Neurologic complications of kidney transplantation
Introduction
Kidney transplantation is the most common solid organ transplant in the United States, with over 17,000 transplants performed annually while over 100,000 patients remain on the waiting list (1). This number has continued to rise over the past years as kidney transplantation offers improved survival and quality of life compared to hemodialysis for the majority of patients with end-stage renal disease, and is ultimately more cost-effective over the course of patients’ lives. However, during the first year after transplant, patients frequently encounter complications requiring hospitalization, typically due to immunosuppressive medications or infection due to chronic immunosuppression. Neurological complications are a common issue after kidney transplant, with between 30–60% of patients experiencing some neurological complication (2). Neurological complications are associated with increased morbidity and mortality and should be kept on the differential for all post-transplant patients. This review discusses the most common etiologies of neurological complication after kidney transplant: infection, malignancy, medication, surgical complications, seizure, and stroke.
Infection
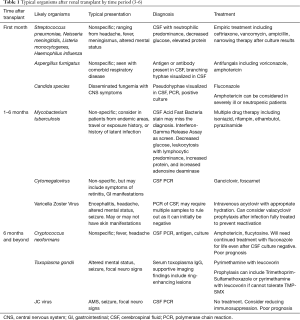
CNS infection accounts for a significant percentage of neurological complications after solid organ transplant, including in kidney recipients. Infection is of specific concern in the post-operative patient as immunosuppressive regimens alter both the typical presentation and types of organisms observed. CNS infection (including meningitis, encephalitis, and abscess) has significant morbidity and mortality in renal transplant patients, and symptoms can range from non-specific (such as headache, fever, and altered mental status) to focal neurologic deficits and coma. When attempting to identify a causative organism, the post-surgical timeline is important as there is a correlation between time post-transplant and likely organisms (3). In general, classification of post-operative infections can be divided into the first month after transplant, one to six months after transplant, and beyond 6 months (Table 1). Management of infection in post-transplant patients typical involves initiation of empiric anti-microbial therapy (antibiotics, antivirals or anti-fungal therapy) with narrowing of agents after the causative organism is determined and decreasing or stopping entirely the immunosuppression regimen (4). As many symptoms of CNS infection in this population are nonspecific, determining whether these symptoms are caused by infection or a non-infectious etiology may be challenging. In general, work-up will include CSF culture and analysis including PCR, blood cultures, and brain imaging.
In the immediate post-operative period, CNS infection is most often caused by typical bacterial organisms although opportunistic infections from environmental organisms and reactivation of latent tuberculosis infection can occur. Typical bacteria causing neurological infection include Streptococcus pneumoniae, Neisseria meningitidis, Listeria monocytogenes, and Haemophilus influenza (5). Common presentation of these patients includes headache, fever, malaise, altered mental status, and meningismus. Opportunistic infections including Mycobacterium tuberculosis, Aspergillus fumigatus, and Candida species can also occur. Tuberculosis is rare as a primary infection post-operatively, but immunosuppression can cause reactivation of latent infection. These patients will have similar presentation to typical bacterial meningitis but may have radiographic evidence of lesions and CSF analysis will reveal very low glucose with a lymphocytic pleocytosis (compared to neutrophilic predominance in typical bacterial infection) with positive acid fast staining and culture (6). Aspergillus fumigatus is a fungus present in the environment, and infections are associated with pre-existing respiratory disease. CNS infection with Aspergillus is associated with multiple lesions on CT or MRI and diagnosis may be made via antigen, serology, or fungal culture. Finally, CNS infection with Candida species can be seen in patients with disseminated fungemia due to immunosuppression.
