Rethinking active surveillance for prostate cancer in African American men
Introduction
Prostate cancer is the most commonly diagnosed solid organ malignancy in men of all races, with approximately 180,000 men diagnosed in the United States in 2017 alone. Prostate cancer is also the second leading cause of cancer-related death in US men (1); however, the ratio of incidence versus death rate in prostate cancer is the largest of any solid organ malignancy. This coupled with the high rate of prostate cancer found on autopsy studies of men without a clinical history of disease underscores the indolent nature of many prostate cancers (2). Evidence gathered from prospective observational series, randomized clinical trials, and large databases have all demonstrated that conservative management of low-risk prostate cancer patients does not significantly impact mortality when compared with active intervention (3,4). These observations have shifted the treatment paradigm for prostate cancer such that healthier men with less aggressive prostate cancers should be encouraged to engage in a protocol of active surveillance (AS) (5). Such a protocol aims to achieve two simultaneous goals: firstly, to maintain quality of life and avoid the burden and complications associated with the overtreatment of clinically insignificant prostate cancer, and secondly, to carefully monitor prostate cancer for the possibility of progression, with the ability to intervene without compromising oncological outcomes. It is important to differentiate AS from “watchful waiting”, which involves no surveillance whatsoever. AS requires repeated biomarker testing, physical examination, imaging, and prostate biopsies to periodically characterize the disease and assess for phenotypic progression. Watchful waiting is a strategy aimed at simply treating symptomatic manifestations of local or metastatic progression of a patient’s prostate cancer.
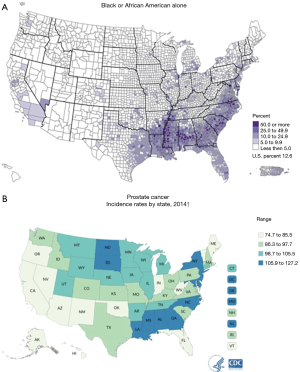
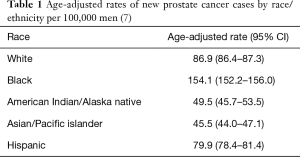
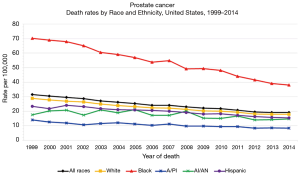
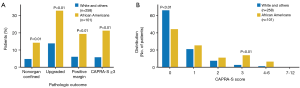
For more than 40 years, it has been theorized that prostate cancer risk can and should be stratified by race, with AA men carrying a higher risk (6,7) (Table 1). Thus, it is not surprising that there is a greater incidence of prostate cancer in regions with higher numbers of AA men (Figure 1) (8,9). The racial differences between AA and Caucasian (CA) men with regard to prostate cancer death can also be quite striking (Figure 2) (10). The overlap of these maps is astonishing, and indicate that within US regions of greater AA populations, there are significantly higher prostate cancer death rates. The states with the greatest populations (per capita) of AA men also have the highest death rates from prostate cancer. AA men are also diagnosed with prostate cancer at a younger age and at a more advanced stage than their CA counterparts (11,12). Similarly, it has been shown that AA men also have higher rates of upstaging, increased risk of biochemical recurrence, higher rates of positive surgical margins and higher 10-year prostate cancer specific mortality, when compared to CA men (Figure 3) (13). Additionally, AA men have an age-adjusted annual death rate from prostate cancer that is 2–3 times that of CA men (1).
Full table
Some of these differences can be attributed to socioeconomic factors. However, despite the availability of several studies specifically addressing these biases, significant differences in outcomes remain. Powell et al. stratified patients into risk categories based on clinical tumor stage, prostate-specific antigen (PSA) at diagnosis, and Gleason grade, and could not account for the racial disparity in progression-free survival in patients with low-risk prostate cancer who underwent radical prostatectomy (14). In another analysis that adjusted for socioeconomic factors, overall survival difference between AA and CA was non-significant, but prostate cancer-specific mortality was higher in AA men (15). These studies strongly suggest a biological difference that is in part responsible for the more aggressive disease observed in AA men (11). When taken together, this leaves practitioners with a challenging conundrum: since prostate cancer is generally more aggressive and deadlier for AA men, is it safe to consider AS surveillance for these “higher” risk patients? And if so, should inclusion criteria be the same for AA when considering AS? Should protocols differ with respect to the frequency of biopsies or biomarkers? Should thresholds be different? In the following review, we will present the available—albeit limited—evidence aimed to address these important questions.
Methods
A literature review was performed in PubMed with the keywords “African American” and/or “Prostate Cancer” and/or “Active Surveillance” and/or “Outcomes” and/or “Molecular Markers”. The articles derived were culled to identify peer-reviewed publications that were relevant to the clinical questions surrounding AS in AA men. These manuscripts were then reviewed to derive other sources of information and data that were relevant to our hypothesis. In our review of the literature, we identified several aspects of prostate cancer that are different in CA and AA men. Specifically, we attempted to focus on these differences that potentially impacted the risks and outcomes associated with observation or AS of men with prostate cancer.
Controversial role of PSA screening
For many years following the widespread adoption of PSA screening in the United States, the incidence of prostate cancer has significantly increased just as prostate cancer-related mortality has decreased (16). However, there is debate about the value of PSA screening. In 2012, the United States Preventative Services Task Force (USPSTF) recommended against PSA screening for men in the United States (17). This recommendation was largely based on two studies: the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) and the European Organization for Research and Treatment of Cancer Trial (EORTC). The American PLCO was a large study which demonstrated little difference between the screening arm and the usual care cohort (18). However, this study was fraught with screening contamination within the control group. Close to 90% of men in the control arm reported having undergone at least one PSA test before or during the trial, and more cumulative PSA testing was performed in this group than men in the intervention group (19). The EORTC, without the degree of contamination seen in the US study, did find that PSA screening resulted in a reduction in mortality from prostate cancer (20). However, this larger European trial did demonstrate a significant possibility for overtreatment. In the initial publication of the EORTC study, it was reported that 48 patients would need to be treated in order to prevent one prostate cancer death; however, subsequent analysis with 12-year follow up data decreased this figure to only 18 patients (21). While these two large studies offer insight, it is difficult to assess the generalizability of those studies to AA men. In the PLCO study, only 4% of those enrolled in the trial were AA. Although no racial demographics were reported in the EORTC trial, it can be assumed that relatively few, if any, AA men were included in this study given the distribution of Europe’s racial demographics.
