Contemporary role of postoperative radiotherapy for prostate cancer
Introduction
Prostate cancer (CaP) is the most common cancer among men (1). Although there is an important progress in decreasing CaP mortality (2), 30,000 men die every year in the United States accounting for 9% of cancer-related deaths in men (1). While there is no high-level evidence showing superiority of surgery over radiation treatment (RT) (3), radical prostatectomy (RP) is the most common treatment option for patients with localized, non-metastatic disease. Nearly 30% of all patients undergoing surgery will develop a biochemical recurrence in 10 years (4). Lately, an increasing number of men with high-risk disease have been primarily treated with RP (5,6), and within this population, more than 50% of patients will experience biochemical recurrence (7) with an increased risk of distant metastasis (DM) and CaP-related death.
Postoperative RT is a potentially curative treatment when either prostate-specific antigen (PSA) is detectable after surgery (salvage radiotherapy, SRT) or when patients have high-risk features for local recurrence (adjuvant radiotherapy, ART). Although efficacious in providing long-term disease control (8), a significant number of men will have disease progression and die of CaP. Conversely, given a heterogeneous course of the disease, many men with high-risk features or manifested biochemical recurrence will not develop clinical disease and hence would be treated unnecessarily. Fortunately, there are a number of advances that will contribute to the optimization of RT in postoperative settings. In this review, we will give an overview on the main topics and latest controversies about the use of radiotherapy after prostatectomy.
What is the role and optimal timing of postoperative radiation?
Adverse pathological factors after RP, such as positive surgical margins (+SM), extracapsular extension (ECE), or seminal vesicle invasion (SVI) increase the likelihood of disease recurrence. Three randomized clinical trials (RCT) have addressed the potential benefits of adjuvant (ART) versus delayed RT after RP for these patents (9-11) (Table 1).
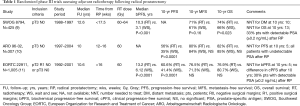
Full table
In 1987, the Southwest Oncology Group (SWOG) initiated the first RCT of ART for pathologic T3N0M0 CaP with ECE, +SM, or SVI (9). They included 425 men who were randomized to RT to the prostatic bed with a dose of 60–64 Gy versus delayed treatment. The primary study endpoint was metastasis-free survival (MFS). Not only MFS (HR 0.71, 95% CI: 0.54–0.94; P=0.016), but overall survival (OS) (HR 0.72, 95% CI: 0.55–0.96; P=0.023) was significantly improved with ART.
The European Organization for Research and Treatment of Cancer (EORTC) 22911 randomized patients to ART vs. wait-and-see (WS) policy until biochemical progression (BP) (11). Patients had a pT2–3 N0 disease, with at least one of the following risk factors: ECE, +SM, or SVI. The primary endpoint was biochemical progression-free survival (BPFS). ART significantly improved BPFS (HR 0.49, 95% CI: 0.41–0.59; P<0.0001). After a median follow up of 10 years, the difference in clinical progression-free survival (PFS) was not maintained between the groups, and no effect on DM or OS was seen.
The ARO 96-02 study included patients with pT3–4 pN0 with +SM (10). Prior to reaching an undetectable PSA after surgery, patients were randomized to either WS or ART. If the undetectable state was not achieved after surgery, patients were excluded by protocol. After a median follow-up of 9.3 years, 10-year PFS was 35% for WS approach versus 56% for ADT (HR 0.51; 95% CI: 0.37–0.70; P<0.0001). The advantage was still more pronounced in the per-protocol group (excluding those patients who did not complete the treatment originally allocated) (HR 0.45; 95% CI: 0.31–0.64; P<0.0001). For the entire eligible cohort (n=385), the HR was 0.71 (95% CI: 0.55–0.92; P=0.0050), favoring ART. ART had no significant impact on DM or OS. However, the study was underpowered for these endpoints.
SWOG 8794 and EORTC 22911 have included approximately 30% of patients with a detectable PSA at the time of adjuvant treatment; therefore, these patients received salvage treatment by definition. The ARO 96-02 trial was the only study that solely included men who achieved an undetectable PSA after RP on top of consistently incorporating three-dimensional conformal treatment planning, allowing a better comparison with more contemporary techniques.
The pathology data from the EORTC 22911 was reviewed with the objective to identify the factors that predict for increased benefit from ART (12). Margin status was the strongest predictor of prolonged BPFS with ART. By year 5, ART could prevent 291 events/1,000 patients with +SM (HR 0.38; 95% CI: 0.26–0.54) vs. 88 events/1,000 patients with negative margins (HR 0.88; 95% CI: 0.53–1.46). This study suggested that ART might not be beneficial in patients with SM.
Despite those three RCT, the use of ART has not increased over the past years (13,14). Many clinicians do not offer ART and recommend SRT at the presence of biochemical or local progression. The main advantage of SRT versus ART is deemed to be the avoidance of a potential overtreatment of patients who would never have disease progression, even in the presence of high-risk pathological features. Interestingly, among the patients randomized to observation in the trials mentioned above, about half (46% to 54%) remained free of biochemical recurrence at 5 years.
Taken together, these RCTs provide level I evidence for improved biochemical control without clear proof of benefit in long-term freedom from metastasis or survival for adjuvant therapy. Three RCTs are underway addressing the comparison between ART versus early salvage: RADICALS (NCT00541047), RAVES (NCT00860652) and GETUG 17 (NCT00667069). These trials recruited men with high-risk disease at RP with a postoperative PSA <0.2 ng/mL and have comparable designs. Men in the control arm all receive prompt SRT in the event of rising PSA (a weakness in the three published trials). A pooled analysis of these trials including >1,200 patients is planned and will hopefully help determine the role of ART.
Until prospective data are available, several retrospective studies and subgroup analysis of RCT have addressed the best timing for initiation of postoperative RT. The patients in the SWOG trial who were originally randomized to the WS approach and later had SRT (30%) were matched with the ART group. For patients with post-RP PSA levels ≤0.2 ng/mL, 5-year PSA-failure-free rate was 77% in the ART group versus 38% in the RT at failure WS group. For patients with PSA 0.2–1.0 ng/mL after RP, the 5-year PSA-failure-free rate was 34% in the ART group versus 17% in the WS group that received RT at failure (15). Fossati et al. compared ART with early SRT (SRT at PSA ≤0.5 ng/mL) in a retrospective cohort of 510 patients with initial undetectable PSA levels. No differences in MFS and OS were observed, although the ART group had more patients with pT3b/pT4 and +SM (16). Briganti et al. also showed no difference in biochemical relapse-free survival (BRFS) between ART versus early SRT (17). Ost et al. in a smaller matched cohort of 178 patients showed that 3-year BRFS was 91% in the ART versus 79% in the SRT group (P<0.05), although SRT was no longer significant on multivariable analysis (18). More recently, Hwang et al. reported on a large multi-institutional propensity-matched cohort of 1,566 patients who received either SRT or ART. The use of ART was associated with reduced biochemical relapses, DM, and overall mortality (19).
