocal findings and by its higher specificity, as it does not readily accumulate in degenerative bone disease (71).
All current Technetium-based PSMA ligands are experimental, with clinical trials ongoing in Europe. In the United States, they remain to be approved for diagnostic purposes, and therefore adoption of the technique has been overshadowed by the larger ongoing trials of PET-based PSMA ligands.
Conclusions
The introduction of PSMA PET ligands has been nothing short of transformational for the imaging of PCa. When comparing PSMA PET ligands to other tracers, substantial advantages to PSMA are seen including higher positive predictive values and higher sensitivity (28). However, there are now many PSMA PET ligands to choose from and it’s impossible to compare them without head-to-head studies which are expensive to conduct. The general impression is that they all perform comparably. Current guidelines are inconsistent and do not reflect the use of PSMA in the United States, with European use in the setting of staging and BCR. The ongoing trials and studies will most likely determine the fate of PSMA compounds and their place in PCa detection and staging. Prior to its broad approval, PSMA PET will remain somewhat of a mystery for most clinicians. Hopefully, this review casts some light on the variety of PSMA-based radionuclides that are now undergoing clinical testing.
Acknowledgements
Funding: The research in this study was funded by the Intramural Research Program of the National Institutes of Health as well as in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclosure: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- O’Keefe DS, Su SL, Bacich DJ, et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim Biophys Acta 1998;1443:113-27. [Crossref] [PubMed]
- Rowe SP, Gage KL, Faraj SF, et al. 18F-DCFBC PET/CT for PSMA-Based Detection and Characterization of Primary Prostate Cancer. J Nucl Med 2015;56:1003-10. [Crossref] [PubMed]
- Zamboglou C, Schiller F, Fechter T, et al. 68Ga-HBED-CC-PSMA PET/CT versus histopathology in primary localized prostate cancer: A voxel-wise comparison. Theranostics 2016;6:1619-28. [Crossref] [PubMed]
- Turkbey B, Mena E, Lindenberg L, et al. 18F-DCFBC Prostate-Specific Membrane Antigen-Targeted PET/CT Imaging in Localized Prostate Cancer: Correlation With Multiparametric MRI and Histopathology. Clin Nucl Med 2017;42:735-40. [Crossref] [PubMed]
- Eder M, Schäfer M, Bauder-Wüst U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 2012;23:688-97. [Crossref] [PubMed]
- Sweat SD, Pacelli A, Murphy GP, et al. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998;52:637-40. [Crossref] [PubMed]
- Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 2013;40:486-95. [Crossref] [PubMed]
- Eder M, Neels O, Müller M, et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals (Basel) 2014;7:779-96. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Representative EBP, et al. EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer. 2017.
- Troyer JK, Beckett ML, Wright GL Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer 1995;62:552-8. [Crossref] [PubMed]
- Taneja SS. ProstaScint(R) Scan: Contemporary Use in Clinical Practice. Rev Urol 2004;6 Suppl 10:S19-28. [PubMed]
- Schuster DM, Nanni C, Fanti S. PET Tracers Beyond FDG in Prostate Cancer. Semin Nucl Med 2016;46:507-21. [Crossref] [PubMed]
- Schmid DT, John H, Zweifel R, et al. Fluorocholine PET/CT in Patients with Prostate Cancer: Initial Experience. Radiology 2005;235:623-8. [Crossref] [PubMed]
- Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2014;41:11-20. [Crossref] [PubMed]
- Carroll PH, Mohler JL. NCCN Guidelines Updates: Prostate Cancer and Prostate Cancer Early Detection. J Natl Compr Canc Netw 2018;16:620-3. [Crossref] [PubMed]
- Janssen JC, Meißner S, Woythal N, et al. Comparison of hybrid 68Ga-PSMA-PET/CT and 99mTc-DPD-SPECT/CT for the detection of bone metastases in prostate cancer patients: Additional value of morphologic information from low dose CT. Eur Radiol 2018;28:610-9. [Crossref] [PubMed]
- Rauscher I, Maurer T, Fendler WP, et al. (68)Ga-PSMA ligand PET/CT in patients with prostate cancer: How we review and report. Cancer Imaging 2016;16:14. [Crossref] [PubMed]
- Rowe SP, Gorin MA, Allaf ME, et al. PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis 2016;19:223-30. [Crossref] [PubMed]
- Schuhmacher J, Maier-Borst W. A new 68Ge/68Ga radioisotope generator system for production of 68Ga in dilute HCl. Int J Appl Radiat Isot 1981;32:31-6. [Crossref]
- Satpati D, Shinto A, Kamaleshwaran KK, et al. Convenient Preparation of [68Ga]DKFZ-PSMA-11 Using a Robust Single-Vial Kit and Demonstration of Its Clinical Efficacy. Mol Imaging Biol 2016;18:420-7. [Crossref] [PubMed]
- Eiber M, Weirich G, Holzapfel K, et al. Simultaneous68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur Urol 2016;70:829-36. [Crossref] [PubMed]
- Herlemann A, Wenter V, Kretschmer A, et al. 68Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients with Prostate Cancer. Eur Urol 2016;70:553-7. [Crossref] [PubMed]
- Budäus L, Leyh-Bannurah SR, Salomon G, et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol 2016;69:393-6. [Crossref] [PubMed]
- Freedland SJ, Presti JC Jr, Amling CL, et al. Time trends in biochemical recurrence after radical prostatectomy: results of the SEARCH database. Urology 2003;61:736-41. [Crossref] [PubMed]
- Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015;42:197-209. [Crossref] [PubMed]
- Verburg FA, Pfister D, Heidenreich A, et al. Extent of disease in recurrent prostate cancer determined by [68Ga]PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. Eur J Nucl Med Mol Imaging 2016;43:397-403. [Crossref] [PubMed]
- Perera M, Papa N, Christidis D, et al. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol 2016;70:926-37. [Crossref] [PubMed]
- Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA-ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 2015;56:668-74. [Crossref] [PubMed]
- Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging 2017;44:1014-24. [Crossref] [PubMed]
- Eiber M, Pyka T, Okamoto S, et al. 68Gallium-HBED-CC-PSMA PET compared to conventional bone scintigraphy for evaluation of bone metastases in prostate cancer patients. Eur Urol Suppl 2016;15. [Crossref]
- Kelly JM, Amor-Coarasa A, Nikolopoulou A, et al. Assessment of PSMA targeting ligands bearing novel chelates with application to theranostics: Stability and complexation kinetics of 68Ga3+, 111In3+, 177Lu3+ and 225Ac3. Nucl Med Biol 2017;55:38-46. [Crossref] [PubMed]
- Ahmadzadehfar H, Rahbar K, Kürpig S, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res 2015;5:114. [Crossref] [PubMed]
- Rahbar K, Schmidt M, Heinzel A, et al. Response and Tolerability of a Single Dose of 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: A Multicenter Retrospective Analysis. J Nucl Med 2016;57:1334-8. [Crossref] [PubMed]
- Benešová M, Schäfer M, Bauder-Wüst U, et al. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J Nucl Med 2015;56:914-20. [Crossref] [PubMed]