Importantly, the USPSTF recommendations from 2012 against PSA screening made no specific mention regarding AA men (22). The data from which they based their guidelines included very few AA men, too few for there to be any reliable data upon which to make any recommendation, and yet no specific caveat was made for this group. In 2017, the USPSTF released a draft to update their recommendations for PSA screenings with a specific discussion regarding AA men. The general update changed the recommendation of PSA screening to grade C or individualized approach. Regarding AA men, the most recent 2018 draft statement notes the following:
“Decision analysis models suggest that given the higher rates of aggressive cancer in African American men, PSA-based screening may provide greater benefit to African American men than the general population. These models also suggest a potential mortality benefit for African American men when beginning screening before age 55 years. The USPSTF believes that a reasonable approach for clinicians is to inform African American men about their increased risk of developing and dying of prostate cancer as well as the potential benefits and harms of screening so they can make an informed, personal decision about whether to be screened.” (23).
The statement also notes it is not possible at this time to make a separate recommendation for PSA screening in AA men given the lack of robust trials demonstrating clinical benefit. It is conceivable that screening may offer greater benefits to AA men compared with the general population.
Diagnostic modalities for prostate cancer in African American (AA) men—is PSA the same in this cohort?
In order to determine the utility of a biomarker, it must specifically be evaluated in the patient population under consideration. PSA is perhaps the most widely studied prostate cancer screening biomarker. It has been evaluated in AA patients, and significant discrepancies between this population and non-AA men have been noted. AA men both with and without prostate cancer have consistently demonstrated higher PSA values than their non-AA counterparts (24,25). As a result, when using traditional PSA cut-off values for prostate biopsy, PSA becomes a less sensitive test for detecting prostate cancer. Although some have called for race-specific PSA thresholds, few clinicians utilize such measures in practice. Similarly, PSA density, when controlling for prostate volume, has been demonstrated to be higher in AA men (25). Clinicians must remain cognizant of these differences in PSA and PSA density during the evaluation of prostate cancer, as well as for men being evaluated for AS.
Several studies have also discovered additional biomarkers that may be particularly applicable in AA men. Feibus et al. noted that when urinary prostate cancer antigen 3 (PCA3), a non-coding mRNA that is overexpressed in prostate cancer patients, is added to standard of care models, it improved the detection of all cancers, including high-grade cancer specifically for AA men (26). Furthermore, a urine test to detect TMPRSS2:ERG gene fusion, a prostate cancer-specific DNA rearrangement in which 5’ untranslated transmembrane protease serine 2 (TMPRSS2) fuses with ETS-related gene (ERG) resulting in the up-regulation of ERG, demonstrated no significant improvement over standard tools for AA men. The 4K score, a non-invasive blood test that combines the levels of four kallikrein proteins [total PSA, free PSA, intact PSA, and human kallikrein-related peptidase 2 (hK2)] with important clinical information (patient age, digital rectal exam findings, and prior biopsy status), was effective in identifying clinically significant cancers (Gleason 7 or greater). A recent prospective trial found that the 4K score outperformed a base model incorporating PSA for AA and demonstrated that the 4K score accurately predicts aggressive prostate cancer and outperforms standard clinical parameters for biopsy decision-making in AA men (27). Prostate health index (PHI) is another measure that has been shown to adequately identify prostate tumors with extraprostatic extension in an evaluation of 80 AA men with prospective evaluation prior to prostatectomy (28). However, no other studies have shown value of PHI as a screening tool. Detection of cancer-specific DNA methylation in negative index biopsy specimens has also been employed in the diagnosis of prostate cancer in AA men. A recent study that incorporated 211 AA men validated this epigenetic assay (ConfirmMDx) that aids clinicians in the decision for repeat biopsy. It was found that ConfirmMDx had a negative predictive value of 78.8% for prostate cancer and 94.2% for Gleason score >7 disease after negative index biopsy (29).
Although it is not strictly a biomarker, magnetic resonance imaging (MRI) has been shown to be a useful modality to diagnosis prostate cancer (30). Multi-parametric MRI (mpMRI) for prostate cancer detection in AA men was evaluated in a retrospective study that included 117 AA and 544 CA men. In this study, there was no racial difference in the detection rate of overall prostate cancers or clinically significant prostate cancers between AA and CA men on mpMRI (31). Another study including 822 men (127 AA) found that MRI/ultrasound-guided fusion biopsy demonstrated increased diagnostic yield in the AA population in an age- and PSA-matched cohort of CA patients (32). This is particularly salient given the fact that studies have shown that AA men are more likely to develop prostate cancer in the transitional zone than CA men (33). The transitional zone is not adequately sampled during standard 12-core transrectal ultrasound guided prostate biopsy, but this area can be visualized well on MRI and targeted based on MRI findings (34).
Treatment and outcome differences in AA men with prostate cancer
It is well established that AA men develop prostate cancer earlier and more often than the general population, but it is less clear why there are striking differences in the types of treatments that AA men receive. Several studies have demonstrated that CA men are more likely to undergo active treatment of their prostate cancer with intent to cure while AA men are more likely to go untreated or initiate watchful waiting (35,36). Furthermore, observational studies have shown that AA men are less likely to receive newer, more expensive imaging and treatments when compared to CA men (37). One prime example is that compared to CA men, AA men are more likely to receive orchiectomy (a relatively cheap modality) and less likely to receive androgen deprivation hormonal therapy (a relatively expensive modality) (36). These differences in treatment utilization have not been adequately explained by variation in clinical factors between AA men and the general population. This suggests that there are other factors likely influencing treatment variation with regards to prostate cancer amongst the AA population.