Risk of infection is highest from one to six months after transplantation as immunosuppression becomes maximally effective and dominant organisms shift to more atypical pathogens. These include viruses and the previously discussed opportunistic bacteria and fungi. Cytomegalovirus (CMV) is the most common opportunistic infection in kidney transplant recipients, present in up to 8% of patients. This prevalence has decreased due to improved recognition of donor and recipient seropositivity and prophylactic treatment (7). Risk is highest with donor seropositivity and recipient seronegativity, induction immunosuppression, and older donors (8). Infection may occur as a primary infection, reinfection of latent recipient infection, or most commonly donor-derived. Symptoms are generally nonspecific in CNS infection, but more characteristic systemic features include leukopenia, thrombocytopenia, and evidence of infection of other tissues with CMV such as retinitis, pneumonitis, or GI disease. Finally, CMV infection has been implicated in case reports of post-operative Guillain Barrè syndrome (9-13). Guillain-Barre Syndrome (GBS) is an auto-immune disease affecting the peripheral nervous system. The exact mechanisms of GBS is unknown but is posited to involve humoral and cell-mediated autoimmunity in response to some antigenic trigger, infectious or otherwise. GBS typically presents as an ascending paralysis and sensory loss with areflexia and can progress to respiratory failure as symptoms spread proximally. Treatment includes respiratory support, rehabilitation, and immunotherapy with plasmapheresis and/or intravenous immunoglobulins (14). Primary infection with Epstein Barr Virus (EBV) is a rare complication after renal transplant, but reactivation can occur and EBV is a significant cause of morbidity and mortality due to its association with post-transplant lymphoproliferative disorder (PTLD) discussed later in this review. Other viruses affecting the nervous system that can sometimes be seen in this post-operative period include human herpes virus 6 (HHV6), varicella zoster (VZV), and BK Polyoma Virus.
After 6 months, immunosuppressive regimens tend to decrease in intensity and overall risk of infection decreases. However, infection with rare atypical organisms can still occur with chronic immunosuppression, and organisms such as Cryptococcus neoformans, Toxoplasma gondii, tuberculosis, and JC virus (causing progressive multifocal leukoencephalopathy) may be seen.
Malignancy
Solid organ transplant recipients have a four-fold increased incidence of malignancy compared to the general population, and this is believed to be due to decreased immune surveillance and an increased susceptibility to oncogenic viruses due to immunosuppression (15). Post-operative primary CNS tumor is uncommon after renal transplant with the exception of PTLD affecting the CNS. While renal transplant is associated with lower rates of PTLD compared to most organ systems, a small percentage (1–2%) of kidney recipients will develop this complication (16). The two largest risk factors for development of PTLD after renal transplant are immunosuppression and Epstein Bar Virus serostatus. Immunosuppression is associated with increases in PTLD after transplant; incidence of PTLD is highest in the first year after transplant, the time of most intense immunosuppression, and rates decline significantly after this. Furthermore, rates of PTLD are significantly higher in solid organ transplants requiring a greater degree of immunosuppression (such as heart) compared to kidney (17). Finally, induction therapy is associated with increased risk of PTLD (18). While the association between EBV and PTLD is now better understood, the pathogenic mechanism is still largely unknown. There is an increased risk (up to twenty-four fold) of PTLD among EBV-negative recipients of EBV-positive donor organs (19). The majority of PTLD tumors in transplant patients contain the EBV genome, suggesting a pathogenic role for the virus. However, EBV negative PTLD has been documented a minority (30%) of PTLD patients and the EBV genome can also be found in patients with this tumor who are not immunosuppressed (20). It is unclear whether these populations represent distinct disease entities.
Medications
Recipients of kidney transplant require immunosuppressive therapy in order to prevent rejection of the transplanted organ. Immunosuppression can be divided into induction and maintenance therapy with additional potential treatment of acute rejection. Induction therapy is given around the time of transplantation, is generally more potent with greater immunosuppression than maintenance therapy, and is done in order to prevent acute rejection. In contrast, maintenance immunosuppression is typically initiated at the time of transplant but continued long-term for the duration of the graft. Typical agents may include glucocorticoids, calcineurin inhibitors (CNIs), anti-metabolic agents, and mTOR inhibitors. Choice of agents will vary based on patients’ individual factors including risk for rejection and balancing potential side effects.
Induction agents
Induction therapy agents work via depletion of T-cells resulting in blunted immune response. Medications used for induction include anti-thymocyte globulins, alemtuzumab (a humanized recombinant monoclonal antibody against CD25), and basiliximab (an IL-2R antagonist). None of these agents have specific neurotoxicities as common adverse effects. Antilymphocyte antibodies (ATG, Atgam, and alemtuzumab) may cause cytokine release syndrome, a systemic inflammatory response caused by initial immune cell activation (21). This is mentioned here as common symptoms (including fever, nausea, vomiting, diarrhea, tachycardia, hypotension, and seizures) are nonspecific and can mimic those of CNS infection after transplant. Basiliximab has not been associated with cytokine release syndrome and is overall well tolerated for kidney transplant induction therapy (22).