- Afshar-Oromieh A, Hetzheim H, Kratochwil C, et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J Nucl Med 2015;56:1697-705. [Crossref] [PubMed]
- Giesel FL, Cardinale J, Schäfer M, et al. 18F-Labelled PSMA-1007 shows similarity in structure, biodistribution and tumour uptake to the theragnostic compound PSMA-617. Eur J Nucl Med Mol Imaging 2016;43:1929-30. [Crossref] [PubMed]
- Liu C, Liu T, Zhang N, et al. 68Ga-PSMA-617 PET/CT: a promising new technique for predicting risk stratification and metastatic risk of prostate cancer patients. Eur J Nucl Med Mol Imaging 2018;45:1852-61. [Crossref] [PubMed]
- Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol 2018;19:825-33. [Crossref] [PubMed]
- Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German Multicenter Study Investigating 177 Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med 2017;58:85-90. [Crossref] [PubMed]
- ClinicalTrials.gov. Study of 177Lu-PSMA-617 In Metastatic Castrate-Resistant Prostate Cancer (VISION). [cited 2018 May 29]. Available online: https://clinicaltrials.gov/ct2/show/NCT03511664
- Weineisen M, Schottelius M, Simecek J, et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J Nucl Med 2015;56:1169-76. [Crossref] [PubMed]
- Herrmann K, Bluemel C, Weineisen M, et al. Biodistribution and radiation dosimetry for a probe targeting prostate-specific membrane antigen for imaging and therapy. J Nucl Med 2015;56:855-61. [Crossref] [PubMed]
- Prasad V, Steffen IG, Diederichs G, et al. Biodistribution of [68Ga]PSMA-HBED-CC in Patients with Prostate Cancer: Characterization of Uptake in Normal Organs and Tumour Lesions. Mol Imaging Biol 2016;18:428-36. [Crossref] [PubMed]
- Derlin T, Weiberg D, von Klot C, et al. 68Ga-PSMA I&T PET/CT for assessment of prostate cancer: evaluation of image quality after forced diuresis and delayed imaging. Eur Radiol 2016;26:4345-53. [Crossref] [PubMed]
- Schmuck S, Mamach M, Wilke F, et al. Multiple time-point68Ga-PSMA I&T PET/CT for characterization of primary prostate cancer value of early dynamic and delayed imaging. Clin Nucl Med 2017;42:e286-93. [Crossref] [PubMed]
- Schmuck S, Nordlohne S, von Klot CA, et al. Comparison of standard and delayed imaging to improve the detection rate of [68Ga]PSMA I&T PET/CT in patients with biochemical recurrence or prostate-specific antigen persistence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging 2017;44:960-8. [Crossref] [PubMed]
- Berliner C, Tienken M, Frenzel T, et al. Detection rate of PET/CT in patients with biochemical relapse of prostate cancer using [68Ga]PSMA I&T and comparison with published data of [68Ga]PSMA HBED-CC. Eur J Nucl Med Mol Imaging 2017;44:670-7. [Crossref] [PubMed]
- McCarthy M, Langton T, Kumar D, et al. Comparison of PSMA-HBED and PSMA-I&T as diagnostic agents in prostate carcinoma. Eur J Nucl Med Mol Imaging 2017;44:1455-62. [Crossref] [PubMed]
- Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res 2011;17:7645-53. [Crossref] [PubMed]
- Szabo Z, Mena E, Rowe SP, et al. Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer. Mol Imaging Biol 2015;17:565-74. [Crossref] [PubMed]
- Plyku D, Mena E, Rowe SP, et al. Combined model-based and patient-specific dosimetry for 18F-DCFPyL, a PSMA-targeted PET agent. Eur J Nucl Med Mol Imaging 2018;45:989-98. [Crossref] [PubMed]
- Bauman G, Martin P, Thiessen JD, et al. [18F]-DCFPyL Positron Emission Tomography/Magnetic Resonance Imaging for Localization of Dominant Intraprostatic Foci: First Experience. Eur Urol Focus 2016. [Epub ahead of print].