Several hypotheses may explain the observed differences in the utilization of different prostate cancer treatment methods in AA men. The first explanation involves cultural and psychosocial considerations. Studies have demonstrated that physicians are less likely to discuss treatment options and side-effects with AA men compared to CA men, which may mar informed decision making (38). AA men are also generally more reticent to discussing their disease, whether it is out of fear or embarrassment, which may hinder the physician-patient therapeutic alliance. In a study conducted by Penson et al. which included 193 family pairs, it was found that shared decision making in treatment selection for prostate cancer was significantly different in AA and CA families. AA men were much less likely to include their family members when discussing potential treatment options for their prostate cancer (39). Socioeconomic status may also play a role, as lower socioeconomic status has been shown to predict lower rates of radical prostatectomy in AA men (40). There may also be a cultural aspect to this phenomenon—AA men may be more concerned about the potential irreversible side-effects of definitive treatment for prostate cancer such as erectile dysfunction and incontinence. This is supported by a study that demonstrated that AA men were twice as likely to choose external beam radiation compared to CA men, while CA were three times more likely to choose radical prostatectomy in that same cohort of patients (41). Access to urologic care is another potential factor. Multiple studies have shown that AA men have less access to high volume urologic care practitioners and are less likely to receive definitive treatment (8).
Regardless of prostate cancer treatment modalities, AA men have worse oncologic outcomes, even when receiving the same treatment as their CA equivalents. Studies have demonstrated that the risk of death from prostate cancer in AA men who received prostatectomy was four times greater than in CA men (42). Race has also been shown to be an independent prognostic factor for disease-free survival among men who had resected specimens with positive surgical margins (43). Another study found that AA men with palpable tumors had significantly higher rates and shorter median time to biochemical recurrence after radical prostatectomy (44). Interestingly, the authors noted that pre-existing comorbidities may not be a driver of racial disparities in overall survival in AA men with prostate cancer, as there were no statistically significant differences in the cause of death among deceased participants. AA men receiving radiation therapy were also found to have a larger proportion of tumors with post-treatment PSA levels greater than 10 ng/mL and lower overall survival (45).
In summary, AA men select different treatment modalities and have different outcomes when the same treatment is selected even when controlling for multiple variables. All of this may inform our understanding of variation in the selection of AS by AA and potentially shed light on differences in the way AS is performed and the outcomes realized.
AS in the general population: outcomes, expanded inclusion criteria and adoption
In recent years, AS has become a commonly accepted management approach for most men with low-risk prostate cancer. The goals of AS are both to avoid the burden of overtreatment of low-risk prostate cancer, and to allow the identification and intervention for prostate cancer when treatment with intent to cure is attainable. Several recent large prospective randomized trials have provided insight into the indolent nature of many prostate cancers and given credence to the notion that AS is a viable option in select patients.
In the literature, there have been six published reports (including a total of over 5,500 patients) that include at least 5-year follow-up data in patients placed on an AS protocol (46-51). Although the inclusion criteria and demographics of these cohorts vary considerably, important trends have emerged from this data. At 5-year follow-up, 24–40% of patients were discontinued on AS and started treatment. For the three studies with 10-year follow-up data, 36–55% of patients received treatment by 10 years. Amongst all six studies, biochemical recurrence occurred in 8–25% of patients after treatment. The proportion of men developing metastatic disease while on AS were low, ranging from 0.1% to 2.8%. And the rates of prostate cancer-specific mortality were similarly low (0–1.5%), even in cohorts with 15-year follow-up data. Although there were differences in the threshold with which to intervene, the preponderance of evidence in these studies support that AS had comparable prostate-cancer-specific mortality to radical prostatectomy for treatment of low-risk prostate cancer (48).
Based largely around these trials, AS is the preferred treatment option for men with low and very low-risk prostate cancer according to the National Comprehensive Cancer Network (NCCN) guidelines without specific exception or discussion about race (5). Emerging evidence suggests that higher-grade tumors can potentially be observed as well. Given the low incidence of adverse oncologic outcomes in prospective AS observational cohorts, several of the aforementioned studies expanded their inclusion criteria to incorporate men with intermediate and high-risk disease. Klotz et al. included 132 men with Gleason 7 prostate cancer and found that only 44% of those who developed metastases came from this group of patients. Furthermore, PSA predicted biochemical recurrence in these patients, while Gleason score at enrollment did not (47). It is important to note, however, that this group with Gleason 7 prostate cancer may include patients with 4+3 or 3+4 tumors, which have clinically significant differences with respect to the likelihood of lethal prostate cancer (52). The results from Klotz’s analysis were mirrored by the study conducted by Selvadurai (51). Another study conducted at the University of California San Francisco (UCSF), started 90 older men on an AS protocol despite the fact that they had higher PSA and greater tumor volume than NCCN guideline-determined low-risk prostate cancer. At follow-up visits 4 years from last positive biopsy, the AS group demonstrated no significant difference in progression free survival, as well as no difference in the rate of being transitioned from surveillance into active treatment (53). In another study conducted at the Cleveland Clinic, a cohort of 117 men with intermediate or high-grade prostate cancers (though all were ≤ Gleason score 7) were initiated into an AS protocol. Cumulative metastasis-free survival at 5 and 10 years was 99% and 98%, respectively. There were no prostate cancer-specific mortalities at 10-year follow-up, though no cancer-specific deaths had been observed to-date. At 10-year follow-up, 51% of patients underwent intervention. Overall cumulative freedom from failure of AS, defined as metastasis or biochemical failure after local therapy with curative intent, was 97% and 91%, at 5 and 10 years, respectively. Of the 117 patients in this cohort, 21 (9.9%) experienced biochemical failure after deferred treatment and the 5-year progression-free probability was 92% (54). Furthermore, the eventual freedom from intervention rates in the low-risk vs. intermediate-risk groups in this study was not significantly different. However, the small number of events and limited follow-up data limited this study. These findings suggest that in carefully selected, cautiously observed patients with well-documented studies, AS is a reasonable option for men with adverse pathologic features such as Gleason pattern 4. Caution should be taken when interpreting the generalizability of these findings, primarily because of the limited number of carefully selected patients with limited follow up. Certainly, some patients with Gleason pattern 4 tumors are reasonable candidates for observation, but tools to select those patients are not yet mature and further investigation is needed. These studies are important for understanding surveillance in AA; although they contain too few AA to draw any conclusions, they do provide insight into the natural history of low-volume, intermediate-risk prostate cancer. If AA really do have higher rates of upgrading, as some have suggested, these studies do provide evidence that at least a fraction of these patients may be safely observed.