Several retrospective studies also suggest that a “very early” administration of SRT resulted in improved outcomes compared to patients with higher PSA levels. A multi-institutional retrospective study of 657 men who underwent SRT with a median follow-up of 9.8 years showed that increasing pre-SRT PSA strongly correlated with BRFS (R2=0.91). On multivariable analysis, a very early SRT (PSA of 0.01 to 0.2 ng/mL) was associated with a two-fold decreased in BF, use of salvage ADT and DM compared to early SRT (PSA of 0.2 to 0.5 ng/mL) (20). These results suggest that the definition of biochemical recurrence as two consecutive PSA levels higher than 0.2 ng/mL by the American Urological Association and European Urology Association guidelines might be too high and that earlier treatment might be optimal. Fossati et al. recently demonstrated that PSA levels were importantly dependent on other adverse pathologic findings. They showed a 10% drop in the 5-year BRFS with every PSA increment of 0.1 ng/mL in patients with two or more high risk factors, whereas for patients with only one or no adverse factors there was a 1.5% drop in 5-year BRFS. In patients with two high risk factors (Gleason ≥8, pT3b/T4, or −SM) postoperative RT should be given at the earliest evidence of PSA increase (21).
Stephenson et al. underlined the importance of pre-SRT PSA, which along with other risk factors, was associated with 6-year PFS of 26% vs. 48% for pre-SRT PSA of ≤0.5 vs. >0.5 ng/mL, respectively (22). Tendulkar et al. recently updated that multicenter cohort showing that 10-year rate of DM increased from 9% to 37% in patients with a pre-SRT PSA level ≤0.20 compared to ≥ 2.00 ng/mL, respectively (23). Furthermore, a pre-SRT PSA >0.5 ng/mL has been shown to independently correlate with BF, DM, cancer-specific mortality and all-cause mortality (24). Therefore, although there is no established PSA threshold, there is a consensus that the early versus late delivery of SRT represents a better treatment option.
Although many studies have correlated the use of early SRT with improved systemic control and CaP specific survival, it is often argued that these results might be due to lead time bias (25). It is claimed that investigators usually calculate the survival period referenced to the time of radiation instead the time of surgery (25), which would artificially skew the results in favor of early SRT. Nevertheless, secondary analysis by Stish et al. (24) suggest that, when considering RP as time zero for survival analyses, significant improvements in DM and cancer-specific survival outcomes were maintained, therefore arguing against prolonged monitoring of detectable post-RP PSA levels that delays initiation of SRT.
Should we combine ADT to postoperative radiation?
Prospective studies have shown that ADT improves local control and OS when combined with primary RT in the treatment of intermediate- and high-risk CaPs (26,27). However, the use of ADT combined with postoperative radiation remains less clear. To date, several retrospective studies (28-32) and two randomized phase III studies have been reported (33,34) (Table 2). In the RTOG 9601 (33), 771 men with an elevated serum PSA following RP were randomly assigned to radiation plus bicalutamide for 2 years or radiation alone. At a median follow up of 12.6 years, a 12-year OS improvement of 5% in the bicalutamide group was observed (HR 0.77; 95% CI: 0.59–0.99; P=0.04). Albeit this trial provides level I evidence for the use of ADT in the salvage setting, there are ongoing discussions on the overall extrapolation of these results due to the high pre-SRT levels as compared to more contemporary early SRT approaches. Approximately 50% of the RTOG 9601 patients had a pre-SRT PSA of 0.2–0.7 ng/mL, and only approximately 5% had a PSA of 0.2 ng/mL. For the first one-third of the trial enrollment the lower PSA limit to enroll was 0.5 ng/mL which explains the higher PSA values pre-SRT. Moreover, 12% of patients had persistently elevated PSAs after prostatectomy of ≥0.5 ng/mL. The largest benefit was derived in men with pre-SRT PSAs of >1.5 ng/mL. In contrast, men with pre-SRT PSAs of <0.7 ng/mL did not have a significant benefit in DM and in fact experienced non-significant worsening of OS [HR 1.13 (0.77–1.65)]. In view of these results, it has been attempted to define subgroups of patients who would benefit the most from ADT in combination with SRT (35), however this topic has created some controversy due to the inconsistent interpretation of unplanned subgroup analysis (36). Subgroup analyses like this are underpowered and should not be taken at face value in making treatment decisions. Men with high risk features and pre-SRT PSAs of <0.7 should still be considered for ADT.

Full table
GETUG-16 is a phase III study that randomized men with biochemical failure after surgery with a serum PSA level ranging from 0.2 to 2.0 ng/mL to SRT alone versus SRT combined with 6 months of Luteinizing hormone-releasing hormone (LHRH) agonist. This study showed a 17.5% improvement in 5-year PFS because of a decrease in biochemical recurrence rates (HR 0.50, 95% CI 0.38–0.66; P<0.0001) (34). LHRH analogs reduce the level of testosterone, which in turn decreases androgen receptor signaling and results in a decreased production of PSA. The initial separation of PFS curves between the ADT treatment and control arms suggests that the difference was related at least in part to the residual castration effects in the treatment arm; the time to testosterone recovery was not reported. Due to robust accrual, the trial accrual goal was raised from 466 to 738 patients, which would result in an 80% power to detect a 10% difference in OS. Given a plethora of new options emerging that extend survival, the likelihood that a difference in OS will be realized is low and awaits long-term follow-up.
Large ongoing studies are addressing the use of ADT combined with salvage radiation. In the phase III RTOG 0534 (NCT00567580), men with a serum PSA ≥0.2 and <2.0 ng/mL have been randomly assigned to salvage radiation to the prostate bed only, salvage radiation to the prostate bed with neoadjuvant and concurrent ADT, or salvage radiation to the prostate bed and pelvic lymph nodes with neoadjuvant and concurrent ADT. The RADICALS (NCT00541047), as previously mentioned, addresses two critical questions: the comparative efficacy of adjuvant versus salvage radiation, and the role of ADT and its treatment duration. Patients receiving radiation (either adjuvant or salvage) are further randomized to three treatment arms: radiation alone, radiation plus 6 months of ADT or radiation plus 2 years of ADT.
ADT is associated with several important adverse-effects (37), therefore the optimization of its use to maximize long-term oncologic benefits in the right patient population is key. Additionally, the overall extrapolation of the RTOG 9601 and GETUG-16 results is often questioned as none of these trials has exclusively focused on the early intervention (PSA <0.5), recommended by most of the international guidelines. As mentioned above, more than 10% of patients included in the RTOG 9601 trial received SRT for post-RP PSA persistence, which is a known risk factor for poorer outcomes (38).