Maintenance agents
Corticosteroids
Glucocorticoids are used in both induction and maintenance therapy for their immunosuppression, as well as for the treatment of acute rejection. These drugs function via multiple pathways, with inhibition of various cytokines including Il-1, Il-2, IL-6, TNF-A, and IFN-gamma. The most commonly used glucocorticoids used for renal transplant patients are oral prednisolone, prednisone, and intravenous methylprednisolone. These agents are not ideal for maintenance therapy because of the well characterized side effects of chronic steroid use, including multiple adverse neurological symptoms. Thankfully, most if not all neurological complications of corticosteroids are reversible with reduction or withdrawal of the offending agent. One of the most common neurological side effects of glucocorticoid use is steroid-induced myopathy. Glucocorticoids have a direct catabolic effect on skeletal muscle (23). Patients with steroid myopathy typically present with gradual onset of proximal muscle weakness with atrophy. Lower extremities are usually affected first and more severely than the upper extremities. It is usually not associated with myalgia or tenderness. Symptoms of the condition can begin any time from days to months after therapy begins and its incidence and severity are related to steroid dose (24). The diagnosis is clinical, and muscle enzymes are typically normal (25). The condition is surprisingly common, with previous studies showing approximately 50% of patients on steroids after solid organ transplant developing symptoms (26). Treatment is supportive for pain control of the myalgia and removal of steroid with full resolution taking months after discontinuation. The neuropsychiatric side effects of corticosteroid therapy are well known. Initially, steroid use is associated with elevation in mood, a potential hypomanic state, and disruption of sleep. Symptoms associated with higher doses for longer duration include sleep disruption, restlessness and akathesia, depression (including increased risk of suicidality), cognitive impairment, and most rarely psychosis (27). The majority of these symptoms are reversible with withdrawal of the agent. Risk of neuropsychiatric side effects is increased with dose, duration of therapy, age of patient, and pre-morbid psychiatric disorder (28). Finally, there have been cases of Idiopathic Intracranial Hypertension associated with long term administration of corticosteroid therapy, but these are rare and reversed with cessation of the agent (29,30).
Calcineurin inhibitors (CNIs)
The most commonly used CNIs for immunosuppression are cyclosporine and tacrolimus. The precise mechanism of CNI neurotoxicity is still unknown. Neurotoxicity associated with cyclosporine and tacrolimus can present in multiple ways ranging from more common mild symptoms to rare, but life-threatening, manifestations. Approximately half of patients will report at least one neurological symptom, with tremor, headache, and fatigue being among the most frequently observed (31). These symptoms are frequently associated with higher drug levels and typically resolve either with time, decrease in dose, or in some cases substitution of cyclosporine with tacrolimus or vice versa (32). Severe neurotoxicity with tacrolimus or cyclosporine is rare in renal transplant, but seizure (33), polyneuropathy (34,35), encephalopathy, visual disturbances, and coma have all been reported (36).
Both tacrolimus and cyclosporine are associated with development of Posterior Reversible Encephalopathy Syndrome (PRES) in renal transplant recipients (37-40). The exact mechanism of PRES is poorly understood but believed to be reversible subcortical brain edema caused by endothelial injury (41). Brain imaging studies reveal vasogenic edema typically (but not exclusively) of the bilateral parietal and occipital regions. Patients present initially with hypertension, headache, altered mental status, and visual disturbances, and may progress to seizures, stupor, and eventually coma. As the name of the condition suggests, most cases are reversible with supportive care including blood pressure control and discontinuation of responsible agents, but delay in diagnosis may lead to permanent deficits and death. Most cases of CNI-associated PRES presented within the first year after transplant, a majority of patients had CNI levels either elevated or high-normal, and a majority had hypertension. However, there have been cases of CNI-associated PRES with normotension, therapeutic levels of CNI, and after years of treatment (42). As such, the entity must remain on the differential for renal transplant patients on a CNI, regardless of the treatment being previously well tolerated.
