Lymph node imaging in testicular cancer
Introduction
Testicular cancer is a relatively rare malignancy affecting younger men with an estimated 9,310 men in the United States diagnosed and 400 going on to die of their disease in 2018 (1,2). A majority (68%) of testicular cancer is diagnosed while still localized (T1 disease), however, 19% will have disease spread to the regional lymph nodes and 12% will have distant metastases at the time of diagnosis (1). The presence, or absence, of disease spread beyond the primary site provides information to both accurately stage and appropriately treat these patients (3-5).
Testicular cancer is often divided into two main categories: germ cell tumors and stromal tumors. Germ cell tumors represent the vast majority of cases (90–95%) and have a good prognosis with modern therapies even at advanced stages (1,6-10). Although the WHO proposed large changes to the classification system in 2016, germ cell tumors have historically been divided into two groups; seminoma and non-seminoma (11,12). Stromal tumors (non-germ cell) are relatively rare, representing only 5–10% of all testicular cancer (13,14). The five-year survival rates at early stages are still high, but for the 10–20% of patients who develop malignant disease, survival is worse than their germ cell counterparts (15-17). Proper staging of patients has tremendous value both in prognostic and therapeutic implications. Here, we review the role of traditional imaging modalities in detecting malignant lymph nodes in patients diagnosed with primary testicular cancer as well as its effects on staging, monitoring during active surveillance, or determining therapeutic response. This review focuses on germ cell tumors due to their greater prevalence.
Lymphatic spread
Malignant testicular cancers metastasize in a predictable fashion through the lymphatic system unless the lymphatic drainage from the testes has been altered from prior procedures. This pattern is especially useful to the clinician when searching for positive lymph nodes. Retroperitoneal nodes are the first landing site of metastatic disease with tumors originating from the right testicle often spreading to the inter-aortocaval lymph nodes while tumors originating in the left testicle will spread to the para-aortic lymph nodes (3,7,18,19). Special attention should be given to the inter-aortocaval lymph nodes with a right sided primary testicular tumor as there is some evidence to suggest that more right sided positive lymph nodes are missed by radiologists than left (20). Patients with a history of inguinal or scrotal surgery, tumor extension through the testicular capsule, or disease involving the epididymis may demonstrate positive inguinal lymph nodes due to altered lymphatic drainage (21,22). Two types of non-seminomatous germ cell tumors (NSGCT), choriocarcinoma and yolk sac, may also rapidly metastasize hematogenously, most commonly to the lungs (3,23,24). These cases may necessitate additional imaging depending on symptomatology or clinical suspicion.
Staging
The TNMS (tumor-node-metastasis-serum markers) system from the American Joint Committee on Cancer is widely used in the staging of testicular cancers: incorporating information from pathology from the primary tumor (T stage), serum markers (S stage), and imaging to determine any lymph node or other sites of metastases (N or M stage) (4). This information is used to appropriately counsel and treat patients with this disease (1,4,5,8). One defining feature of early stage (less than stage II) testicular cancers is that they lack lymph node involvement. The presence of a positive lymph node in the retroperitoneum elevates the disease to stage II, in which additional treatment is needed and active surveillance is no longer an option (4,8,10). These cancers have penetrated local tissue, as may be seen in stage I, but additionally have spread to at least one local lymph node. The TNMS staging system considers local lymph nodes to be located in the retroperitoneum and all others, for example supraclavicular or chest, are considered to be distant metastases (4). The size of positive lymph nodes further stratifies stage II patients between IIA (1–5 total enlarged nodes and none are larger than 2 cm), IIB (more than 5 nodes are enlarged and/or the nodes are 2–5 cm in size) and IIC [enlarged node(s) are larger than 5 cm) (4). Lymph node specific criteria used to stage testicular cancer are summarized in Table 1. The 8th edition of the AJCC staging manual was implemented on January 1st, 2018. The clinical staging of testicular cancer remained unchanged from the seventh edition, however, updates to the pathologic staging of testicular cancers was implemented. Notably, stage I pure seminoma tumors were divided into stage T1a and T1b based on a size cutoff of 3 cm. Additionally, epididymal, hilar soft tissue and lymphovascular invasion (LVI) of the spermatic cord without parenchymal invasion is now pT2. Lastly, discontinuous invasion of the spermatic cord and soft tissue via LVI is now considered metastatic invasion (25).