Retrospective studies have shown that ADT improves outcomes only in selected group of patients. Gandaglia et al. (39) retrospectively reviewed 525 patients who received SRT at PSA levels ≤2 ng/mL to assess the impact of ADT according to the risk of metastasis. Median PSA and RT dose were 0.42 ng/mL and 66 Gy, respectively. At a median follow-up of 104 months, 71 patients experienced DM. On the multivariable model, the impact of ADT significantly differed according to the risk of DM. When considering patients treated with early SRT (PSA <0.5), ADT was associated with a reduction in DM rate only in patients with more adverse features (i.e., pT3b/4 and grade group ≥4, or pT3b/4 and pre-SRT PSA ≥0.4ng/mL). Therefore, the use of ADT in the context of postoperative ADT should be carefully considered as many patients with low PSA levels and favorable pathological features could be spared from the ADT-related side effects.
What is the optimal dose to the prostate bed?
The use of dose escalation in the context of radical treatment to the prostate improved local control in multiple randomized trials (40-44). However, currently no randomized trial has been published on the benefits of dose escalation after prostatectomy, although radiobiological models and retrospective series have supported this approach. King et al. (45) showed that patients treated with 70 Gy had a 5-year BRFS of 58% compared to 25% when treated with 60 Gy (P<0.0001). In a multivariate analysis, higher dose [P=0.012, HR 0.48 (95% CI, 0.27–0.87)] was an independent factor for superior BRFS together with pre-RT PSA value ≤1 ng/mL [P<0.0001, HR 0.28 (95% CI, 0.16–0.48)], and lack of seminal vesicle involvement [P=0.009, HR 0.44 (95% CI, 0.26–0.77)]. Ost et al. (46) obtained similar results employing 76 Gy to the prostate bed in 136 patients. The 5-year actuarial BRFS and clinical relapse-free survival (CRFS) were 56% and 86%, respectively. Goenka et al. (29) assessed 285 patients receiving SRT, of whom 270 patients (95%) were treated to a dose ≥66 Gy, and 205 (72%) received doses ≥70 Gy. Thirty-one percent received additional ADT. The median follow-up was 60 months. After 7 years, BRFS was 37% and DMFS was 77%. In this series, SRT dose ≥70 Gy was not associated with improvement in biochemical control. However, in patients with macroscopic recurrence at time of salvage, there was a trend in favor of dose ≥70 Gy (7 years: 90% vs. 79.1%, P=0.07). Pisansky et al. (47) assessed the data of 1,108 patients (+SM, pre-RT PSA ≤2.0 ng/mL, without ADT) who underwent SRT at 10 American academic centers. With a 65.2-month follow-up, a SRT dose of ≥66 Gy was associated with a reduced cumulative incidence of BF, but not of DM (47). Ohri et al. (48) performed a meta-analysis along with a radiobiological modelling including 25 studies with 3,828 patients to identify predictors of BRFS and late toxicity. Five-year BRFS ranged from 25% to 70%. Severe (grade
Cozzarini et al. (50) studied more than 700 postoperative RT patients to identify potential predictors of long-term severe GU sequelae. Patients either received ART (n=556; median dose, 70.2 Gy) or SRT (n=186; median dose, 72 Gy) intent. After a median follow-up of 99 months, the 8-year risk of grade
The Swiss SAKK 09/10 is a prospective phase III study that randomized 350 patients to SRT to the prostate bed with 64 vs. 70 Gy. A recent analysis on the acute toxicity and early quality of life has shown acceptable results (51). In the 64 Gy arm, acute grade 2 and 3 GU toxicity was observed in 13.0% and 0.6%, respectively; whereas in the 70 Gy arm, 16.6% and 1.7%, respectively. In the 64 Gy arm, acute grade 2 and 3 GI toxicity was observed in 16.0% and 0.6%, respectively; whereas in the 70 Gy arm, 15.4% and 2.3%, respectively. There was no significant difference in Common Terminology Criteria for Adverse Effects (CTCAE)-based acute toxicity rates. However, patient-reported health-related quality of life was worse for GU symptoms in the 70 Gy arm, although this was deemed to be small. The primary endpoint analysis (freedom from biochemical failure) is expected to be published in 2019.
What is the status of hypofractionation in the postoperative setting?
Not only dose escalation but also hypofractionation has an attractive role in post-prostatectomy irradiation. Exploiting the radiobiological properties of CaP, hypofractionation uses larger daily fraction sizes (i.e., 2 to 5 Gy) to deliver the treatment over a shorter duration. The potential advantages of hypofractionation include increased convenience to patients, reduced costs to patients and society and improved resource utilization.
Although hypofractionation in the radical treatment of the prostate is gaining traction due to its safety and comparable efficacy demonstrated in RCTs with over 5 years of follow up (52), the experience with hypofractionation in the postoperative setting is more limited. While the treatment target in the radical treatment is the prostatic tissue, in the postoperative setting dose is delivered to the prostatic bed which includes a more significant volume of normal tissues. Most of the prostate bed recurrences occur at the level of the vesicourethral anastomosis therefore the coverage of this region is essential. Despite a striking progress of RT delivery techniques [i.e., intensity-modulated RT (IMRT)] with significant improvement in conformality, the impact of hypofractionation in long-term urinary toxicity is unknown.
In terms of biochemical control rates, retrospective series on hypofractionation to the prostate bed seem to report similar results as compared to normal fractionation, 74–85% at 3 years (53,54) and 67–75% at 4 years (53,55-57). Nevertheless, toxicity rates overall tend to be higher than from normal fractionation. Currently, few retrospective studies with limited follow-up have reported on the late toxicity of hypofractionation following RP. Kruser et al. studied 108 patients who underwent hypofractionated SRT delivering 65 Gy in 26 fractions. At a median follow up of 32 months, the incidence of late grade 2 GU toxicity was 15%, while no late grade ≥3 urinary toxicities were documented (55). Lee et al. used 50–52.5 Gy in 20 fractions, and after a median follow-up of 3 years, reported no acute or late grade ≥3 toxicity (53).
A recent systematic review included 14 studies with 918 patients treated with hypofractionation in the postoperative setting (57). The total dose to the prostate bed ranged from 72.8 Gy in 29 fractions to 50 Gy in 20 fractions; the median dose per fraction delivered was 2.5 Gy (range, 2.3–3.4 Gy). Assuming an α/β value of 3 Gy for late toxicity, the equivalent dose in 2 Gy fractions (EQD2Gy) ranged from 55 to 80 Gy, while the corresponding values for an α/β value =1.5 Gy for CaP cells ranged from 57 to 83 Gy. Overall, rates of GU and GI acute toxicities were mild to moderate with similar rates across the studies. Despite an important difference in follow up time among the studies (6 to 98 months, median: 36 months), the reported late toxicity was mild (i.e., grade 1–2).