A distinct pain syndrome has been reported with both tacrolimus and cyclosporine use (43). Termed Calcineurin Inhibitor Induced Pain Syndrome (CIPS), the entity is associated with bilateral, symmetric distal leg pain sparing hip areas and usually involving the feet, ankles, and knees. CIPS will affect some 2–15% of renal transplant patients on CNIs, and usually presents within the first year after kidney transplant (44). The pain is worsened with activity and improved with rest. Labs will show increased alkaline phosphatase and calcium, and imaging studies (MRI) reveal marrow edema in the regions of pain (45). CIPS is usually self-limited but can recur. Interestingly, it is associated with good long term prognosis of the graft. Treatment can be supportive for pain-management, removal of the CNI and replacement with non-CNI immunosuppression (46), or treatment with calcium channel blocking agents (47). There is one case report of rapid improvement in symptoms with one-time infusion of iloprost (48).
Other agents
Bortezomib is a small molecule proteasome inhibitor approved by the FDA for the treatment of multiple myeloma that is now finding increased usage as immunosuppressive therapy for renal transplant patients. Side effects in the renal transplant population are similar to multiple myeloma patients (49). The significant neurological side effect of bortezomib is a distal peripheral neuropathy and it is fairly common, affecting 20–40% of renal patients (50). Symptoms initially begin in a “stocking glove” distribution before spreading more proximally. The neuropathy is typically a painful, sensory polyneuropathy; motor neuropathy is rare (51). While reversible, neuropathy symptoms take a median of three months to resolve and may persist for longer (52). The most significant risk factor for development of neuropathy associated with bortezomib is pre-existing peripheral neuropathy (53). There are no established agents for treatment of bortezomib-induced polyneuropathy, and the mainstay of therapy is discontinuation or dose reduction of the offending agent. Some evidence exists suggesting reduction of incidence with concomitant dexamethasone dosing (54).
Acute neuropathy
While peripheral neuropathy after kidney transplant can present secondary to medications used for immunosuppression and antibiotic prophylaxis, acute neuropathy can also occur as a complication of surgery. Acute femoral neuropathy (AFN) is a relatively uncommon complication, with incidence ranging 2% to 4% (55,56). The pathophysiology is believed to involve direct compression and ischemia of the nerve, possibly caused by steal phenomenon following anastomosis of the graft renal artery to the iliac artery. Method and duration of arterial anastomosis have both been correlated with increased incidence of neuropathy, with the highest risk occurring in surgeries with internal iliac ligation with external iliac anastomosis and prolonged anastomosis time greater than 40 minutes (57). Presentation of AFN can vary depending on where along the nerve’s course the damage occurs. Motor complications are more common, with the majority of patients presenting with weakness of hip flexion and knee extension either immediately after surgery or in the days following. Atrophy of these muscles can occur in severe or prolonged cases. Sensory impairment occurs less frequently and presents as numbness and/or paresthesia of the anterior-medial thigh (58). EMG studies confirm a femoral neuropathy. Overall prognosis of AFN is good, and recovery of neurologic function is the most frequent outcome, but symptoms may last weeks to months. Treatment includes physical therapy and medications for neuropathic pain in cases of painful sensory neuropathy. Prevention can be achieved with careful positioning of retractors, avoiding anastomoses associated with increased risk, and careful surgery to prevent hematoma formation.
Seizure
Causes of seizure after renal transplant include drug toxicity, metabolic disorders (electrolyte disturbances), organ dysfunction, CNS infection, ischemic and hemorrhagic stroke, and PRES. Thankfully, the majority of seizures after renal transplant are isolated events and patients will not go on to develop a seizure disorder (59). Therapy is therefore focused on treating the underlying etiology. There is no role for primary seizure prophylaxis for all post-transplant patients, but patients who do have a seizure should be started on an anti-epileptic regimen. Levetiracetam is the drug of choice for seizure prevention, with lorazepam and fosphenytoin being used for status epilepticus. Levetiracetam is ideal due to its lack of interactions between it and the common immunosuppressive. As it is excreted renally, levetiracetam dosage must be adjusted according to renal function.
Stroke
Cerebrovascular disease is seen frequently in patients who have undergone kidney transplantation, with an incidence of stroke or TIA of 5% in the first year and 9.4% in the second year (60). A large proportion of the incidence can be explained by frequent comorbidities of renal transplant patients; diabetes, age, post-transplant kidney function, and previous stroke are all risk factors for ischemic stroke after transplant while diabetes, PKD, and hypertension are associated with hemorrhagic stroke (61). Renal patients also may develop post-transplant polycythemia and hypercoagulability which increase the risk of stroke. Prevention is aimed at control of risk factors (cholesterol, hyperglycemia, and hypertension) as well as prevention via antiplatelet agents such as aspirin. Control of cholesterol is especially important as some of the immunosuppressive regimens may cause hypercholesterolemia (62). Prophylactic anticoagulation is not a mainstay of therapy for all patients post-transplant.