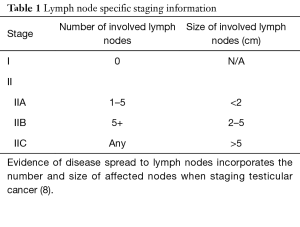
Full table
Identification of nodal invasion for staging of germ cell tumors
Computed tomography (CT)
CT remains the modality of choice when assessing retroperitoneal lymph nodes (8,10). Large positive lymph nodes can be well visualized on CT and may even provide some clues as to the cancer type, although not definitively. The presence of necrosis can indicate possible seminoma and large heterogenous masses (tissue and cystic components) can indicate possible NSGCTs (3). While CT provides excellent spatial resolution, it is unable to discern benign from positive lymph nodes in smaller nodes based on tissue characteristics alone. Instead, lymph node size is used to stratify stage II patients based on measured size and number of nodes (4). This solution proves complicated as benign lymph nodes can vary widely in size and shape, oftentimes with sizes that overlap substantially with those of metastatic lymph nodes (26,27).
CT reportedly detects around 70–80% of positive retroperitoneal lymph nodes, although this number can fluctuate with different size cut-offs (8,28-32). Discerning size cutoff on CT to delineate malignant versus benign lymph nodes has important clinical ramifications as this determines stage and therefore treatment options, most importantly whether surveillance remains an option or not. A study by Hilton et al. tested various size cut-offs by giving 70 retroperitoneal lymph node dissection (RPLND) patients pre-operative CT scans (29). They reported a sensitivity of 37% and specificity of 100% after labeling lymph nodes 10 mm or larger on CT as positive (29). A more contemporary series by Hudolin et al. performed a similar experiment by correlating lymph node size to presence of positive nodes in 85 RPLND patients. They reported that a 1 cm cut-off would miss 60% of positive lymph nodes, and that decreasing the cutoff to 7–8 mm will provide a specificity and sensitivity of 70% (31). Furthermore, lowering the cut-off size to >3 mm on CT to indicate a positive node in a tumor landing zone can reportedly increase sensitivity and negative predictive value to >90%, but, predictably, the specificity suffered greatly, falling to 58% (28,29). While still lacking consensus, it is generally recommended that lymph nodes 8–10 mm or larger be considered suspicious, especially in higher risk patients (3,7). However, even with appropriate imaging there is evidence to suggest that around 25–30% of patients have positive nodes or metastases that are not visible on CT (micro-metastases) (33,34).
If any lymph nodes are detected on abdominopelvic CT, it is recommended to obtain a chest CT to look for distant metastasis (M) (10). In the presence of suspicion of metastases to other organs (brain, liver, bone), additional imaging is often obtained (8).
Magnetic resonance imaging (MRI)
MRI and CT provide similar results, and suffer from similar constraints, when assessing lymph nodes during testicular cancer staging (35,36). Two studies directly compared the ability of CT and MRI to detect positive retroperitoneal lymph nodes in patients with testicular germ cell tumors based on size criteria. Ellis et al. reported on 25 RPLND patients that received both preoperative CT and MRI. CT correctly reported lymph node status in 88% of patients and correctly staged 84%, compared to MRI which correctly reported 84% and 80%, respectively (35). In a more contemporary series, Sohaib et al. reported that MRI has a comparable sensitivity to CT across 3 readers (36). In addition to reporting similar specificity and sensitivity, MRI shares a similar constraint with CT: it, too, is unable to definitively discern disease spread in lymph nodes based on tissue characteristics (3). However, in larger positive nodes, MRI has reportedly demonstrated an ability to differentiate germ cell tumor subtypes. In a small series of patients, Johnson et al. identified imaging features and patterns that led to the correct histological diagnosis in 13 of 15 cases (37). Although not essential for staging, early suspicion of the disease subtype may provide some utility to the clinician as treatment options differ between the two.
Research investigating the ability of combining MRI with lymphotropic nanoparticles to detect positive lymph nodes in many cancers has yielded promising results (38-40). This imaging technique would serve to potentially replace the size criteria, and its inherent shortcomings, by looking directly for disease in lymph nodes. Harisinghani et al. reported that lymphotropic nanoparticle enhanced MRI demonstrated a higher sensitivity (88.2% vs. 70.5%) and specificity (92% vs. 68%) in detecting positive lymph nodes in testicular cancer patients than unenhanced MRI using traditional size criteria cut-offs (41). While this study reports a significant improvement, it was limited due to the small sample size (n=18) and use of CT-guided lymph node biopsy (n=17) instead of RPLND. This MRI technique boasts encouraging results but has several limitations; it requires two imaging sessions 24–36 hours apart, the nanoparticle agent is expensive, and the study authors reported an increased number of adverse events (39). Despite the limitations associated with MRI with lymphotropic nanoparticles, the associated cost and benefits could be considered in patients with metastatic testicular cancer.
Overall, MRI is not routinely used in the staging of testicular cancers as it is costly, time consuming, and lacks physicians experienced in its interpretation (3,7,8). However, MRI does provide utility to patients with a CT contrast allergy, a concern of a high radiation dose (young patients), or an inconclusive CT scan (7,8,35,42).