In contrast, some series have shown greater long-term GU toxicity. Syndikus et al. (58) published a series with a follow up >7 years for patients receiving 50–55 Gy in 16–20 fractions. The crude incidence of late grade 3–4 GU sequelae was 19% in the 89 patients receiving ART and 27% in the 26 patients treated for a biopsy-proven local recurrence. Remarkably, after a median follow-up time of 68 months (range, 54–81 months), Cozzarini et al. (59) reported a 5-year risk for grade 3 or higher late GU toxicity of 18.1%. Among the group of 115 patients with late grade 3 GU toxicity, 68 underwent surgical interventions for urethral stenosis and/or bladder neck strictures, 30 patients received blood transfusions and/or hyperbaric oxygen therapy for severe and persistent gross hematuria, and 47 patients reported a post-irradiation onset or worsening of grade 3 urinary incontinence. Salvage cystectomy was undertaken in 5 patients who suffered of grade 4 GU toxicity. These data serve as a warning that long follow-up is essential to adequately assess the impact of hypofractionation.
Ongoing prospective trials will shed further light on the role of hypofractionation in the postoperative setting. The SHARP trial (NCT02976402) is a phase I study that addresses the use of extreme hypofractionation. Patients treated in the adjuvant setting receive 31 Gy in 5 fractions of 6.2 Gy (68.2 Gy EQD2Gy; α/β=1.5 Gy), while for the salvage setting patients are to be treated with 32.5 Gy in 5 fractions of 6.5 Gy (74.3 Gy EQD2Gy; α/β=1.5 Gy), both delivered in 5 daily consecutive fractions. The PRIAMOS trial (NCT01620710) is a phase II trial that uses 54 Gy in 18 fractions to the prostatic bed. Results on late severe toxicity are expected to be published in 2018. Another study from Virginia University (NCT01868386) explores four dose escalation schemes in a phase I-II trial (from 65 Gy in 26 fractions of 2.5 Gy up to 42.6 Gy in 10 fractions of 4.26 Gy). This trial was started in May 2013 and first results are expected to be published soon.
NRG-GU 003 (NCT03274687) is an ongoing randomized phase III non-inferiority trial that is randomizing 282 patients to 66.6 Gy in 37 fractions versus 62.5 Gy in 25 fractions. The primary objective is to demonstrate that hypofractionated post-operative RT does not increase patient-reported GI and GU symptoms (Expanded CaP Index, EPIC) over conventionally fractionated post-prostatectomy at the 2-year time point. One could argue that a 2-year endpoint is inadequate to describe the late effects of postoperative hypofractionated radiotherapy.
In summary, current data on hypofractionation after prostatectomy suggest favorable acute toxicity profile and similar biochemical control rates; however, there is lack of long-term safety data and its use should be considered investigational until reports of long-term follow up are available.
Elective treatment of pelvic nodes
The above-mentioned randomized trials evaluating ART vs observation limited treatment to the prostate bed only (9-11), precluding evaluation of whether elective pelvic lymph node radiotherapy (PLNRT) improves outcomes. RT to the pelvic lymph nodes could potentially eradicate micrometastatic disease that is not detected at the time of staging and treatment. In fact, patters of recurrence after SRT to the prostate bed evaluated with new imaging modalities show a significant number of relapses in the pelvic lymph nodes (60). Conversely, three randomized trials failed to demonstrate an effect on clinical relapse or survival in men treated primarily for CaP (61-63). NRG RTOG 0924 (NCT01368588) is currently recruiting patients to readdress this question because of concerns that the prostate doses used in the past were insufficient to control the primary, resulting in a lack of power to detect a benefit from PLNRT. The PIVOTAL trial (NCT01685190) is examining the toxicity from PLNRT.
In the postoperative setting, several small retrospective studies have presented conflicting results regarding the utilization of elective treatment of the pelvic lymph nodes (64-67). Recently, the largest series investigating the roles of supplemental pelvic treatment and/or ADT combined with post-prostatectomy radiation was reported by Ramey et al. (68). More than 1,800 patients were assessed to explore whether pelvic treatment and/or ADT added to prostate bed RT improves freedom from biochemical failure or DM. Overall, both ADT and pelvic irradiation were associated with improved freedom from biochemical failure in multivariable analysis. Subgroup analyses demonstrated that the addition of pelvic treatment was associated with 14% absolute improvement in 5-year freedom from biochemical failure in patients with Gleason score (GS) 7 and 16% improvement in patients with GS 8–10. The highest rate of freedom from biochemical failure was seen in patients treated with PLNRT combined with ADT. No improvement in DM or OS was observed.
Although many clinicians have routinely used PLNRT treatment in the postoperative setting in order to optimize oncologic outcomes (69), more definite measures of treatment efficacy and toxicity of elective pelvic treatment should be drawn by the RTOG 0534 study. This large phase III trial includes patients with biochemical failure who were randomly assigned to prostate bed RT vs. prostate bed and 4–6 months of ADT vs. prostate bed, PLNRT and ADT.
How to integrate new imaging modalities (i.e., PET-CT) into clinical practice?
The current use of postoperative RT assumes that most of the recurrences occur in the prostate bed (15). Largely this was the rationale for the formulation of contouring guidelines that encompass the CaP surgical bed (70-73). However, these guidelines have been developed in the era of conventional imaging modalities with relative low sensitivity for the detection of prostate bed and/or pelvic lymph node disease. Perhaps this explains the fact that SRT, as per current standards, only provides long-term biochemical control in less than half of all patients post-prostatectomy.
Lately the use of new imaging modalities has increasingly changed the management of patients after RP. The impact of choline PET/CT imaging on SRT planning has been assessed in several retrospective studies (74-78). These studies found that addition of choline PET/CT to SRT planning changed the initial plan in 357 of 1,083 patients (33%). Comparative studies indicate that 68Ga-prostate specific membrane antigen (PSMA)-11 PET/CT is superior to Choline-PET (79) and 18F-fluciclovine PET/CT (80), therefore the impact of 68Ga-PSMA-11 PET/CT is expected to be even more important.
Calais et al. (81) assessed 270 patients with BP after RP and PSA <1.0 ng/mL. Patients underwent 68Ga-PSMA-11 PET/CT at a median PSA of 0.48 ng/mL (range, 0.03–1 ng/mL). One hundred and thirty-two patients (49%) had a positive 68Ga-PSMA-11 PET/CT. In 52 patients (19%) at least one lesion was found to be located outside the consensus clinical tumor volume (CTV) recommended by RTOG contouring guidelines. Extra-pelvic PSMA-positive lesions was identified in 33 patients (12%) and pelvic lesions outside the CTV was found in 19 (7%). The two most frequent sites not covered by recommended CTV were bone (23/52, 44%) and perirectal lymph nodes (16/52, 31%). Post-hoc analysis of 68Ga-PSMA-11 PET/CT implied a major impact on RT planning in 52 patients (19%) with biochemical recurrence (PSA<1.0 ng/mL). Furthermore, 32 patients (24% of the 132 PSMA-positive patients) had PSMA-positive lesions isolated to the prostate bed alone, while 67/132 patients (51%) had PSMA-positive lesions within the pelvis but without DM. This work also underscores the potential benefit of treating the pelvic lymph nodes, which is being addressed in NRG RTOG 0534 (NCT00567580).