- Gorin MA, Rowe SP, Patel HD, et al. Prostate Specific Membrane Antigen Targeted18F-DCFPyL Positron Emission Tomography/Computerized Tomography for the Preoperative Staging of High Risk Prostate Cancer: Results of a Prospective, Phase II, Single Center Study. J Urol 2018;199:126-32. [Crossref] [PubMed]
- Dietlein M, Kobe C, Kuhnert G, et al. Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Mol Imaging Biol 2015;17:575-84. [Crossref] [PubMed]
- Rowe SP, Macura KJ, Ciarallo A, et al. Comparison of Prostate-Specific Membrane Antigen-Based 18F-DCFBC PET/CT to Conventional Imaging Modalities for Detection of Hormone-Naive and Castration-Resistant Metastatic Prostate Cancer. J Nucl Med 2016;57:46-53. [Crossref] [PubMed]
- Rowe SP, Macura KJ, Mena E, et al. PSMA-Based [(18)F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Mol Imaging Biol 2016;18:411-9. [Crossref] [PubMed]
- Mehralivand S, Shih JH, Rais-Bahrami S, et al. A Magnetic Resonance Imaging-Based Prediction Model for Prostate Biopsy Risk Stratification. JAMA Oncol 2018;4:678-85. [Crossref] [PubMed]
- Harmon SA, Bergvall E, Mena E, et al. A Prospective Comparison of 18F-Sodium Fluoride PET/CT and PSMA-targeted 18F-DCFBC PET/CT in Metastatic Prostate Cancer. J Nucl Med 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Giesel FL, Hadaschik B, Cardinale J, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging 2017;44:678-88. [Crossref] [PubMed]
- Kesch C, Vinsensia M, Radtke JP, et al. Intraindividual Comparison of 18F-PSMA-1007 PET/CT, Multiparametric MRI, and Radical Prostatectomy Specimens in Patients with Primary Prostate Cancer: A Retrospective, Proof-of-Concept Study. J Nucl Med 2017;58:1805-10. [Crossref] [PubMed]
- Giesel FL, Kesch C, Yun M, et al. 18F-PSMA-1007 PET/CT Detects Micrometastases in a Patient With Biochemically Recurrent Prostate Cancer. Clin Genitourin Cancer 2017;15:e497-9. [Crossref] [PubMed]
- Paddubny K, Freitag MT, Kratochwil C, et al. Fluorine-18 Prostate-specific Membrane Antigen-1007 Positron Emission Tomography/Computed Tomography and Multiparametric Magnetic Resonance Imaging in Diagnostics of Local Recurrence in a Prostate Cancer Patient After Recent Radical Prostatectomy. Clin Genitourin Cancer 2018;16:103-5. [Crossref] [PubMed]
- Giesel FL, Will L, Kesch C, et al. Biochemical Recurrence of Prostate Cancer: Initial Results with [18F]PSMA-1007 PET/CT. J Nucl Med 2018;59:632-5. [Crossref] [PubMed]
- Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J Nucl Med 2016;57:1006-13. [Crossref] [PubMed]
- Yoo I, Choi EK, Chung YA. The Current Status of SPECT or SPECT/CT in South Korea. Nucl Med Mol Imaging 2017;51:101-5. [Crossref] [PubMed]
- Vallabhajosula S, Nikolopoulou A, Babich JW, et al. 99mTc-Labeled Small-Molecule Inhibitors of Prostate-Specific Membrane Antigen: Pharmacokinetics and Biodistribution Studies in Healthy Subjects and Patients with Metastatic Prostate Cancer. J Nucl Med 2014;55:1791-8. [Crossref] [PubMed]
- Goffin KE, Joniau S, Tenke P, et al. Phase 2 study of 99mTc-Trofolastat SPECT/CT to identify and localize prostate cancer in intermediate- and high-risk patients undergoing radical prostatectomy and extended pelvic LN dissection. J Nucl Med 2017;58:1408-13. [Crossref] [PubMed]
- Reinfelder J, Kuwert T, Beck M, et al. First Experience With SPECT/CT Using a 99mTc-Labeled Inhibitor for Prostate-Specific Membrane Antigen in Patients With Biochemical Recurrence of Prostate Cancer. Clin Nucl Med 2017;42:26-33. [Crossref] [PubMed]
- Schmidkonz C, Hollweg C, Beck M, et al. 99mTc-MIP-1404-SPECT/CT for the detection of PSMA-positive lesions in 225 patients with biochemical recurrence of prostate cancer. Prostate 2018;78:54-63. [Crossref] [PubMed]
- Pyka T, Okamoto S, Dahlbender M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging 2016;43:2114-21. [Crossref] [PubMed]
- Rathke H, Afshar-Oromieh A, Giesel FL, et al. Intraindividual comparison of Tc-99m-MDP bone scan and the PSMA-ligand Tc-99m-MIP-1427 in patients with osseous metastasized prostate cancer. J Nucl Med 2018;59:1373-9. [Crossref] [PubMed]


