
Urologic issues in pediatric transplant recipients
Introduction
Currently, patients in waiting list for organ transplantation overpass 100,000 people. Although efforts have been made for increasing organ donation and transplantation, there continues to be a gap between supply and demand. According to Organ Procurement and Transplantation Network (OPTN), in 2015, there were 122,071 people waiting, whereas only 30,975 transplants were performed. In 2018, more than 95,000 chronic kidney patients are in waiting list only in United States (http://optn.transplant.hrsa.gov). Of those in waiting transplant list, children are a significant percentage, presenting a high mortality rate due to the limited number of organs available for this special population.
The most common causes of pediatric end-stage renal disease (ESRD) are urinary tract congenital anomalies (children aged <12 years) and glomerulonephritis. This second condition is usually seen in the adolescent population (1,2). Regarding preoperative care, patients on dialysis need to be controlled for metabolic acidosis, electrolyte abnormalities, fluid overload and hypertension, and symptomatic uremia (3). Secondary disorders from chronic kidney disease (CKD), such as anemia, nutritional imbalance (particularly protein energy wasting and cachexia), CKD-mineral bone disorder, and cardiovascular health disserve also special attention. Before transplantation, urinary tract abnormalities should be careful evaluated, as it may impact on graft and patient survival. Due to improvements on ESRD care, data from NAPRTCS registry has shown that survival while on dialysis has improved in the last decades (4).
The aim of this review is focus on urologic issues in pediatric kidney transplants. Preoperative evaluation and urinary tract abnormalities correction, surgical technique, graft survival, and postoperative complications will be discussed.
Preoperative evaluation
All children that are candidates for kidney transplantation should be submitted to abdominal ultrasound to evaluate upper urinary tract system, bladder morphology, and post-void residual. If children present a past medical history of urinary tract infection, incontinence or urological surgery or instrumentation, a voiding cystourethrography is indicated. In case of bladder dysfunction (i.e., spinal dysraphism), a urodynamic study should also be performed before kidney transplantation. Inferior urinary tract abnormalities have to be recognized and corrected before kidney transplantation (5).
Taghizadeh et al. reported their experience with 18 renal transplants that were performed in 16 children, 10 after bladder augmentation and 8 before bladder augmentation. There was only one graft loss in patients submitted to bladder augmentation before kidney transplantation, while there was four graft loss in patients who were transplanted first. Therefore, authors concluded that bladder augmentation before renal transplant does not increase complications and might better protect the renal graft (6). Although it seems logical to perform bladder augmentation before kidney transplantation, some authors have reported good outcomes in children transplanted first. Basiri et al. reported similar graft survival rate and febrile UTI in children who underwent augmentation cystoplasty before and after kidney transplantation (7).
Based on our experience we recommend performing the bladder augmentation before a kidney is transplanted. The child has not the inconvenience of a procedure under immunosuppression and the kidney is already allocated into an adequate urinary reservoir. In general, bladder augmentation is performed 3 to 4 months before the transplant. In case of anuria the reservoir is irrigated three or four times a week during the period of dialysis to maintain adequate bladder volume and remove any enteric secretion (8). If child is waiting a graft from a cadaveric donor, the reservoir may be reduced during the waiting time, but it is not a problem, because it will quickly achieve an adequate capacity after the transplantation. We had performed in some cases the bladder augmentation after the transplant with an uneventful evolution. In these cases, the bladder had been initially considered adequate to receive the graft, however the evolution was unfavorable with upper urinary dilation.
Bladder augmentation
The goal of bladder augmentation is creating a low-pressure reservoir with a good capacity and an adequate drainage by regular micturition with a Valsalva maneuver or clean intermittent catheterization (CIC). CIC may be performed safely even in patients under immunosuppression (9). The child or some familiar member needs to be trained and committed about the importance of adequate bladder drainage before the transplantation.