Grubmuller et al. retrospectively assessed 117 consecutive patients with biochemical recurrence who had 68Ga-PSMA 11 PET/CT (n=46) or PET/MRI (n=71). For patients with PSA value of 0.2–0.5, 0.5–1, 1–2 and ≥2 ng/mL, detection rates were 65%, 85.7%, 85.7% and 100%. PSMA-PET detected lesions in 67 patients (57.3%) who had no suspicious correlates according to the Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 criteria on MRI or CT. A change in treatment management occurred in 74.6% of these 67 patients (P<0.001), with 86% of them being considered for metastases-directed therapies (82).
Interestingly, some have argued that as imaging techniques improve, it is possible that salvage treatment could be deferred until the site of recurrent disease is detected. Men with metastatic disease could then avoid local or loco-regional RT, whereas RT could be tailored according to the presence of localized or oligometastatic disease. The former counterintuitive argument suggests that patients with metastatic disease will show up later than potentially curable patients with microscopic pelvic only disease. Likewise, the latter argument that only the PSMA-PET disease needs to be addressed assumes that PSMA-PET is a perfect test, which is an assumption known to be false. This is a point of important controversy as, although CaPs can importantly differ clinically, the efficacy (or non-inferiority) of such strategy has never been prospectively proven. The bulk of evidence favors early interventions and, while novel imaging modalities have shown improved detection rates, they do not provide optimal sensitivity at very low PSA levels. Recently, Emmet et al. showed that PSMA PET is independently predictive of treatment response to radiation, and stratifies men into a high treatment response to SRT (negative or fossa confined PSMA) versus men with poor response to SRT (nodes or distant disease PSMA). A negative PSMA-PET therefore was a strong predictor of a high response to SRT to the prostate bed (83).
Of note, as with any biomarker study, a change in management does not necessarily mean improved clinical outcomes. Therefore, randomized trials incorporating new imaging modalities are urgently warranted in the postoperative setting.
Biomarkers for the management of postoperative patients
CaP is a heterogeneous disease at clinical and molecular/genomic levels. Many patients treated with RP who have high-risk factors at final pathology or present postoperative detectable PSA may never develop clinical disease progression. Conversely, a substantial number of men are significantly impacted by the burden of metastatic disease or CaP deaths. Although conventional clinicopathologic features (PSA, T category and GS) and current nomograms have helped clinicians identify patients who require more intensive treatments, there is a need for better prognostic and predictive tools for treatment personalization.
Several tissue-based biomarkers have been progressively introduced into clinical practice. A wide range of tools have been reported to improve the discrimination of various disease-related outcomes in addition and/or independent to the conventional clinicopathologic parameters. It is important, nevertheless, to clarify that prognostic biomarkers inform about a likely cancer outcome (e.g., clinical progression, death) independently of the received treatment. A biomarker is predictive if the treatment effect (experimental compared with control) is different for biomarker-positive patients compared with biomarker-negative patients (84). Unfortunately, the vast majority of available biomarkers have only provided prognostic information and do not inform on the specific treatment selection.
Decipher (GenomeDx Biosciences, Vancouver, BC, Canada) is a tissue-based genomic classifier (GC) that was the result of large-scale analysis. It is based on 22 RNA biomarkers related to androgen receptor signaling, cell proliferation, differentiation, motility and immune modulation (85). Decipher generates a risk score between 0-1 in increments of 0.1 and stratifies patients in three risk groups (low risk =0 to 0.45; average risk =0.45 to 0.60 and high risk =0.60 to 1.0) for DM following RP (86-89). A meta-analysis of five retrospective studies including 855 men with adverse pathology at RP assessed the performance of the GC in predicting DM (90). Low-, intermediate-, and high-risk Decipher categories showed 10-year cumulative incidences of metastasis of 5.5%, 15.0%, and 26.7%, respectively. Decipher was an independent predictor of metastasis adjusting for clinicopathological parameters and adjuvant treatments.
Decipher could potentially guide treatment decisions after RP. Den et al. (86) analyzed a cohort of 188 high risk patients who underwent RP followed by RT. GC had had a higher ability to predict DM (AUC =0.83–0.85) than clinicopathological parameters alone (AUC =0.66). Patients with a GC of ≥0.4 undergoing ART or SRT showed a cumulative incidence of 5-year DM of 6% or 23%, respectively (P=0.008). Dalela et al. (91) developed a risk stratification tool (score 1–4) to potentially identify men who could benefit from ART versus observation. ART significantly reduced the 10-year clinical recurrence rate only in patients with a risk score of ≥2, suggesting that patients with unfavorable pathological characteristics and a higher Decipher score should be considered for ART.
Overall, patients with persistent PSA after RP (as opposed to undetectable and subsequent rising) have worse clinical outcomes (38). Decipher has been recently investigated in this patient population and has shown to independently predict for DM (92). In this study, only GC high-risk, detectable PSA after RP, and positive lymph node(s) remained prognostic factors for DM. Among patients with detectable PSA after RP, the 5-year DM rate was 0.90% for GC low/intermediate and 18% for GC high risk (P<0.001). Prospective validation of the GC as a predictor of DM in patients with persistent PSA after RP will be performed as part of the ongoing randomized trial NRG GU-002 (NCT03070886).
Zhao et al. assessed a gene expression signature to predict the specific benefits of postoperative RT (93). A 24-gene Post-Operative Radiation Therapy Outcomes Score (PORTOS) was initially evaluated in a training cohort (n=196). In patients with a high PORTOS score (n=39), those who had RT had a lower incidence of DM than did patients who did not have RT (5% vs. 63%). In patients with a low PORTOS (n=157), patients who had RT has a statistically higher rate of DM as compared to those not treated with RT. A validation cohort (n=330) confirmed that RT was associated with lower DM rates only in patients with high PORTOS score. It is unclear why patients with low-risk PORTOS score have a higher incidence of DM and do not benefit from postoperative RT. Nevertheless, these results should be interpreted with caution while PORTOS undergoes further validation.
Oncotype Dx Genomic Prostate Score (GPS; Genomic Health Inc., Redwood City, CA, USA) is a quantitative real-time PCR assay based on needle biopsy tissue. The assay comprises 12 cancer-related genes involved in androgen pathway, cellular organization, proliferation, and stromal response as well as five reference housekeeping genes (94). The GPS is expressed on a scale of 0–100 and has been originally investigated as a predictor of adverse pathology at RP; therefore, it may guide clinicians towards active surveillance versus therapeutic intervention. Its role in the postoperative setting is less clear. Retrospective studies have shown that the GPS is a good predictor of adverse pathological features at RP (94) and time to biochemical recurrence (95). The lack of large and prospective evaluations and its correlation with oncological outcomes may limit the real clinical benefit of this assay.