Other modalities for staging
Ultrasound is used for initial visualization of tumors in the testes and to examine the contralateral testes (8). US may also be used to examine young male patients with metastatic disease, but in every other scenario it is not recommended for staging or the assessment of regional lymph nodes as far superior modalities exist to detect disease spread (3,7,8,10,43).
Currently, there is insufficient evidence to suggest that positron emission tomography (PET) scans should be used to stage testicular cancers (8). While PET scans may provide slightly higher specificity, sensitivity, PPV and NPV than CT, they have still been unable to detect occult metastatic disease and patients stratified with this modality continue to have high relapse rates, at least in NSGCT patients (44,45). The greatest utility of a PET scan may be in its ability to demonstrate the absence of residual disease after chemotherapy in seminoma patients, although physicians should be cautious of automatically interpreting a positive PET scan as evidence of residual disease (46,47).
Imaging for active surveillance
Survival for stage I testicular cancer is nearly 100% irrespective of initial post-orchiectomy therapy options such as RPLND, adjuvant radiotherapy, adjuvant chemotherapy or active surveillance (48). As such, the high survival rate and potential to avoid short and long-term morbidity and mortality makes active surveillance an attractive option among patients and physicians (49,50). Nearly 70–75% of stage I NSGCT, and 83% of stage I seminoma patients, are cured with radical orchiectomy alone. Therefore, many patients may opt to forego the morbidity of chemotherapy or RPLND and pursue active surveillance for which imaging plays a central role (8,10). CT imaging of the retroperitoneum diagnoses more instances of relapses in seminoma and NSGCT patients than any other method of surveillance, and thus is the central pillar of active surveillance protocols (48). Of those that do relapse, salvage chemotherapy will cure nearly all of the rest (48). Clinical visits, blood serum markers and imaging (CT and chest X-ray) are used to monitor patients on active surveillance. Currently, there is no consensus on the optimal strategy of imaging in active surveillance patients. While protocols may differ depending on institution, all are geared toward finding disease relapse early, usually in the lymph nodes, as anything indicating disease spread will prompt salvage therapy.
Seminoma
The most common site of relapse for stage I seminoma patients on active surveillance is the retroperitoneal lymph nodes (10). In the case of seminoma, the relapse rate is highest within the first two years but decreases annually until it becomes 0.3% after year 5 (51). Thus, many active surveillance protocols begin with more frequent imaging that slowly decreases until it is stopped sometime between the 5th and 10th year. The National Comprehensive Cancer Network (NCCN) recommends chest X-rays in years 1–5 and abdominal/pelvic CT every 6 months for the first 2 years, every 6–12 months for year 3 and then annually through year 5 (10). In another study, Martin et al. recommend that the frequency of imaging and visits be based on the annual risk of relapse; patients should be imaged every 4 months between years 0–2, every 6 months during years 3–4, and annually until year 10 (51). A meta-analysis by Groll et al. reported on their institution’s protocol of CT of the abdomen and pelvis every 4 months during years 0–3, then every 6 months during years 4–7, then annually during years 7–16. Groll et al. reported chest X-rays were performed at 8, 16, 24 months and then annually with CT scans until year 16 (48). Overall, these strategies follow the general understanding that relapse rates are highest initially within 2 years and decline over time, but there is no consensus on how long these patients should be followed for.
NSGCT
Approximately 30% of NSGCT present as clinical stage I and regardless of initial treatment, survival is between 95–100% (48,52). The median time to progression in NGCST is shorter than in seminoma, with most relapses occurring within the first year (48). A meta-analysis reported that when relapse is detected, only 54% were seen in the retroperitoneal nodes (52). Additionally, there is evidence to show that up to 30% of stage I NSGCT patients are staged incorrectly on CT after correlation to disease detected in nodes at the time of RPLND (52). Due to the higher rate of relapse in a shorter time frame and a high false negative rate of CT scans, active surveillance imaging and screening tests of NSGCT are more frequent. The NCCN recommends chest X-ray every 1–2 months the first year, every 2 months the second year, every 3 months the 3rd year, every 4 months the 4th year, every 6 months the 5th year and annually thereafter. Abdominal and pelvic CT scan should be performed every 3–4 months the first year, every 4–6 months the second year, every 6–12 months the third and fourth years, every 12 months the fifth year and then every 1–2 years thereafter (10). Groll et al. reported on their institution’s protocol for NSGCT surveillance: chest X-rays every 2 months in years 0–2, every 4 months in year 3, every 6 months in year 4 and then annually. However, they only recommended CT abdomen and pelvis every 4 months the first two years, stopping after that (48). A meta-analysis by Sternberg et al. recommend CT abdomen and pelvis every month for the first year and then every 3–4 months for the second year.