Several augmentation procedures have been described. In our opinion, the native ureter should be used as first choice to bladder reconstruction when it was available. One or both dilated ureter may be incorporated to the bladder without risk of electrolyte and acid-base disturbances and malignization (Figure 1). Unfortunately, this approach is feasible in few patients, who have an adequate ureter and did not have been submitted to ureteral reimplantation (8). When the upper urinary tract is not dilated, bladder augmentation with an intestinal segment (ileum or sigmoid) is the best option. Enterocystoplasty (Figure 2) is the most commonly used technique. The ileum segment is the preferred due to its abundance and easier manipulation. Long-term studies have demonstrated that enterocystoplasty improves bladder capacity and compliance and that these changes are maintained over time (10,11). We reported previously our experience with 305 pediatric kidney transplantations; 96 of 305 children presented with a urological cause of ESRD and 31 (10%) required bladder augmentation. Most of cases were submitted to an ileal cystoplasty (18 cases, 58%), although the ureter was our first choice when it was available (11 cases, 35.5%). One patient (3.23%) underwent a bladder augmentation with sigmoid because the ileum did not reach the bladder without tension, and 1 patient (3.23%) underwent a bladder auto-augmentation (5). All children with augmented bladder due to neuropathic bladder, or with difficulties in spontaneous drainage, were trained in CIC before transplantation. In three patients the inferior urinary tract was not feasible to be reconstructed and a continent urinary diversion was created with a Mitrofanoff or Monti procedure.
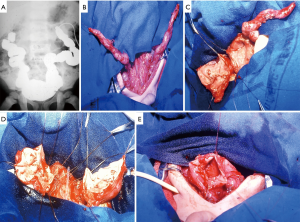
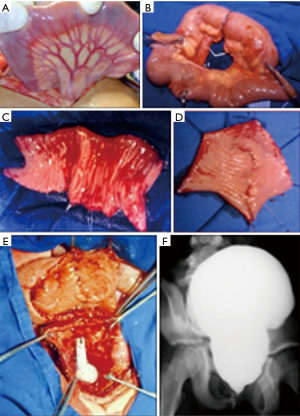
Surgical technique
Kidney transplantation in children can be performed by transperitoneal or extraperitoneal access. We prefer the extraperitoneal approach even in low weight children, as it seems to have many advantages compared with the transperitoneal approach. It provides excellent exposure of the inferior vena cava, aorta and bladder, avoid intestinal manipulation, and enables native ureter exposure if it is needed (Figure 3). In addition, extraperitoneal access may facilitate postoperative management of patients who present with surgical complication or need a percutaneous biopsy, as this procedure become easier (12).
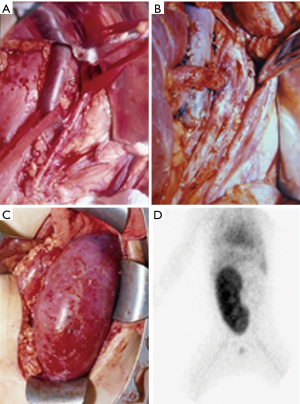
Venous anastomosis is performed end-to-side to the inferior vena cava, common iliac vein or external iliac vein based on the weight of the child. After venous anastomosis is done, a vascular occlusion clamp is applied over the renal vein, restoring central vein drainage. The renal artery is then anastomosed end-to-side to the aorta, common iliac artery, or external iliac artery. An alternative is an end-to-end anastomosis with the internal iliac artery. The ureter is implanted into the bladder by an extravesical ureteroneocystostomy technique. When performing the ureteric implantation in an augmented bladder, it should make a more lateral-posterior dissection of the reservoir, getting access to the native bladder wall and performing an easier reimplantation with an anti-reflux mechanism. It is very important in such group of patients, because it prevents a higher risk of upper urinary tract infection, especially in children who require performing CIC. When the bladder reservoir is not feasible to be dissected an option is to perform an uretero-ureteral anastomosis with the native non-refluxing ureter. Usually a Foley catheter is left for 5 days in regular bladder and for 10 days in augmented bladder.
We have described this extraperitoneal access in 46 children with less than 20 kg. In 6 patients there were 7 surgical complications, including 2 urinary fistulas, 2 superficial wound infection and 3 vascular complications. Only one graft was lost due to a venous thrombosis (12). Furness et al. reported their experience with 29 children weighing less than 15 kg. All children underwent allograft placement extraperitoneally and the rate of surgical complications was 10%, including 3% vascular and 7% nonvascular (13). These numbers are similar to others published previously in the literature (12,14-16).
Graft survival and postoperative complications
According to last OPTN report, graft survival continued to improve over the past decade. Renal transplantation in children with urological disease does not carry a higher risk of graft loss, if urinary tract abnormalities are recognized and treated adequately. Many studies have demonstrated no significant difference in graft survival and renal function between patients with a reconstructed bladder and those with a normal bladder (5,6,17-21). The most common complication in patients with augmented bladder is urinary tract infection, however an early diagnosis and prompt treatment avoid graft and patient survival impairment (5,22,23).