Prolaris test is a 46-gene panel [31 cell-cycle progression (CCP) genes and 15 housekeeping genes] developed by Myriad Genetics (Salt Lake City, UT, USA). The Prolaris tissue-based assay (prostate biopsy or RP specimens) that could in the decision active surveillance versus active treatment, and it may also suggest the use of ART in high-risk patients with adverse pathological features after RP. Cuzick et al. (96) investigated the impact of the Prolaris assay in two different populations of CaP patients treated with RP (n=366) or transurethral resection of the prostate (n=337). The CCP score was associated with the risk of PSA recurrence after RP and the 10-year specific mortality in patients conservatively managed. Bishoff et al. (97) showed that pre-treatment Prolaris assay (biopsy-based) was a significant predictor of PSA recurrence after RP in a multi-institutional cohort. It was also a significant predictor of DM on univariate analysis. Cooperberg et al. (98) confirmed the ability of the Prolaris test to predict postoperative PSA recurrence and found that a combined model incorporating Prolaris test with CAPRA score improved the prognostic accuracy.
CaP biological behavior has been hypothesized to be similar to breast cancer. PAM50 classifier, a molecular panel used in breast cancer, has been recently assessed in 3,782 RP samples (99). PAM50 consistently segregated patients into luminal A, luminal B, and basal-like subtypes, which are associated with different lineage markers. Patients with luminal tumors exhibit increased androgen signaling, and those with luminal B tumors have poorer outcomes but may respond more efficiently to postoperative ADT. PAM50 thus provide a potential clinical tool to tailor ADT use after surgery. Prospective validation of the PAM50 classifier in post-prostatectomy patients will take place in the NRG GU 006 (NCT03371719).
The future is bright but there is a lot of work ahead
There have been several endeavors to optimize the use of postoperative radiation including PLNRT, dose escalation, altered fractionation schemes, novel RT techniques, and the concomitant use of ADT. While data from large randomized trials are maturing to finally prove or refute the efficacy of those approaches, novel imaging modalities and biomarkers have shown a promising role for better patient stratification. Due to this rapidly evolving knowledge, it will be rather interesting to interpret data from forthcoming RCTs in the context of more contemporary approaches. Nevertheless, before widespread implementation, robust validation and evidence-based approaches are critical in the implementation of new imaging methods and genomic biomarkers.
Novel drugs have significantly improved OS in patients with metastatic disease and the early use of these agents hold promise in the treatment of patients after prostatectomy. However, predictive biomarkers are key for an efficient patient selection and cost-effective approaches.
Finally, the use of postoperative RT should be made in the context of multidisciplinary discussions and shared-decision making. Patients should be actively involved in the decision process with clear understanding on the balances between maximizing oncologic benefits (potential overtreatment) and quality of life optimization (risk of undertreatment). Patient’s life expectancy and preferences must be incorporated into the final treatment recommendation and, when available, participation in clinical trials should be emphasized. The future is certainly bright in view of state-of-the-art RT, novel systemic therapies, better imaging modalities and enhanced genomic profiling.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Sartor O, de Bono JS. Metastatic Prostate Cancer. N Engl J Med 2018;378:645-57. [Crossref] [PubMed]
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415-24. [Crossref] [PubMed]
- Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol 2007;51:1175-84. [Crossref] [PubMed]
- Gontero P, Spahn M, Tombal B, et al. Is there a prostate-specific antigen upper limit for radical prostatectomy? BJU Int 2011;108:1093-100. [Crossref] [PubMed]
- Yossepowitch O, Eggener SE, Serio AM, et al. Secondary therapy, metastatic progression, and cancer-specific mortality in men with clinically high-risk prostate cancer treated with radical prostatectomy. Eur Urol 2008;53:950-9. [Crossref] [PubMed]
- Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol 2016;69:428-35. [Crossref] [PubMed]
- Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008;299:2760-9. [Crossref] [PubMed]
- Thompson IM Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 2006;296:2329-35. [Crossref] [PubMed]
- Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol 2009;27:2924-30. [Crossref] [PubMed]
- Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 2012;380:2018-27. [Crossref] [PubMed]
- Van der Kwast TH, Bolla M, Van Poppel H, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol 2007;25:4178-86. [Crossref] [PubMed]
- Ghia AJ, Shrieve DC, Tward JD. Adjuvant radiotherapy use and patterns of care analysis for margin-positive prostate adenocarcinoma with extracapsular extension: postprostatectomy adjuvant radiotherapy: a SEER analysis. Urology 2010;76:1169-74. [Crossref] [PubMed]
- Hoffman KE, Nguyen PL, Chen MH, et al. Recommendations for post-prostatectomy radiation therapy in the United States before and after the presentation of randomized trials. J Urol 2011;185:116-20. [Crossref] [PubMed]
- Swanson GP, Hussey MA, Tangen CM, et al. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol 2007;25:2225-9. [Crossref] [PubMed]
- Fossati N, Karnes RJ, Boorjian SA, et al. Long-term Impact of Adjuvant Versus Early Salvage Radiation Therapy in pT3N0 Prostate Cancer Patients Treated with Radical Prostatectomy: Results from a Multi-institutional Series. Eur Urol 2017;71:886-93. [Crossref] [PubMed]
- Briganti A, Wiegel T, Joniau S, et al. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol 2012;62:472-87. [Crossref] [PubMed]
- Ost P, De Troyer B, Fonteyne V, et al. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2011;80:1316-22. [Crossref] [PubMed]
- Hwang WL, Tendulkar RD, Niemierko A, et al. Comparison Between Adjuvant and Early-Salvage Postprostatectomy Radiotherapy for Prostate Cancer With Adverse Pathological Features. JAMA Oncol 2018. [Crossref] [PubMed]
- Abugharib A, Jackson WC, Tumati V, et al. Very Early Salvage Radiotherapy Improves Distant Metastasis-Free Survival. J Urol 2017;197:662-8. [Crossref] [PubMed]