Conclusions
As immunosuppression has improved, organ transplant has become more common and safer overall. As more patients experience improved survival, the total number of patients at risk for chronic complications increases as well. Neurological symptoms are a significant contributor to morbidity and mortality after kidney transplant. Serious neurological complications result in hospitalization, and therefore physicians must be aware of the conditions unique to this population.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- U.S. Department of Health & Human Services. Organ Procurement and Transplantation Network. Retrieved from OPTN: Organ Procurement and Transplantation Network. Available online: https://optn.transplant.hrsa.gov/
- Ponticelli C, Campise MR. Neurological complications in kidney transplant recipients. J Nephrol 2005;18:521-8. [PubMed]
- Rubin RH, Wolfson JS, Cosimi AB, et al. Infection in the renal transplant recipient. Am J Med 1981;70:405-11. [Crossref] [PubMed]
- Zunt JR. Central nervous system infection during immunosuppression. Neurol Clin 2002;20:1-22. v. [Crossref] [PubMed]
- Anastasopoulos NA, Duni A, Peschos D, et al. The Spectrum of Infectious Diseases in Kidney Transplantation: A Review of the Classification, Pathogens and Clinical Manifestations. In Vivo 2015;29:415-22. [PubMed]
- Leonard JM. Central Nervous System Tuberculosis. Microbiol Spectr 2017;5. [Crossref] [PubMed]
- Fehr T, Cippà PE, Mueller NJ. Cytomegalovirus post kidney transplantation: prophylaxis versus pre-emptive therapy? Transpl Int 2015;28:1351-6. [Crossref] [PubMed]
- Requião-Moura LR, Dematos AC, Pacheco-silva A. Cytomegalovirus infection in renal transplantation: clinical aspects, management and the perspectives. Einstein (Sao Paulo) 2015;13:142-8. [Crossref] [PubMed]
- El-Sabrout RA, Radovancevic B, Ankoma-Sey V, et al. Guillain-Barré syndrome after solid organ transplantation. Transplantation 2001;71:1311-6. [Crossref] [PubMed]
- Donaghy M, Gray JA, Squier W, et al. Recurrent Guillain-Barré syndrome after multiple exposures to cytomegalovirus. Am J Med 1989;87:339-41. [Crossref] [PubMed]
- Keithi-Reddy SR, Chakravarthi RM, Hussaini SM, et al. Cytomegalovirus disease with Guillain-Barré syndrome in a cadaver renal allograft recipient: cause or coincidence. Int Urol Nephrol 2007;39:967-70. [Crossref] [PubMed]
- Shaban E, Gohh R, Knoll BM. Late-onset cytomegalovirus infection complicated by Guillain-Barre syndrome in a kidney transplant recipient: case report and review of the literature. Infection 2016;44:255-8. [Crossref] [PubMed]
- Merzkani M, Israel E, Sachdeva M. Primary Cytomegalovirus Infection Causing Guillain-Barré Syndrome in a Living Renal Allograft Recipient. Case Rep Transplant 2017;2017:7264793. [Crossref] [PubMed]
- van Doorn PA. Diagnosis, treatment and prognosis of Guillain-Barré syndrome (GBS). Presse Med 2013;42:e193-201. [Crossref] [PubMed]
- Penn I. Post-transplant malignancy: the role of immunosuppression. Drug Saf 2000;23:101-13. [Crossref] [PubMed]
- Gill D, Juffs HG, Herzig KA, et al. Durable and high rates of remission following chemotherapy in posttransplantation lymphoproliferative disorders after renal transplantation. Transplant Proc 2003;35:256-7. [Crossref] [PubMed]
- Opelz G, Schwarz V, Henderson R, et al. Non-Hodgkin's lymphoma after kidney or heart transplantation: frequency of occurrence during the first posttransplant year. Transpl Int 1994;7 Suppl 1:S353-6. [Crossref] [PubMed]
- Bustami RT, Ojo AO, Wolfe RA, et al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant 2004;4:87-93. [Crossref] [PubMed]