Repeated prospective observations demonstrating the safety of AS have led not only to changes in guidelines, but to real-world changes in the management of the majority of men with low-risk prostate cancer. In 2001, only 6.2% of men with low-risk prostate cancer were initiated on AS or watchful waiting. By 2010, 40% of all low-risk prostate cancer have been placed on AS, and this figure was up to 76% in men over 75, with low-risk disease, as demonstrated in a large multi-center clinical study (55). In a collaborative urologic study of low-risk prostate cancer in patients in Michigan, roughly 50% were placed on initial AS. The use of AS has also expanded outside of the United States, including Sweden, Japan, and Australia (56). While these studies demonstrate a growing trend in the urologic community to perform AS protocols for low-risk prostate cancer, they do not provide specific insight into the trends of AS for AA men. It is most likely that as AS criteria expand and AS becomes the de facto treatment for low-risk prostate cancer, more urologists will offer and encourage their low-risk AA patients to pursue a similar strategy.
Evidence for and adoption of AS in AA men
The American Urological Association (AUA) has long recognized AA race as a risk factor for prostate cancer and incorporated this into their PSA screening recommendations. This consideration is based on a myriad of evidence that show that AA men have higher prostate cancer incidence as well as mortality compared to non-AA counterparts (57). Unfortunately, racial diversity in the large AS cohorts are lacking. AA men were particularly underrepresented in these studies (only 7–13% of patients), which diminishes the generalizability of their findings with regards to AS efficacy in AA men. Although the data is limited, we highlight several retrospective, prospective observational, and prospective randomized studies that incorporated AA men.
Retrospective studies of low-risk patients undergoing prostatectomy
Potential candidates for AS who elect for radical prostatectomy and then retrospectively have their prostate specimens reviewed to determine the correlation between the initial biopsy and the final pathologic specimen have been considered in evaluating candidacy for AS and outcomes. While valuable and insightful, this data must be critiqued for the “what if this patient had opted for AS?” nature of their findings. Finding minute amounts of Gleason pattern 4 at radical prostatectomy cannot provide adequate surrogacy for how these patients may have done if placed on AS protocols. Furthermore, AS requires repeat evaluation of the prostate with imaging, biomarker assessment, and tissue acquisition specifically because there is known initial understaging of disease.
Despite these limitations, the retrospective studies that have included AA men on AS have yielded mixed results. Seven retrospective studies including a total of over 4,500 AA men have been conducted in men diagnosed with very low-risk prostate cancer who underwent radical prostatectomy (13,58-62). In these cohorts, AA men were more likely to have pathologic Gleason upgrading, pathologic stage T3a, positive surgical margins, and adverse surgical histology. Additionally, AA men diagnosed with very low- or low-risk disease had biochemical recurrence-free survival similar to CA men of low- and intermediate-risk groups, respectively. A study conducted at UCSF involving 191 AA men found that when NCCN risk criteria were used, the incidence of upstaging was significantly higher in AA men compared to CA men (63). Results from the Surveillance, Epidemiology, and End Results (SEER) database compared prostate cancer-specific mortality results of low-risk AA (n=7,523) and CA populations (n=43,792) who would have qualified for AS. Five-year prostate cancer-specific mortality rates were significantly higher in the AA population compared with CA population (1.0 vs. 0.64%, P=0.019) (64). One of the aforementioned studies conducted by Sundi et al. revealed that AA men with very low-risk prostate cancer at diagnosis had significantly higher prevalence of anterior zone foci that were of higher grade and larger volume compared to CA men (58). This disparity in the location of dominant and potentially higher grade tumor foci, may result in undersampling during index or surveillance biopsy, as the anterior zone is the most difficult site to sample with 12-core biopsy techniques (65).
There have also been at least four other robust retrospective studies with contrasting findings. One study that utilized data from the Shared Equal Access Regional Cancer Hospital (SEARCH) cohort demonstrated that in 355 AA and 540 CA men with low-risk tumors who were followed a median of 6.3 years following adjustment for relevant covariates, AA race was not significantly associated with pathological upgrading (OR 1.33, P=0.12), major upgrading (OR 0.58, P=0.10), up-staging (OR 1.09, P=0.73) or positive surgical margins (OR 1.04, P=0.81). Five-year recurrence-free survival rates were 73.4% in AA men and 78.4% in CA men (log rank P=0.18). In a Cox proportional hazards analysis model, AA race was not significantly associated with biochemical recurrence (HR 1.11, P=0.52) (66). Similarly, another retrospective study that included 345 CA and 58 AA men with prostate cancer found that AA men with clinical AUA low-risk prostate cancer were less likely to be Gleason score upgraded (29.8% of AA vs. 44.5% of CA, P<0.04) on radical prostatectomy compared to CA men. However, AA men were less likely to be clinically diagnosed with low-risk prostate cancer overall (33.1% of AA vs. 41.7% of CA, P<0.05) (67). Resnick et al. also noted no racial differences in Gleason upgrading, extracapsular extension, positive surgical margins, seminal vesicle invasion, or tumor volume in a study of 1,146 patients (144 AA) with low-risk disease who underwent radical prostatectomy (68). Schreiber et al. similarly found no difference in pathologic Gleason score, pathologic extent of disease, positive margins, CAPRA-S score, or adverse features when comparing AA and CA men (69).