- Fossati N, Karnes RJ, Cozzarini C, et al. Assessing the Optimal Timing for Early Salvage Radiation Therapy in Patients with Prostate-specific Antigen Rise After Radical Prostatectomy. Eur Urol 2016;69:728-33. [Crossref] [PubMed]
- Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 2007;25:2035-41. [Crossref] [PubMed]
- Tendulkar RD, Agrawal S, Gao T, et al. Contemporary Update of a Multi-Institutional Predictive Nomogram for Salvage Radiotherapy After Radical Prostatectomy. J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Stish BJ, Pisansky TM, Harmsen WS, et al. Improved Metastasis-Free and Survival Outcomes With Early Salvage Radiotherapy in Men With Detectable Prostate-Specific Antigen After Prostatectomy for Prostate Cancer. J Clin Oncol 2016;34:3864-71. [Crossref] [PubMed]
- Seisen T, Trinh QD, Abdollah F. Could lead-time bias explain the apparent benefits of early salvage radiotherapy? Nat Rev Urol 2017;14:193-4. [Crossref] [PubMed]
- Locke JA, Dal Pra A, Supiot S, et al. Synergistic action of image-guided radiotherapy and androgen deprivation therapy. Nat Rev Urol 2015;12:193-204. [Crossref] [PubMed]
- Nguyen PL. Optimization of the Radiation Management of High-Risk Prostate Cancer. Semin Radiat Oncol 2017;27:43-9. [Crossref] [PubMed]
- Jang JW, Hwang WT, Guzzo TJ, et al. Upfront androgen deprivation therapy with salvage radiation may improve biochemical outcomes in prostate cancer patients with post-prostatectomy rising PSA. Int J Radiat Oncol Biol Phys 2012;83:1493-9. [Crossref] [PubMed]
- Goenka A, Magsanoc JM, Pei X, et al. Long-term outcomes after high-dose postprostatectomy salvage radiation treatment. Int J Radiat Oncol Biol Phys 2012;84:112-8. [Crossref] [PubMed]
- Soto DE, Passarelli MN, Daignault S, et al. Concurrent androgen deprivation therapy during salvage prostate radiotherapy improves treatment outcomes in high-risk patients. Int J Radiat Oncol Biol Phys 2012;82:1227-32. [Crossref] [PubMed]
- Parekh A, Chen MH, Graham P, et al. Role of androgen deprivation therapy in early salvage radiation among patients with prostate-specific antigen level of 0.5 or less. Clin Genitourin Cancer 2015;13:e1-6. [Crossref] [PubMed]
- Ervandian M, Hoyer M, Petersen SE, et al. Salvage radiation therapy following radical prostatectomy. A national Danish study. Acta Oncol 2016;55:598-603. [Crossref] [PubMed]
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med 2017;376:417-28. [Crossref] [PubMed]
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol 2016;17:747-56. [Crossref] [PubMed]
- Spratt DE, Dess RT, Zumsteg ZS, et al. A Systematic Review and Framework for the Use of Hormone Therapy with Salvage Radiation Therapy for Recurrent Prostate Cancer. Eur Urol 2018;73:156-65. [Crossref] [PubMed]
- Spears MR, James ND, Sydes MR. 'Thursday's child has far to go'-interpreting subgroups and the STAMPEDE trial. Ann Oncol 2017;28:2327-30. [Crossref] [PubMed]
- Higano CS. Side effects of androgen deprivation therapy: monitoring and minimizing toxicity. Urology 2003;61:32-8. [Crossref] [PubMed]
- Wiegel T, Bartkowiak D, Bottke D, et al. Prostate-specific antigen persistence after radical prostatectomy as a predictive factor of clinical relapse-free survival and overall survival: 10-year data of the ARO 96-02 trial. Int J Radiat Oncol Biol Phys 2015;91:288-94. [Crossref] [PubMed]
- Gandaglia G, Fossati N, Karnes RJ, et al. Use of Concomitant Androgen Deprivation Therapy in Patients Treated with Early Salvage Radiotherapy for Biochemical Recurrence After Radical Prostatectomy: Long-term Results from a Large, Multi-institutional Series. Eur Urol 2018;73:512-8. [Crossref] [PubMed]
- Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67-74. [Crossref] [PubMed]
- Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol 2010;28:1106-11. [Crossref] [PubMed]
- Heemsbergen WD, Al-Mamgani A, Slot A, et al. Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol 2014;110:104-9. [Crossref] [PubMed]
- Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 2014;15:464-73. [Crossref] [PubMed]
- Michalski JM, Moughan J, Purdy J, et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol 2018;4. [Crossref] [PubMed]
- King CR, Spiotto MT. Improved outcomes with higher doses for salvage radiotherapy after prostatectomy. Int J Radiat Oncol Biol Phys 2008;71:23-7. [Crossref] [PubMed]
- Ost P, Lumen N, Goessaert AS, et al. High-dose salvage intensity-modulated radiotherapy with or without androgen deprivation after radical prostatectomy for rising or persisting prostate-specific antigen: 5-year results. Eur Urol 2011;60:842-9. [Crossref] [PubMed]
- Pisansky TM, Agrawal S, Hamstra DA, et al. Salvage Radiation Therapy Dose Response for Biochemical Failure of Prostate Cancer After Prostatectomy-A Multi-Institutional Observational Study. Int J Radiat Oncol Biol Phys 2016;96:1046-53. [Crossref] [PubMed]
- Ohri N, Dicker AP, Trabulsi EJ, et al. Can early implementation of salvage radiotherapy for prostate cancer improve the therapeutic ratio? A systematic review and regression meta-analysis with radiobiological modelling. Eur J Cancer 2012;48:837-44. [Crossref] [PubMed]
- King CR. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys 2012;84:104-11. [Crossref] [PubMed]
- Cozzarini C, Fiorino C, Da Pozzo LF, et al. Clinical factors predicting late severe urinary toxicity after postoperative radiotherapy for prostate carcinoma: a single-institute analysis of 742 patients. Int J Radiat Oncol Biol Phys 2012;82:191-9. [Crossref] [PubMed]
- Ghadjar P, Hayoz S, Bernhard J, et al. Acute Toxicity and Quality of Life After Dose-Intensified Salvage Radiation Therapy for Biochemically Recurrent Prostate Cancer After Prostatectomy: First Results of the Randomized Trial SAKK 09/10. J Clin Oncol 2015;33:4158-66. [Crossref] [PubMed]
- Datta NR, Stutz E, Rogers S, et al. Conventional Versus Hypofractionated Radiation Therapy for Localized or Locally Advanced Prostate Cancer: A Systematic Review and Meta-analysis along with Therapeutic Implications. Int J Radiat Oncol Biol Phys 2017;99:573-89. [Crossref] [PubMed]
- Lee LW, McBain CA, Swindell R, et al. Hypofractionated radiotherapy as salvage for rising prostate-specific antigen after radical prostatectomy. Clin Oncol (R Coll Radiol) 2004;16:517-22. [Crossref] [PubMed]
- Koukourakis MI, Papadopoulou A, Abatzoglou I, et al. Postoperative pelvic hypofractionated accelerated radiotherapy with cytoprotection (HypoARC) for high-risk or recurrent prostate cancer. Anticancer Res 2012;32:4561-8. [PubMed]