- Walker RC, Marshall WF, Strickler JG, et al. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis 1995;20:1346-53. [Crossref] [PubMed]
- Morscio J, Tousseyn T. Recent insights in the pathogenesis of post-transplantation lymphoproliferative disorders. World J Transplant 2016;6:505-16. [Crossref] [PubMed]
- Hardinger KL. Rabbit antithymocyte globulin induction therapy in adult renal transplantation. Pharmacotherapy 2006;26:1771-83. [Crossref] [PubMed]
- Ramirez CB, Marino IR. The role of basiliximab induction therapy in organ transplantation. Expert Opin Biol Ther 2007;7:137-48. [Crossref] [PubMed]
- Konagaya M, Bernard PA, Max SR. Blockade of glucocorticoid receptor binding and inhibition of dexamethasone-induced muscle atrophy in the rat by RU38486, a potent glucocorticoid antagonist. Endocrinology 1986;119:375-80. [Crossref] [PubMed]
- Bowyer SL, Lamothe MP, Hollister JR. Steroid myopathy: incidence and detection in a population with asthma. J Allergy Clin Immunol 1985;76:234-42. [Crossref] [PubMed]
- Askari A, Vignos PJ, Moskowitz RW. Steroid myopathy in connective tissue disease. Am J Med 1976;61:485-92. [Crossref] [PubMed]
- Wijdicks EF. Neurologic Complications in Organ Transplant Recipients: Blue Books of Practical Neurology, Volume 21. Boston: Butterworth-Heinemann, 1999.
- Brown ES, Chandler PA. Mood and Cognitive Changes During Systemic Corticosteroid Therapy. Prim Care Companion J Clin Psychiatry 2001;3:17-21. [Crossref] [PubMed]
- Bolanos SH, Khan DA, Hanczyc M, et al. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol 2004;92:500-5. [Crossref] [PubMed]
- Vyas CK, Talwar KK, Bhatnagar V, et al. Steroid-induced benign intracranial hypertension. Postgrad Med J 1981;57:181-2. [Crossref] [PubMed]
- Sasisekhar TV. Benign Intracranial Hypertension Secondary to Prolonged Steroid Usage: A Case Report. International Journal of Scientific Study 2015;2:204-7.
- Veroux P, Veroux M, Puliatti C, et al. Tacrolimus-induced neurotoxicity in kidney transplant recipients. Transplant Proc 2002;34:3188-90. [Crossref] [PubMed]
- Böttiger Y, Brattström C, Tydén G, et al. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br J Clin Pharmacol 1999;48:445-8. [Crossref] [PubMed]
- Chegounchi M, Hanna MG, Neild GH. Progressive neurological disease induced by tacrolimus in a renal transplant recipient: case presentation. BMC Nephrol 2006;7:7. [Crossref] [PubMed]
- Arnold R, Pussell BA, Pianta TJ, et al. Association between calcineurin inhibitor treatment and peripheral nerve dysfunction in renal transplant recipients. Am J Transplant 2013;13:2426-32. [Crossref] [PubMed]
- Bhagavati S, Maccabee P, Muntean E, et al. Chronic sensorimotor polyneuropathy associated with tacrolimus immunosuppression in renal transplant patients: case reports. Transplant Proc 2007;39:3465-7. [Crossref] [PubMed]
- Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int 2000;13:313-26. [Crossref] [PubMed]
- Schwartz RB, Bravo SM, Klufas RA, et al. Cyclosporine neurotoxicity and its relationship to hypertensive encephalopathy: CT and MR findings in 16 cases. AJR Am J Roentgenol 1995;165:627-31. [Crossref] [PubMed]
- Haughey D, Narsipur SS. Posterior reversible encephalopathy syndrome after renal transplant: a simple solution for a complicated patient. Case Rep Nephrol Dial 2014;5:20-5. [Crossref] [PubMed]
- Song T, Rao Z, Tan Q, et al. Calcineurin Inhibitors Associated Posterior Reversible Encephalopathy Syndrome in Solid Organ Transplantation: Report of 2 Cases and Literature Review. Medicine (Baltimore) 2016;95:e3173. [Crossref] [PubMed]
- Bartynski WS, Tan HP, Boardman JF, et al. Posterior reversible encephalopathy syndrome after solid organ transplantation. AJNR Am J Neuroradiol 2008;29:924-30. [Crossref] [PubMed]