Mendizal et al. reported their experience with kidney transplantation in 15 patients with 6 to 18 years old carrying severe abnormalities of the lower urinary tract. A total of 18 renal transplants were performed in 15 children. There were only three graft losses related to urological disease. The graft survival at 1, 5 and 10 years in the group of patients with bladder dysfunction were 77%, 62% and 30%, respectively, with a median of 79 months. The patient survival at 1, 5 and 10 years in the group with bladder dysfunction vs. the control group was 100%, 93% and 92% vs. 88%, 92% and 82%, respectively, with no significant differences (log rank test 0.62, P=0.43) (21). We reported our experience with 305 pediatric kidneys transplants and mean follow-up was 11 years. Overall graft and patient survival rates were in 1, 5, and 10 years 87.0%, 68.7%, and 58.6%, and 95.9%, 91.2%, and 85.7%, respectively, with no difference if the child had been submitted or not to a bladder augmentation. Arterial and venous thrombosis rates were 1.6% and 2.3%, respectively, while urinary fistula and vesicoureteral reflux were noted in 2.9% and 3.6% of cases, respectively. Patients with arterial stenosis (1.6%) were clinically managed, but one patient required an arterial stent placement. Patients with vascular thrombosis were submitted to transplantectomy. All urinary fistulas were successfully treated with ureteral reimplantation or uretero-ureteral anastomosis with the native ureter. Children with pyelonephritis and vesicoureteral reflux (3.6%) were submitted to polymer injection and antibiotic prophylaxis (24). When looking at factors impacting on graft survival rate, we found only the type of donor to be significant. Regarding the urological complication rate, it is similar to previous reports in literature (23,25).
Lastly, there is a risk of malignancy in bladders augmented with intestinal segments. There are several risk factors, including chronic inflammation and immunosuppressive therapy. According to some authors, the prevalence risk for bladder cancer is 1% to 2% over 10 years and 4.5% after a mean follow-up of 32 years (8,26-28).
Conclusions
Pediatric kidney transplantation in children with urological cause of ERSD may achieve similar outcomes when compared with those from nonurological causes. Careful preoperative evaluation and correction of urinary tract abnormalities is the key for success transplantation. Restoring urinary reservoir condition and urinary emptying is mandatory. Extraperitoneal access to graft implant is feasible even in small children. Complication rate is low and graft survival has been increasing.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Warady BA, Neu AM, Schaefer F. Optimal care of the infant, child, and adolescent on dialysis: 2014 update. Am J Kidney Dis 2014;64:128-42. [Crossref] [PubMed]
- Leonard MB, Donaldson LA, Ho M, et al. A prospective cohort study of incident maintenance dialysis in children: an NAPRTC study. Kidney Int 2003;63:744-55. [Crossref] [PubMed]
- Chua AN, Warady BA. Care of the Pediatric Patient on Chronic Dialysis. Adv Chronic Kidney Dis 2017;24:388-97. [Crossref] [PubMed]
- Weaver DJ Jr, Somers MJG, Martz K, et al. Clinical outcomes and survival in pediatric patients initiating chronic dialysis: a report of the NAPRTCS registry. Pediatr Nephrol 2017;32:2319-30. [Crossref] [PubMed]
- Torricelli FC, Watanabe A, Piovesan AC, et al. Urological complications, vesicoureteral reflux, and long-term graft survival rate after pediatric kidney transplantation. Pediatr Transplant 2015;19:844-8. [Crossref] [PubMed]
- Taghizadeh AK, Desai D, Ledermann SE, et al. Renal transplantation or bladder augmentation first? A comparison of complications and outcomes in children. BJU Int 2007;100:1365-70. [Crossref] [PubMed]
- Basiri A, Otookesh H, Hosseini R, et al. Kidney transplantation before or after augmentation cystoplasty in children with high-pressure neurogenic bladder. BJU Int 2009;103:86-8; discussion 88. [Crossref] [PubMed]