Given the lack of consensus, it is challenging to garner actionable clinical insight from these studies. It is important to note that all the patients included in these studies had treatment, and as such, the findings do not provide appropriate insight into the natural history of prostate cancer survival or morbidity while on AS. Additionally, some of the studies demonstrating increased adverse pathologic findings do translate into a difference in survival.
Prospective observational studies of AA men on AS
While there have been several well-designed prospective observational studies of men who elected for AS, studies to-date contain relatively few AA men. This data is more informative than the “what if” studies listed above; however, they are limited by lack of uniformity with regards to the inclusion criteria and follow-up regimens. Recently, several authors have suggested that AA race may be a risk factor for disease progression or discontinuation of AS (70,71). The University of Miami reported on their cohort of 249 patients who underwent at least one surveillance biopsy. Of the 249 patients, 24 (9.6%) were AA and were found to be four times more likely to progress (increased grade, number of positive cores, or volume of positive cores) than their CA counterparts, with a median follow-up of 2.9 years. The study was primarily limited by its small size and retrospective nature (70). Abern et al. reported on the AS cohort at Duke University, which consisted of 145 patients (32 were AA), and noted that within 23 months of the median follow-up, AA were almost three times as likely to receive treatment. Of those who underwent at least one confirmatory biopsy, AA were more likely to have progression as compared with CA, although this did not reach significance given the small sample size (71). This study found that AA race was associated with earlier discontinuation of AS, and the authors suggest that this may be secondary to the increased prostate cancer growth rates in AA. This study is also limited by its small size, and patients may elect to discontinue AS for many reasons that have little to do with progression of disease. A recent multi-institutional study conducted by Odom et al. stratified AS outcomes by race in 139 patients (48% AA) with low-risk prostate cancer. Similarly, with a limited median follow-up of 34 months, AA were more likely to experience disease progression and receive definitive treatment (72).
Although the aforementioned studies suggest that AA men may be higher risk patients on AS protocol, there have also been two studies that showed that race had no impact on survival rates in men who elected AS. However, it is important to note that the protocols used in these two studies were more consistent with watchful waiting and delayed intervention rather than AS. The cohort examined by Cullen et al. defined AS as the absence of treatment for a minimum of 9 months after prostate cancer diagnosis (73). Similarly, in the cohort presented by Koscuiszka et al., the majority of the patients had intermediate- or high-risk disease, and primary use of androgen deprivation was included within the definition of a deferred primary treatment (74). Both of these are more consistent with a deferred primary treatment rather than an AS cohort.
Although the preponderance of evidence suggests that there may be earlier discontinuation or more progression in AA who elect AS, the available data is limited and mixed in nature. Progression while on AS surveillance may be a result of mischaracterization of initial grade and volume versus growth and dedifferentiation of disease. These studies that suggest greater rates of progression for AA patients have limited follow up, and although rapid progression of disease is possible, a more likely explanation is biopsy mischaracterization.
Prospective randomized studies of AA men on AS
Although there are no published reports of prospective randomized studies of AS in AA men, we can gain valuable insights from the Prostate Cancer Intervention versus Observation Trial (PIVOT); the single prospective randomized trial in the PSA era that has reported outcomes of radical prostatectomy to observation, rather than AS (3,75). Although patients in the observation arm did not undergo repeat PSA testing, physical examination, or prostate biopsies as in the AS cohorts, data extrapolated from this trial provides insights into the natural history of PSA-detected prostate cancer. Inclusion criteria were men with newly diagnosed prostate cancer, aged <75 years, PSA level <50 ng/mL, and any Gleason score. Importantly, in this Veterans Affairs-based study, 232 (31.7%) of the total 731 men in the study were AA, which is a higher number of AA men than in any of the published prospective observational studies cited above. With more than 10 years of follow-up data, no differences in overall or prostate cancer-specific mortality between the surgery and observation groups have been found. There was, however, a significant reduction in bone metastasis in the radical prostatectomy group, with a relative risk reduction of 0.44 (95% CI, 0.25–0.76; P=0.001). Sub-group analyses demonstrated radical prostatectomy resulted in significant improvement of prostate cancer-specific survival rates for patients with aggressive disease. Interestingly, although also underpowered, when analyses were limited to AA regardless of disease characteristics, no significant benefit was found for radical prostatectomy in the overall survival rate, disease-specific survival rate, or bone metastases. For CA, there was a significant advantage in terms of reduction in bone metastases with radical prostatectomy. Given the more aggressive nature of prostate cancer in AA men, these findings might be unexpected and may be secondary to underpowered subset analysis or the poor health of the overall cohort. This may be particularly relevant because the mean age at enrollment in the PIVOT was 67 years, which approaches the overall survival rate of AA in the United States (75.5 years) (76). Additionally, inclusion criteria required predicted 10-year survival; however, at 10 years follow-up in both the treatment and observation cohort, the overall mortality approached 50% (3,77). These findings highlight how poorly physicians may be at predicting 10-year survival of their patients, which has been demonstrated in other investigations as well (64).
To-date, the most robust prospective randomized study that measured outcomes of AS is the Prostate Testing for Cancer and Treatment (ProtecT) trial. This trial randomized 545 men to active monitoring, 553 men to surgery, and 545 men to radiotherapy. Results from this study demonstrated that at a median of 10 years follow-up, prostate-cancer-specific mortality was low irrespective of the treatment assigned, with no significant difference among treatments. Surgery and radiotherapy were associated with lower incidences of disease progression and metastases than was active monitoring. Unfortunately, less than 1% of the participants enrolled in ProtecT were AA, thus significantly limiting the generalizability of these results to this patient population (48). Future studies that aim to replicate the findings found in the ProtecT trial should aim to enroll a greater number of AA patients.