There are several considerations for potential active surveillance patients. Due to the rigorous imaging and examination requirements, it may be best to consider adjuvant therapy right away in patients suspected to be at risk of poor adherence to active surveillance protocols (52). Compliance to active surveillance protocols is reportedly poor. Yu et al. reported that 30% of all active surveillance patients received no imaging or tumor marker tests within the first year of diagnosis (53). For the patients that do adhere to the active surveillance schedule, there is some concern about the radiation dose received due to the frequency of CT scans (54). EAU guidelines recommend MRI as an alternative in place of a CT when either patients or physicians are concerned about the radiation dose they are receiving as part of their active surveillance scheduled CT imaging (8). Tarin et al. reported on the risk of malignancy, mainly lung and colon, after 5 years on the NCCN active surveillance protocol being 1.9% for an 18-year-old compared to 1.2% for a 40-year-old diagnosed with NSGCT (55). This risk increased to 2.6% if chest CT was performed at the same time (56). The risk of secondary malignancies cannot be ignored by urologists caring for these patients, however, with MRIs costing over twice the cost of CT, the burden on the healthcare system must also be acknowledged when following these men (57).
Imaging and treatment selection for advanced disease
Patients in whom positive lymph nodes are identified, either at initial staging or those who progressed while on active surveillance, are elevated to stage II and require additional treatment. With surveillance no longer an option, imaging plays a role in identifying positive lymph node size and location to select appropriate treatment and then monitoring for therapeutic response.
If imaging identifies a seminoma patient as stage II with low serum tumor makers (< S2), treatment recommendations vary based on lymph node size: IIA, IIB or IIC. One treatment option for stage IIA and IIB patients is radiation therapy, which has a 5-year disease free survival of over 90% (10,58). This is typically low dose treatment to the pelvis, para-aortic lymph nodes, and any other site of positive nodes. Chemotherapy is both a primary treatment option but may typically be reserved for relapses in these patients. The recommended treatment for selected stage IIB, all IIC and all stage III seminoma patients is chemotherapy (10). These chemotherapy regimens have also shown excellent outcomes, with over 90% success in long term relapse free survival (5). Overall, seminomas carry an extremely favorable prognosis, reporting a 5-year survival rate above 95% (2). Traditionally, there are two risk categories applied to metastatic (> stage I) seminomas by the International Germ Cell Cancer Collaborative Group (IGCCCG): good risk and intermediate risk (5). Patients are identified as “good risk”, even if they have distant metastases, so long as the cancer is confined to the lymph nodes or lungs. Conversely, patients are identified as “intermediate risk” if they have distant metastases to sites other than the lymph nodes or lungs. Within the study originally outlining this risk stratification system, it was found that of the men with advanced seminoma, about 90% fell within the “good risk” category. Within this group, multimodal treatment conferred an excellent prognosis, with 5-year progression free survival (PFS) at 82%, and overall survival at 86%. Of those with intermediate risk advanced seminoma, the 5-year PFS was 67%, and overall survival was 72% (Table 2) (5).

Full table
Conversely, stage IIA NSGCT patients with negative serum tumor markers post radical orchiectomy may choose between nerve sparing RPLND or primary chemotherapy. Alternatively, EAU guidelines propose a potential 6-week surveillance period in stage IIA NSGCT patients to clarify stage before treatment (8). Stage IIB patients with negative serum tumor markers and positive lymph nodes in landing zones may also choose between primary chemotherapy or RPLND. However, if imaging has identified stage IIB positive lymph nodes outside of expected areas, the patient should receive primary chemotherapy for treatment (10). NSGCT patients with persistent serum tumor markers in stage IIA or IIB patients, lymph node metastases identified outside landing zones, stage IIC disease, or Stage III disease should receive primary chemotherapy (10). In stage III patients, only chemotherapy, or clinical trials (IIIC) are recommended (10). Metastatic NSGCTs have a similar IGCCCG risk classification pattern as seminomas, with slightly worse outcomes, but also have a poor “prognosis group” with a 5-year survival rate of less than 50% (5). Good prognosis patients have metastases confined to the lungs or lymph nodes, but low (S0 or S1) levels of serum tumor markers. Intermediate risk patients may have the same metastasis pattern, but higher levels of serum tumor markers (S2). Poor risk patients have either metastases identified outside of the lungs or nodes, or high levels of serum tumor markers (S3). For NSGCT patients, PFS rates are 89%, 75%, and 41% for good, intermediate, and poor prognosis groups respectively and the 5-year survival rates are 92%, 80%, and 48%, respectively (5). The presence of NSGCT in the liver, bone, brain, viscera or a primary tumor site in the mediastinum are indicators of a very poor prognosis (5-year survival rates below 50%) and are often resistant to chemotherapy (Table 2) (5).