- Pereira PL, Urrutia MJ, Lobato R, et al. Renal transplantation in augmented bladders. Curr Urol Rep 2014;15:431. [Crossref] [PubMed]
- Traxel E, DeFoor W, Minevich E, et al. Low incidence of urinary tract infections following renal transplantation in children with bladder augmentation. J Urol 2011;186:667-71. [Crossref] [PubMed]
- Hayashi Y, Yamataka A, Kaneyama K, et al. Review of 86 patients with myelodysplasia and neurogenic bladder who underwent sigmoidocolocystoplasty and were followed more than 10 years. J Urol 2006;176:1806-9. [Crossref] [PubMed]
- Lopez Pereira P, Moreno Valle JA, Espinosa L, et al. Enterocystoplasty in children with neuropathic bladders: long-term follow-up. J Pediatr Urol 2008;4:27-31. [Crossref] [PubMed]
- Nahas WC, Mazzucchi E, Scafuri AG, et al. Extraperitoneal access for kidney transplantation in children weighing 20 kg. or less. J Urol 2000;164:475-8. [Crossref] [PubMed]
- Furness PD 3rd, Houston JB, Grampsas SA, et al. Extraperitoneal placement of renal allografts in children weighing less than 15 kg. J Urol 2001;166:1042-5. [Crossref] [PubMed]
- Melter M, Briscoe DM. Challenges after pediatric transplantation. Semin Nephrol 2000;20:199-208. [PubMed]
- Sheldon CA, Churchill BM, Khoury AE, et al. Complications of surgical significance in pediatric renal transplantation. J Pediatr Surg 1992;27:485-90. [Crossref] [PubMed]
- Tanabe K, Takahashi K, Kawaguchi H, et al. Surgical complications of pediatric kidney transplantation: a single center experience with the extraperitoneal technique. J Urol 1998;160:1212-5. [Crossref] [PubMed]
- Rigamonti W, Capizzi A, Zacchello G, et al. Kidney transplantation into bladder augmentation or urinary diversion: long-term results. Transplantation 2005;80:1435-40. [Crossref] [PubMed]
- Nahas WC, Mazzucchi E, Arap MA, et al. Augmentation cystoplasty in renal transplantation: a good and safe option--experience with 25 cases. Urology 2002;60:770-4. [Crossref] [PubMed]
- Basiri A, Hosseini Moghaddam S, Khoddam R. Augmentation cystoplasty before and after renal transplantation: long-term results. Transplant Proc 2002;34:2106-8. [Crossref] [PubMed]
- Lopez Pereira P, Ortiz Rodriguez R, Fernandez Camblor C, et al. Renal transplant outcome in children with an augmented bladder. Front Pediatr 2013;1:42. [Crossref] [PubMed]
- Mendizabal S, Estornell F, Zamora I, et al. Renal transplantation in children with severe bladder dysfunction. J Urol 2005;173:226-9. [Crossref] [PubMed]
- Nahas WC, Lucon M, Mazzucchi E, et al. Clinical and urodynamic evaluation after ureterocystoplasty and kidney transplantation. J Urol 2004;171:1428-31. [Crossref] [PubMed]
- Praz V, Leisinger HJ, Pascual M, et al. Urological complications in renal transplantation from cadaveric donor grafts: a retrospective analysis of 20 years. Urol Int 2005;75:144-9. [Crossref] [PubMed]
- Antonopoulos IM, Piovesan AC, Falci R Jr, et al. Transurethral injection therapy with carbon-coated beads (Durasphere(R)) for treatment of recurrent pyelonephritis in kidney transplant patients with vesico-ureteral reflux to the allograft. Clin Transplant 2011;25:329-33. [Crossref] [PubMed]
- Almeida F, Branco F, Cavadas V, et al. Urological complications after 134 pediatric kidney transplants: a single-center study. Transplant Proc 2013;45:1096-8. [Crossref] [PubMed]
- Soergel TM, Cain MP, Misseri R, et al. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol 2004;172:1649-51; discussion 1651-2. [Crossref] [PubMed]
- Husmann DA, Rathbun SR. Long-term follow up of enteric bladder augmentations: the risk for malignancy. J Pediatr Urol 2008;4:381-5; discussion 386. [Crossref] [PubMed]
- Nahas WC, Iizuka FH, Mazzucchi E, et al. Adenocarcinoma of an augmented bladder 25 years after ileocecocystoplasty and 6 years after renal transplantation. J Urol 1999;162:490-1. [Crossref] [PubMed]