Monitoring protocol on AS
Unfortunately, a standardized protocol for AS has failed to reach consensus. AS follow-up involves serial PSA measurements and repeat physical exams, including digital rectal exam. A repeat or confirmatory biopsy after the initial diagnostic biopsy is useful for more accurate grading cancer in patients. MRI prostate imaging and guided confirmatory biopsies are increasing in prevalence, and because of the high negative predictive value for upgrading, utilizing this modality may be of particular use in the early stages of AS.
Our Tulane University institutional AS protocol, similar to many others, involves mpMRI within 6 months of initial diagnosis followed by directed biopsies, with repeat physical examination and PSA obtained every 6 months, and repeat imaging and biopsy at 24 months. The NCCN guidelines state that reconsideration of biopsy may be warranted up to every 12 months while on AS (78). Utilizing MRI may be particularly valuable in AA men potentially because of the differing location of tumors (anterior versus transition zone) and because of potential, yet controversial, increased upgrading rates. Several authors have confirmed the utility of MRI fusion biopsies in AA (32). It remains unclear if or how surveillance strategies should be modified for AA patients.
Accounting for non-prostate cancer specific survival
Even low-grade, slow-growing, and clinically localized prostate cancer retains metastatic potential. The inherent presumption of AS when compared with watchful waiting is that repeat evaluations will allow detection of progression of the prostate cancer with time to intervene. However, the often underappreciated and difficult-to-assess factor is mortality from non-prostate cancer specific causes. While most AS protocols focus on repeat assessment of oncologic factors with methods such as repeat biopsies, it must be noted that non-oncologic overall survival is not static and requires repeat assessment as well. However, determining 10-year mortality remains extremely challenging. The NCCN guidelines recommend using the Social Security Administration (SSA) tables and adding 50% for patients in the top quartile and subtracting 50% for patients in the lowest quartile of health (78). While this may be some of the clearest and most specific recommendations, they leave much to be desired. Clinicians with various biases and experience are the ones to determine the patient’s quartile of health. This method fails to account for various demographic influences that are not directly related to health but may influence mortality such as race. According to the Centers for Disease Control and Prevention (CDC), the age-adjusted death rate was 1.2 times greater for the non-Hispanic AA population and the life expectancy was 3.6 years lower compared to CA men. According to 2015 CDC data, life expectancy of a CA 65-year-old man is 18 years compared to 16.4 years for an AA man of the same age (76). Additionally, using the SSA method would likely lead practitioners to overtreat men with prostate cancer; for example, a 75-year-old man has a life expectancy of 11 years, suggesting that all but the least healthy quartile should consider pursuing a surgical approach to their prostate cancer (79).
Conclusions
The essential question bears repeating: if prostate cancer is generally more aggressive and deadlier for AA men, is it safe to consider AS for these “higher” risk patients? The preponderance of evidence demonstrates that AA have a greater incidence of prostate cancer and are more likely to die from it, typical biomarker screening tools such as PSA are less accurate in AA without making meaningful adjustments, and AA are at greater risk of failure from local treatment when compared to adjusted CA counterparts. Large prospective studies supporting the safety of AS have included few AA men, and AA men diagnosed with low-risk prostate cancer may be more likely to harbor aggressive elements. However, given the often-indolent nature of lower risk prostate cancer, it is the opinion of the authors that AS remains a useful tool to prevent overtreatment without sacrificing oncologic outcomes in AA men. Utilizing MRI and directed biopsies to more accurately characterize the disease may be important strategies to mitigate progression. More prospective observational studies are required before definitive conclusions can be reached.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst 2013;105:1050-8. [Crossref] [PubMed]
- Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012;367:203-13. [Crossref] [PubMed]
- Albertsen PC. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-101. [Crossref] [PubMed]
- Carroll PH, Mohler JL. NCCN Guidelines Updates: Prostate Cancer and Prostate Cancer Early Detection. J Natl Compr Canc Netw 2018;16:620-3. [Crossref] [PubMed]
- Franks LM. Proceedings: Etiology, epidemiology, and pathology of prostatic cancer. Cancer 1973;32:1092-5. [Crossref] [PubMed]
- Kilpinen JT. Percent Black or African American, 2010. Available online: https://scholar.valpo.edu/usmaps/9/
- Moses KA, Paciorek AT, Penson DF, et al. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol 2010;28:1069-74. [Crossref] [PubMed]
- United States Cancer Statistics. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, 2017.