Monitoring therapeutic response after primary treatment
Approximately 10–30% of patients receiving primary treatment will relapse, and CT remains the modality of choice to assess for response to treatment and to identify recurrent disease (3,7,59). The reduction of lymph node or metastatic focus size on CT is indicative of a positive response to therapy. These findings are often interpreted in conjunction with serum tumor marker levels for a more robust assessment of disease response (10). Changes in the tumor appearance on CT may also help clinicians characterize the residual mass. For example, cystic changes seen on CT post chemotherapy may indicate a teratoma, necessitating additional surgery to remove the teratomatous mass (3).
Seminoma
Metastatic seminomas generally respond well to chemotherapy or radiation treatment. Imaging is used to monitor the residual mass and inform clinicians’ decisions on the necessity of additional therapy or not (Figure 1). A residual mass less than 3 cm in size is likely to be fibrotic and necrotic tissue and continued surveillance is recommended (10). A growing mass or rising serum tumor markers, likely represents disease progression, which prompts either second line chemotherapy or surgical salvage in select patients. However, a residual mass greater than 3 cm, but without positive serum tumor markers, requires additional imaging to decide between additional therapy or continued surveillance. In this scenario, a PET scan is indicated to rule out disease. The SEMPET trial evaluated the utility of PET scans, 4–12 weeks post chemotherapy, to correctly predict absence of disease and potentially spare certain patients from overtreatment. They reported that PET scan produced a specificity and sensitivity of 100% and 80%, compared to 74% and 70% with CT, respectively (46). This indicates that surgery can safely be avoided in patients with a residual mass >3 cm and a negative PET scan, and is recommended for this use by both the NCCN and EAU (8,10,46). The SEMPET trial reported no false positive PET scans, prompting researchers at Indiana University to perform a retrospective review. Lewis et al. reported an NPV of 100% and a PPV of 67%, confirming that a negative PET scan likely represents no disease, but a positive PET scan should not confer the same level of confidence that disease is present (47). The SEMPET trial also reported that three of 37 (8%) nodes <3 cm were harboring residual tumor—a PET scan is optional in this scenario (8).
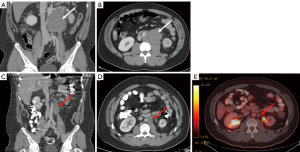
NSGCT
For patients with NSGCT who undergo primary chemotherapy, a “complete response” is defined as a residual mass <1 for NSGCT patients. However, one study with a median follow-up of 15.5 years, reported that 12/141 (9%) patients eventually relapsed with no evidence of disease on CT (60). Although 8 of 12 of these patients were cured, other studies suggest that RPLND always be performed after chemotherapy as residual disease or teratoma may be found in up to one third of patients whose largest node is less than 2 cm (61). Regardless, patients achieving a complete response to primary therapy undergo a follow-up period to evaluate for recurrent disease. Chest X-rays are performed with the same frequency as clinical exam and serum tumor markers: every 2–3 months in years 1 and 2, every 3–6 months in year 3, every 6 months in year 4, every 6–12 months in year 5 and annually after that (10). Abdominal and pelvic CT scans are slightly less frequent, every 6 months in year 1, every 6–12 months in year 2 and annually thereafter (10). Any evidence of recurrent disease prompts additional treatment. Metastatic NSGCT patients with a residual retroperitoneal mass post chemotherapy have different considerations.
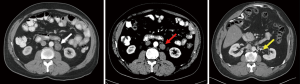
In patients without a “complete response”, a residual mass in NSGCT patients has been estimated to contain a mature teratoma in 30–40% of patients and residual disease in up to 20% (62). Additionally, teratomas do not respond to chemotherapy and may undergo a malignant transformation over time (Figure 2) (63). To further complicate this, PET scans are not indicated to investigate NSGCT post chemotherapy as mature teratomas have variable or no levels of uptake. Furthermore, necrosis and fibrosis cannot be differentiated on this modality (3,64). As such, the decision to undergo surgery or surveillance relies on residual mass size criteria as measured on CT. RPLND is indicated for a residual mass >1 cm on CT with negative tumor markers and surveillance or RPLND are options for a residual mass <1 cm (8,10).
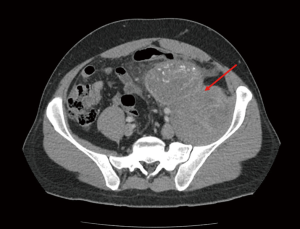
After RPLND, patients with pathologically-confirmed N1 or N2 disease may decide between adjuvant chemotherapy or surveillance. The NCCN recommends that for patients choosing RPLND, a baseline CT should be performed and then again at any point if clinically indicated (10). Evidence of recurrence should prompt salvage chemotherapy. In both cases of primary chemotherapy or RPLND, imaging is involved in following these patients up as disease can still present at a later date (Figure 3).