- Fischer M, Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol 2017;264:1608-16. [Crossref] [PubMed]
- Wu Q, Marescaux C, Wolff V, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol 2010;64:169-77. [Crossref] [PubMed]
- Collini A, De Bartolomeis C, Barni R, et al. Calcineurin-inhibitor induced pain syndrome after organ transplntation. Kidney Int 2006;70:1367-70. [Crossref] [PubMed]
- Grotz WH, Breitenfeldt MK, Braune SW, et al. Calcineurin-inhibitor induced pain syndrome (CIPS): a severe disabling complication after organ transplantation. Transpl Int 2001;14:16-23. [Crossref] [PubMed]
- Chapin RW, Chua E, Simmons J, et al. Case report: imaging features in a renal transplant patient with calcineurin inhibitor-induced pain syndrome (CIPS). Skeletal Radiol 2013;42:1311-5. [Crossref] [PubMed]
- Prates LC, Rigatto SZ, Lutaif AC, et al. Pain syndrome induced by calcineurin inhibitor and resolved by conversion to sirolimus in a child after kidney transplantation: a case report. Transplant Proc 2012;44:2510-1. [Crossref] [PubMed]
- Prommer E. Calcineurin-inhibitor pain syndrome. Clin J Pain 2012;28:556-9. [Crossref] [PubMed]
- Tillmann FP, Jäger M, Blondin D, et al. Intravenous iloprost: a new therapeutic option for patients with post-transplant distal limb syndrome (PTDLS). Am J Transplant 2007;7:667-71. [Crossref] [PubMed]
- Schmidt N, Alloway RR, Walsh RC, et al. Prospective evaluation of the toxicity profile of proteasome inhibitor-based therapy in renal transplant candidates and recipients. Transplantation 2012;94:352-61. [Crossref] [PubMed]
- Argyriou AA, Iconomu G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood 2008;112:1593-9. [Crossref] [PubMed]
- Bilińska M, Usnarska-zubkiewicz L, Pokryszko-dragan A. Bortezomib-induced painful neuropathy in patients with multiple myeloma. Contemp Oncol (Pozn) 2013;17:421-6. [Crossref] [PubMed]
- Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol 2009;144:895-903. [Crossref] [PubMed]
- Lanzani F, Mattavelli L, Frigeni B, et al. Role of a pre-existing neuropathy on the course of bortezomib-induced peripheral neurotoxicity. J Peripher Nerv Syst 2008;13:267-74. [Crossref] [PubMed]
- Kumar SK, Laubach JP, Giove TJ, et al. Impact of concomitant dexamethasone dosing schedule on bortezomib-induced peripheral neuropathy in multiple myeloma. Br J Haematol. 2017;178:756-63. [Crossref] [PubMed]
- Li QS, Huo WQ, Nie ZL, et al. Acute femoral neuropathy following renal transplantation: a retrospective, multicenter study in China. Transplant Proc 2010;42:1699-703. [Crossref] [PubMed]
- Sharma KR, Cross J, Santiago F, et al. Incidence of acute femoral neuropathy following renal transplantation. Arch Neurol 2002;59:541-5. [Crossref] [PubMed]
- Meech PR. Femoral neuropathy following renal transplantation. Aust N Z J Surg 1990;60:117-9. [Crossref] [PubMed]
- H Van veer H, Coosemans W, Pirenne J, et al. Acute femoral neuropathy: a rare complication after renal transplantation. Transplant Proc 2010;42:4384-8.
- Senzolo M, Marco S, Ferronato C, et al. Neurologic complications after solid organ transplantation. Transpl Int 2009;22:269-78. [Crossref] [PubMed]
- United States Renal Data System. 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2017.
- Ferro CJ, Karim A, Farrugia D, et al. Stroke-Related Hospitalization and Mortality After a Kidney Allograft: A Population-Cohort Study. Exp Clin Transplant 2016;14:50-7. [PubMed]
- Satterthwaite R, Aswad S, Sunga V, et al. Incidence of new-onset hypercholesterolemia in renal transplant patients treated with FK506 or cyclosporine. Transplantation 1998;65:446-9. [Crossref] [PubMed]