- Prostate Cancer Rates by Race and Ethnicity 1999-2014. Available online: https://www.cdc.gov/cancer/prostate/statistics/race.htm
- Chornokur G, Dalton K, Borysova ME, et al. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate 2011;71:985-97. [Crossref] [PubMed]
- Oakley-Girvan I, Kolonel LN, Gallagher RP, et al. Stage at diagnosis and survival in a multiethnic cohort of prostate cancer patients. Am J Public Health 2003;93:1753-9. [Crossref] [PubMed]
- Sundi D, Ross AE, Humphreys EB, et al. African American Men With Very Low–Risk Prostate Cancer Exhibit Adverse Oncologic Outcomes After Radical Prostatectomy: Should Active Surveillance Still Be an Option for Them? J Clin Oncol 2013;31:2991-7. [Crossref] [PubMed]
- Powell IJ, Dey J, Dudley A, et al. Disease-free survival difference between African Americans and whites after radical prostatectomy for local prostate cancer: a multivariable analysis. Urology 2002;59:907-12. [Crossref] [PubMed]
- Evans S, Metcalfe C, Ibrahim F, et al. Investigating Black-White differences in prostate cancer prognosis: A systematic review and meta-analysis. Int J Cancer 2008;123:430-5. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524-8. [Crossref] [PubMed]
- Shenoy D, Packianathan S, Chen AM, et al. Do African-American men need separate prostate cancer screening guidelines? BMC Urol 2016;16:19. [Crossref] [PubMed]
- Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 2012;104:125-32. [Crossref] [PubMed]
- Shoag JE, Mittal S, Hu JC. Reevaluating PSA Testing Rates in the PLCO Trial. N Engl J Med 2016;374:1795-6. [Crossref] [PubMed]
- Schröder FH. Screening and Prostate-Cancer Mortality in a Randomized European Study. N Engl J Med 2009;360:1320-8. [Crossref] [PubMed]
- Loeb S, Vonesh EF, Metter EJ, et al. What is the true number needed to screen and treat to save a life with prostate-specific antigen testing? J Clin Oncol 2011;29:464-7. [Crossref] [PubMed]
- Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:762-71. [Crossref] [PubMed]
- Bibbins-Domingo K, Grossman DC, Curry SJ. The US Preventive Services Task Force 2017 Draft Recommendation Statement on Screening for Prostate Cancer: An Invitation to Review and Comment. JAMA 2017;317:1949-50. [Crossref] [PubMed]
- Morgan TO, Jacobsen SJ, McCarthy WF, et al. Age-Specific Reference Ranges for Serum Prostate-Specific Antigen in Black Men. N Engl J Med 1996;335:304-10. [Crossref] [PubMed]
- Henderson RJ, Eastham JA, Culkin DJ, et al. Prostate-specific antigen (PSA) and PSA density: racial differences in men without prostate cancer. J Natl Cancer Inst 1997;89:134-8. [Crossref] [PubMed]
- Feibus AH, Sartor O, Moparty K, et al. Clinical Use of PCA3 and TMPRSS2:ERG Urinary Biomarkers in African-American Men Undergoing Prostate Biopsy. J Urol 2016;196:1053-60. [Crossref] [PubMed]
- Punnen S, Freedland SJ, Polascik TJ, et al. A Multi-institutional Prospective Trial in the Veterans Affairs Health System confirms the 4Kscore maintains its Predictive value among African American Men. J Urol 2018;199:1459-63. [Crossref] [PubMed]
- Schwen ZR, Tosoian JJ, Sokoll LJ, et al. Prostate Health Index (PHI) Predicts High-stage Pathology in African American Men. Urology 2016;90:136-40. [Crossref] [PubMed]
- Waterhouse RL Jr, Van Neste L, Moses KA, et al. Evaluation of an Epigenetic Assay for Predicting Repeat Prostate Biopsy Outcome in African American Men. Urology 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Kasivisvanathan V. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med 2018;378:1767-77. [Crossref] [PubMed]
- Shin T, Smyth TB, Ukimura O, et al. Detection of prostate cancer using magnetic resonance imaging/ultrasonography image-fusion targeted biopsy in African-American men. BJU Int 2017;120:233-8. [Crossref] [PubMed]
- George A, Rais-Bahrami S, Rothwax J, et al. Using MRI/ultrasound fusion biopsy to detect clinically significant prostate cancer in the African American population. J Clin Oncol 2014;32:abstr 57.
- Tiguert R, Gheiler EL, Tefilli MV, et al. Racial differences and prognostic significance of tumor location in radical prostatectomy specimens. Prostate 1998;37:230-5. [Crossref] [PubMed]
- Volkin D, Turkbey B, Hoang AN, et al. Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int 2014;114:E43-9. [Crossref] [PubMed]
- Morris CR, Snipes KP, Schlag R, et al. Sociodemographic factors associated with prostatectomy utilization and concordance with the physician data query for prostate cancer (United States). Cancer Causes Control 1999;10:503-11. [Crossref] [PubMed]
- Shavers VL. Racial and Ethnic Disparities in the Receipt of Cancer Treatment. J Natl Cancer Inst 2002;94:334-57. [Crossref] [PubMed]
- Gordetsky JB, Saylor B, Bae S, et al. Prostate cancer management choices in patients undergoing multiparametric magnetic resonance imaging/ultrasound fusion biopsy compared to systematic biopsy. Urol Oncol 2018;36:241.e7-e13. [Crossref] [PubMed]
- Zeliadt SB, Ramsey SD, Penson DF, et al. Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer 2006;106:1865-74. [Crossref] [PubMed]
- Zeliadt SB, Penson DF, Moinpour CM, et al. Provider and partner interactions in the treatment decision-making process for newly diagnosed localized prostate cancer. BJU Int 2011;108:851-6. [PubMed]
- Cooperberg MR, Lubeck DP, Meng MV, et al. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol 2004;22:2141-9. [Crossref] [PubMed]
- Peay K, Elsamanoudi S, Lockhart R, et al. Race, treatment choice, and health-related quality of life in patients undergoing counseling for prostate cancer. Urology 2009;4:26. [Crossref]
- Gilliland FD, Hunt WC, Key CR. Ethnic variation in prostate cancer survival in New Mexico. Cancer Epidemiol Biomarkers Prev 1996;5:247-251. [PubMed]
- Shekarriz B, Tiguert R, Upadhyay J, et al. Impact of location and multifocality of positive surgical margins on disease-free survival following radical prostatectomy: a comparison between African-American and white men. Urology 2000;55:899-903. [Crossref] [PubMed]