Conclusions
While rare, testicular cancers typically affect younger men and have an excellent prognosis for most patients due to well-defined treatment algorithms and use of salvage therapies. The imaging of lymph nodes plays a unique role in this disease as lymph nodes are often consistently the first site of spread or disease recurrence. Features detected on imaging directly affect treatment selection and imaging studies are the backbone of current active surveillance protocols. CT remains the primary modality used to stage and monitor testicular cancer, however, in certain cases MRI may be used as a reliable substitute. Currently, both modalities are limited in their detection of low volume disease spread and differentiation of disease subtypes, although there is ongoing research investigating the use of different techniques to address these shortcomings. As research continues to progress, we may expect clearer answers about the frequency with which imaging should be performed while on active surveillance schedules to maximize disease detection while minimizing radiation exposure. Overall, imaging remains a cornerstone in the treatment of testicular cancers, and will continue to be for the foreseeable future.
Acknowledgements
This research was supported by the Intramural Research Program of the National Cancer Institute, NIH. This research was also made possible through the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, the Colgate-Palmolive Company, Elsevier, alumni of student research programs, and other individual supporters via contributions to the Foundation for the National Institutes of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- SEER Cancer Stat Facts: Testicular Cancer. National Cancer Institute, Bethesda, MD. 2018. Available online: https://seer.cancer.gov/statfacts/html/testis.html. Accessed 24 April, 2018 2018.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Sohaib SA, Koh DM, Husband JE. The role of imaging in the diagnosis, staging, and management of testicular cancer. AJR Am J Roentgenol 2008;191:387-95. [Crossref] [PubMed]
- Amin MB, Edge SB, Greene FL, et al. editors. AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017.
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997;15:594-603. [Crossref] [PubMed]
- Ye H, Ulbright TM. Difficult differential diagnoses in testicular pathology. Arch Pathol Lab Med 2012;136:435-46. [Crossref] [PubMed]
- Kreydin EI, Barrisford GW, Feldman AS, et al. Testicular cancer: what the radiologist needs to know. AJR Am J Roentgenol 2013;200:1215-25. [Crossref] [PubMed]
- Albers P., Albrecht W, Algaba F, et al. EAU guidelines on testicular cancer: 2011 update. Eur Urol 2011;60:304-19. [Crossref] [PubMed]
- Howitt BE, Berney DM. Tumors of the Testis: Morphologic Features and Molecular Alterations. Surg Pathol Clin 2015;8:687-716. [Crossref] [PubMed]
- Motzer RJ, Agarwal N, Beard C, et al. Testicular Cancer. J Natl Compr Canc Netw 2012;10:502-35. [Crossref] [PubMed]
- Williamson SR, Delahunt B, Magi-Galluzzi C, et al. The World Health Organization 2016 classification of testicular germ cell tumours: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology 2017;70:335-46. [Crossref] [PubMed]
- Cheville JC. Classification and pathology of testicular germ cell and sex cord-stromal tumors. Urol Clin North Am 1999;26:595-609. [Crossref] [PubMed]
- Idrees MT, Ulbright TM, Oliva E, et al. The World Health Organization 2016 classification of testicular non-germ cell tumours: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology 2017;70:513-21. [Crossref] [PubMed]
- Dilworth JP, Farrow GM, Oesterling JE. Non-germ cell tumors of testis. Urology 1991;37:399-417. [Crossref] [PubMed]
- Banerji JS, Odem-Davis K, Wolff EM, et al. Patterns of Care and Survival Outcomes for Malignant Sex Cord Stromal Testicular Cancer: Results from the National Cancer Data Base. J Urol 2016;196:1117-22. [Crossref] [PubMed]
- Featherstone JM, Fernando HS, Theaker JM, et al. Sex cord stromal testicular tumors: a clinical series--uniformly stage I disease. J Urol 2009;181:2090-6; discussion 2096. [Crossref] [PubMed]
- Silberstein JL, Bazzi WM, Vertosick E, et al. Clinical outcomes of local and metastatic testicular sex cord-stromal tumors. J Urol 2014;192:415-9. [Crossref] [PubMed]
- Yeh SD, Morse MJ, Grando R, et al. Lymphoscintigraphic studies of lymphatic drainage from the testes. Clin Nucl Med 1986;11:823-7. [Crossref] [PubMed]
- Wood DP Jr, Herr HW, Heller G, et al. Distribution of retroperitoneal metastases after chemotherapy in patients with nonseminomatous germ cell tumors. J Urol 1992;148:1812-5; discussion 1815-6.