- Iselin CE, Box JW, Vollmer RT, et al. Surgical control of clinically localized prostate carcinoma is equivalent in African-American and White males. Cancer 1998;83:2353-60. [Crossref] [PubMed]
- Kim JA, Kuban DA, El-Mahdi AM, et al. Carcinoma of the prostate: race as a prognostic indicator in definitive radiation therapy. Radiology 1995;194:545-9. [Crossref] [PubMed]
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol 2015;33:3379-85. [Crossref] [PubMed]
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272-7. [Crossref] [PubMed]
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415-24. [Crossref] [PubMed]
- Godtman RA, Holmberg E, Khatami A, et al. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Goteborg randomised population-based prostate cancer screening trial. Eur Urol 2013;63:101-7. [Crossref] [PubMed]
- Welty CJ, Cowan JE, Nguyen H, et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol 2015;193:807-11. [Crossref] [PubMed]
- Selvadurai ED, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol 2013;64:981-7. [Crossref] [PubMed]
- Stark JR, Perner S, Stampfer MJ, et al. Gleason Score and Lethal Prostate Cancer: Does 3 + 4 = 4 + 3? J Clin Oncol 2009;27:3459-64. [Crossref] [PubMed]
- Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol 2011;29:228-34. [Crossref] [PubMed]
- Nyame YA, Almassi N, Haywood SC, et al. Intermediate-Term Outcomes for Men with Very Low/Low and Intermediate/High Risk Prostate Cancer Managed by Active Surveillance. J Urol 2017;198:591-9. [Crossref] [PubMed]
- Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990-2013. JAMA 2015;314:80-2. [Crossref] [PubMed]
- Tosoian JJ, Carter HB, Lepor A, et al. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol 2016;13:205-15. [Crossref] [PubMed]
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol 2013;190:419-26. [Crossref] [PubMed]
- Sundi D, Kryvenko ON, Carter HB, et al. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol 2014;191:60-7. [Crossref] [PubMed]
- Faisal FA, Sundi D, Cooper JL, et al. Racial disparities in oncologic outcomes after radical prostatectomy: long-term follow-up. Urology 2014;84:1434-41. [Crossref] [PubMed]
- Vora A, Large T, Aronica J, et al. Predictors of Gleason score upgrading in a large African-American population. Int Urol Nephrol 2013;45:1257-62. [Crossref] [PubMed]
- Yamoah K, Deville C, Vapiwala N, et al. African American men with low-grade prostate cancer have increased disease recurrence after prostatectomy compared with Caucasian men. Urol Oncol 2015;33:70.e15-22. [Crossref] [PubMed]
- Weiner AB, Patel SG, Etzioni R, et al. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol 2015;193:95-102. [Crossref] [PubMed]
- Ha YS, Salmasi A, Karellas M, et al. Increased incidence of pathologically nonorgan confined prostate cancer in African-American men eligible for active surveillance. Urology 2013;81:831-5. [Crossref] [PubMed]
- Mahal BA, Aizer AA, Ziehr DR, et al. Racial disparities in prostate cancer-specific mortality in men with low-risk prostate cancer. Clin Genitourin Cancer 2014;12:e189-95. [Crossref] [PubMed]
- Komai Y, Numao N, Yoshida S, et al. High diagnostic ability of multiparametric magnetic resonance imaging to detect anterior prostate cancer missed by transrectal 12-core biopsy. J Urol 2013;190:867-73. [Crossref] [PubMed]
- Leapman MS, Freedland SJ, Aronson WJ, et al. Pathological and Biochemical Outcomes among African-American and Caucasian Men with Low Risk Prostate Cancer in the SEARCH Database: Implications for Active Surveillance Candidacy. J Urol 2016;196:1408-14. [Crossref] [PubMed]
- Qi R, Moul J. African American Men With Low-Risk Prostate Cancer Are Candidates for Active Surveillance: The Will-Rogers Effect? Am J Mens Health 2017;11:1765-71. [Crossref] [PubMed]
- Resnick MJ, Canter DJ, Guzzo TJ, et al. Does race affect postoperative outcomes in patients with low-risk prostate cancer who undergo radical prostatectomy? Urology 2009;73:620-3. [Crossref] [PubMed]
- Schreiber D, Chhabra A, Rineer J, et al. A Population-Based Study of Men With Low-Volume Low-Risk Prostate Cancer: Does African-American Race Predict for More Aggressive Disease? Clin Genitourin Cancer 2015;13:e259-64. [Crossref] [PubMed]
- Iremashvili V, Soloway MS, Rosenberg DL, et al. Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol 2012;187:1594-9. [Crossref] [PubMed]
- Abern MR, Bassett MR, Tsivian M, et al. Race is associated with discontinuation of active surveillance of low-risk prostate cancer: results from the Duke Prostate Center. Prostate Cancer Prostatic Dis 2013;16:85-90. [Crossref] [PubMed]
- Odom BD, Mir MC, Hughes S, et al. Active surveillance for low-risk prostate cancer in African American men: a multi-institutional experience. Urology 2014;83:364-8. [Crossref] [PubMed]
- Cullen J, Brassell SA, Chen Y, et al. Racial/Ethnic Patterns in Prostate Cancer Outcomes in an Active Surveillance Cohort. Prostate Cancer 2011;2011. [Crossref] [PubMed]
- Koscuiszka M, Hatcher D, Christos PJ, et al. Impact of race on survival in patients with clinically nonmetastatic prostate cancer who deferred primary treatment. Cancer 2012;118:3145-52. [Crossref] [PubMed]
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N Engl J Med 2017;377:132-42. [Crossref] [PubMed]
- Kochanek KD, Anderson RN, Arias E. Leading Causes of Death Contributing to Decrease in Life Expectancy Gap Between Black and White Populations: United States, 1999-2013. NCHS Data Brief 2015.1-8. [PubMed]
- Sartor O. Implications of the prostate intervention versus observation trial (PIVOT). Asian J Androl 2012;14:803-4. [Crossref] [PubMed]
- NCCN Guidelines, Version 2.2018, Prostate Cancer. National Comprehensive Cancer Network 2018.
- Kent M, Penson DF, Albertsen PC, et al. Successful external validation of a model to predict other cause mortality in localized prostate cancer. BMC Med 2016;14:25. [Crossref] [PubMed]