- Munechika H, Cohan RH, Dunnick NR. Non-seminomatous testicular tumors: effect of lesion side on CT detection of lymph node metastasis. Comput Med Imaging Graph 1988;12:343-8. [Crossref] [PubMed]
- Daugaard G, Karas V, Sommer P. Inguinal metastases from testicular cancer. BJU Int 2006;97:724-6. [Crossref] [PubMed]
- Capelouto CC, Clark PE, Ransil BJ, et al. A review of scrotal violation in testicular cancer: is adjuvant local therapy necessary? J Urol 1995;153:981-5. [Crossref] [PubMed]
- Bahrami A, Ro JY, Ayala AG. An overview of testicular germ cell tumors. Arch Pathol Lab Med 2007;131:1267-80. [PubMed]
- Dalal PU, Sohaib SA, Huddart R. Imaging of testicular germ cell tumours. Cancer Imaging 2006;6:124-34. [Crossref] [PubMed]
- Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol 2018;73:560-9.
- Dorfman RE, Alpern MB, Gross BH, et al. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology 1991;180:319-22. [Crossref] [PubMed]
- McMahon CJ, Rofsky NM, Pedrosa I. Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology 2010;254:31-46. [Crossref] [PubMed]
- Leibovitch L, Foster RS, Kopecky KK, et al. Improved accuracy of computerized tomography based clinical staging in low stage nonseminomatous germ cell cancer using size criteria of retroperitoneal lymph nodes. J Urol 1995;154:1759-63. [Crossref] [PubMed]
- Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. AJR Am J Roentgenol 1997;169:521-5. [Crossref] [PubMed]
- Strohmeyer T, Geiser M, Ackermann R, et al. Value of computed tomography in the staging of testicular tumors. Urol Int 1988;43:198-200. [Crossref] [PubMed]
- Hudolin T, Kastelan Z, Knezevic N, et al. Correlation between retroperitoneal lymph node size and presence of metastases in nonseminomatous germ cell tumors. Int J Surg Pathol 2012;20:15-8. [Crossref] [PubMed]
- Richie JP, Garnick MB, Finberg H. Computerized tomography: how accurate for abdominal staging of testis tumors? J Urol 1982;127:715-7. [Crossref] [PubMed]
- Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year follow up. J Urol 1995;154:1045-9. [Crossref] [PubMed]
- Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol 1988;6:1597-603. [Crossref] [PubMed]
- Ellis JH, Bies JR, Kopecky KK, et al. Comparison of NMR and CT imaging in the evaluation of metastatic retroperitoneal lymphadenopathy from testicular carcinoma. J Comput Assist Tomogr 1984;8:709-19. [Crossref] [PubMed]
- Sohaib SA, Koh DM, Barbachano Y, et al. Prospective assessment of MRI for imaging retroperitoneal metastases from testicular germ cell tumours. Clin Radiol 2009;64:362-7. [Crossref] [PubMed]
- Johnson JO, Mattrey RF, Phillipson J. Differentiation of seminomatous from nonseminomatous testicular tumors with MR imaging. AJR Am J Roentgenol 1990;154:539-43. [Crossref] [PubMed]
- Pandharipande PV, Mora JT, Uppot RN, et al. Lymphotropic nanoparticle-enhanced MRI for independent prediction of lymph node malignancy: a logistic regression model. AJR Am J Roentgenol 2009;193. [Crossref] [PubMed]
- Anzai Y, Piccoli CW, Outwater EK, et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology 2003;228:777-88. [Crossref] [PubMed]
- Mouli SK, Zhao LC, Omary RA, et al. Lymphotropic nanoparticle enhanced MRI for the staging of genitourinary tumors. Nat Rev Urol 2010;7:84-93. [Crossref] [PubMed]
- Harisinghani MG, Saksena M, Ross RW, et al. A pilot study of lymphotrophic nanoparticle-enhanced magnetic resonance imaging technique in early stage testicular cancer: a new method for noninvasive lymph node evaluation. Urology 2005;66:1066-71. [Crossref] [PubMed]
- Hogeboom WR, Hoekstra HJ, Mooyaart EL, et al. The role of magnetic resonance imaging and computed tomography in the treatment evaluation of retroperitoneal lymph-node metastases of non-seminomatous testicular tumors. Eur J Radiol 1991;13:31-6. [Crossref] [PubMed]
- Sohaib SA, Cook G, Koh DM. Imaging studies for germ cell tumors. Hematol Oncol Clin North Am 2011;25:487-502. vii. [Crossref] [PubMed]
- Huddart RA, O'Doherty MJ, Padhani A, et al. 18fluorodeoxyglucose positron emission tomography in the prediction of relapse in patients with high-risk, clinical stage I nonseminomatous germ cell tumors: preliminary report of MRC Trial TE22--the NCRI Testis Tumour Clinical Study Group. J Clin Oncol 2007;25:3090-5. [Crossref] [PubMed]
- de Wit M, Brenner W, Hartmann M, et al. [18F]-FDG-PET in clinical stage I/II non-seminomatous germ cell tumours: results of the German multicentre trial. Ann Oncol 2008;19:1619-23. [Crossref] [PubMed]
- De Santis M, Becherer A, Bokemeyer C, et al. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J Clin Oncol 2004;22:1034-9. [Crossref] [PubMed]
- Lewis DA, Tann M, Kesler K, et al. Positron emission tomography scans in postchemotherapy seminoma patients with residual masses: a retrospective review from Indiana University Hospital. J Clin Oncol 2006;24:e54-5. [Crossref] [PubMed]
- Groll RJ, Warde P, Jewett MA. A comprehensive systematic review of testicular germ cell tumor surveillance. Crit Rev Oncol Hematol 2007;64:182-97. [Crossref] [PubMed]
- Alomary I, Samant R, Genest P, et al. The preferred treatment for stage I seminoma: a survey of Canadian radiation oncologists. Clin Oncol (R Coll Radiol) 2006;18:696-9; discussion 693-5. [Crossref] [PubMed]
- Huddart RA, Joffe JK. Preferred treatment for stage I seminoma: a survey of Canadian radiation oncologists. Clin Oncol (R Coll Radiol) 2006;18:693-5. [Crossref] [PubMed]
- Martin JM, Panzarella T, Zwahlen DR, et al. Evidence-based guidelines for following stage 1 seminoma. Cancer 2007;109:2248-56. [Crossref] [PubMed]
- Sternberg CN. The management of stage I testis cancer. Urol Clin North Am 1998;25:435-49. [Crossref] [PubMed]
- Yu HY, Madison RA, Setodji CM, et al. Quality of surveillance for stage I testis cancer in the community. J Clin Oncol 2009;27:4327-32. [Crossref] [PubMed]
- Chamie K, Kurzrock EA, Evans CP, et al. Secondary malignancies among nonseminomatous germ cell tumor cancer survivors. Cancer 2011;117:4219-30. [Crossref] [PubMed]
- Tarin TV, Sonn G, Shinghal R. Estimating the risk of cancer associated with imaging related radiation during surveillance for stage I testicular cancer using computerized tomography. J Urol 2009;181:627-32; discussion 632-3. [Crossref] [PubMed]
- de Cobelli O, Terracciano D, Tagliabue E, et al. Predicting Pathological Features at Radical Prostatectomy in Patients with Prostate Cancer Eligible for Active Surveillance by Multiparametric Magnetic Resonance Imaging. PLoS One 2015;10. [Crossref] [PubMed]
- Su D, Faiena I, Tokarz R, et al. Comparative analysis of the risk of radiation exposure and cost of reduced imaging intensity for surveillance of early-stage nonseminomatous germ cell tumors. Urology 2015;85:141-6. [Crossref] [PubMed]
- Wood L, Kollmannsberger C, Jewett M, et al. Canadian consensus guidelines for the management of testicular germ cell cancer. Can Urol Assoc J 2010;4:e19-38. [Crossref] [PubMed]
- Shahidi M, Norman AR, Dearnaley DP, et al. Late recurrence in 1263 men with testicular germ cell tumors. Multivariate analysis of risk factors and implications for management. Cancer 2002;95:520-30. [Crossref] [PubMed]
- Ehrlich Y, Brames MJ, Beck SD, et al. Long-term follow-up of Cisplatin combination chemotherapy in patients with disseminated nonseminomatous germ cell tumors: is a postchemotherapy retroperitoneal lymph node dissection needed after complete remission? J Clin Oncol 2010;28:531-6. [Crossref] [PubMed]
- Oldenburg J, Alfsen GC, Lien HH, et al. Postchemotherapy retroperitoneal surgery remains necessary in patients with nonseminomatous testicular cancer and minimal residual tumor masses. J Clin Oncol 2003;21:3310-7. [Crossref] [PubMed]
- Heidenreich A, Pfister D, Witthuhn R, et al. Postchemotherapy retroperitoneal lymph node dissection in advanced testicular cancer: radical or modified template resection. Eur Urol 2009;55:217-24. [Crossref] [PubMed]
- Motzer RJ, Amsterdam A, Prieto V, et al. Teratoma with malignant transformation: diverse malignant histologies arising in men with germ cell tumors. J Urol 1998;159:133-8. [Crossref] [PubMed]
- Cremerius U, Effert PJ, Adam G, et al. FDG PET for detection and therapy control of metastatic germ cell tumor. J Nucl Med 1998;39:815-22. [PubMed]