- Kruser TJ, Jarrard DF, Graf AK, et al. Early hypofractionated salvage radiotherapy for postprostatectomy biochemical recurrence. Cancer 2011;117:2629-36. [Crossref] [PubMed]
- Fersino S, Tebano U, Mazzola R, et al. Moderate Hypofractionated Postprostatectomy Volumetric Modulated Arc Therapy With Daily Image Guidance (VMAT-IGRT): A Mono-institutional Report on Feasibility and Acute Toxicity. Clin Genitourin Cancer 2017;15:e667-73. [Crossref] [PubMed]
- Picardi C, Perret I, Miralbell R, et al. Hypofractionated radiotherapy for prostate cancer in the postoperative setting: What is the evidence so far? Cancer Treat Rev 2018;62:91-6. [Crossref] [PubMed]
- Syndikus I, Pickles T, Kostashuk E, et al. Postoperative radiotherapy for stage pT3 carcinoma of the prostate: improved local control. J Urol 1996;155:1983-6. [Crossref] [PubMed]
- Cozzarini C, Fiorino C, Deantoni C, et al. Higher-than-expected severe (Grade 3-4) late urinary toxicity after postprostatectomy hypofractionated radiotherapy: a single-institution analysis of 1176 patients. Eur Urol 2014;66:1024-30. [Crossref] [PubMed]
- Parker WP, Evans JD, Stish BJ, et al. Patterns of Recurrence After Postprostatectomy Fossa Radiation Therapy Identified by C-11 Choline Positron Emission Tomography/Computed Tomography. Int J Radiat Oncol Biol Phys 2017;97:526-35. [Crossref] [PubMed]
- Lawton CA, DeSilvio M, Roach M 3rd, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys 2007;69:646-55. [Crossref] [PubMed]
- Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol 2007;25:5366-73. [Crossref] [PubMed]
- Asbell SO, Krall JM, Pilepich MV, et al. Elective pelvic irradiation in stage A2, B carcinoma of the prostate: analysis of RTOG 77-06. Int J Radiat Oncol Biol Phys 1988;15:1307-16. [Crossref] [PubMed]
- Moghanaki D, Koontz BF, Karlin JD, et al. Elective irradiation of pelvic lymph nodes during postprostatectomy salvage radiotherapy. Cancer 2013;119:52-60. [Crossref] [PubMed]
- Waldstein C, Dorr W, Potter R, et al. Postoperative radiotherapy for prostate cancer: Morbidity of local-only or local-plus-pelvic radiotherapy. Strahlenther Onkol 2018;194:23-30. [Crossref] [PubMed]
- Byun SJ, Kim YS, Ahn H, et al. Image-guided, whole-pelvic, intensity-modulated radiotherapy for biochemical recurrence following radical prostatectomy in high-risk prostate cancer patients. PLoS One 2018;13. [Crossref] [PubMed]
- Spiotto MT, Hancock SL, King CR. Radiotherapy after prostatectomy: improved biochemical relapse-free survival with whole pelvic compared with prostate bed only for high-risk patients. Int J Radiat Oncol Biol Phys 2007;69:54-61. [Crossref] [PubMed]
- Ramey SJ, Agrawal S, Abramowitz MC, et al. Multi-institutional Evaluation of Elective Nodal Irradiation and/or Androgen Deprivation Therapy with Postprostatectomy Salvage Radiotherapy for Prostate Cancer. Eur Urol 2018;74:99-106. [Crossref] [PubMed]
- Moghanaki D, Urdaneta AI, Karlin JD, et al. Management of Postprostatectomy Biochemical Relapse With Salvage Radiotherapy: Results of an International Survey. Am J Clin Oncol 2016;39:64-8. [Crossref] [PubMed]
- Sidhom MA, Kneebone AB, Lehman M, et al. Post-prostatectomy radiation therapy: consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother Oncol 2008;88:10-9. [Crossref] [PubMed]
- Poortmans P, Bossi A, Vandeputte K, et al. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol 2007;84:121-7. [Crossref] [PubMed]
- Wiltshire KL, Brock KK, Haider MA, et al. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys 2007;69:1090-9. [Crossref] [PubMed]
- Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010;76:361-8. [Crossref] [PubMed]
- Sobol I, Zaid HB, Haloi R, et al. Contemporary Mapping of Post-Prostatectomy Prostate Cancer Relapse with (11)C-Choline Positron Emission Tomography and Multiparametric Magnetic Resonance Imaging. J Urol 2017;197:129-34. [Crossref] [PubMed]
- Souvatzoglou M, Krause BJ, Purschel A, et al. Influence of (11)C-choline PET/CT on the treatment planning for salvage radiation therapy in patients with biochemical recurrence of prostate cancer. Radiother Oncol 2011;99:193-200. [Crossref] [PubMed]
- Goldstein J, Even-Sapir E, Ben-Haim S, et al. Does Choline PET/CT Change the Management of Prostate Cancer Patients With Biochemical Failure? Am J Clin Oncol 2017;40:256-9. [Crossref] [PubMed]
- Jereczek-Fossa BA, Rodari M, Bonora M, et al. [11C]choline PET/CT impacts treatment decision making in patients with prostate cancer referred for radiotherapy. Clin Genitourin Cancer 2014;12:155-9. [Crossref] [PubMed]
- Lamanna G, Tabouret-Viaud C, Rager O, et al. Long-term Results of a Comparative PET/CT and PET/MRI Study of 11C-Acetate and 18F-Fluorocholine for Restaging of Early Recurrent Prostate Cancer. Clin Nucl Med 2017;42:e242-6. [Crossref] [PubMed]
- Calais J, Cao M, Nickols NG. The Utility of PET/CT in the Planning of External Radiation Therapy for Prostate Cancer. J Nucl Med 2018;59:557-67. [Crossref] [PubMed]
- Calais J, Fendler WP, Herrmann K, et al. Head-to-head comparison of (68)Ga-PSMA-11 PET/CT and (18)F-Fluciclovine PET/CT in a case series of 10 patients with prostate cancer recurrence. J Nucl Med 2018;59:789-94. [Crossref] [PubMed]
- Calais J, Czernin J, Cao M, et al. (68)Ga-PSMA-11 PET/CT Mapping of Prostate Cancer Biochemical Recurrence After Radical Prostatectomy in 270 Patients with a PSA Level of Less Than 1.0 ng/mL: Impact on Salvage Radiotherapy Planning. J Nucl Med 2018;59:230-7. [Crossref] [PubMed]
- Grubmuller B, Baltzer P, D'Andrea D, et al. (68)Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy - diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging 2018;45:235-42. [Crossref] [PubMed]
- Emmett L, van Leeuwen PJ, Nandurkar R, et al. Treatment Outcomes from (68)Ga-PSMA PET/CT-Informed Salvage Radiation Treatment in Men with Rising PSA After Radical Prostatectomy: Prognostic Value of a Negative PSMA PET. J Nucl Med 2017;58:1972-6. [Crossref] [PubMed]
- Ballman KV. Biomarker: Predictive or Prognostic? J Clin Oncol 2015;33:3968-71. [Crossref] [PubMed]
- Erho N, Crisan A, Vergara IA, et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS One 2013;8. [Crossref] [PubMed]
- Den RB, Yousefi K, Trabulsi EJ, et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. J Clin Oncol 2015;33:944-51. [Crossref] [PubMed]
- Karnes RJ, Bergstralh EJ, Davicioni E, et al. Validation of a genomic classifier that predicts metastasis following radical prostatectomy in an at risk patient population. J Urol 2013;190:2047-53. [Crossref] [PubMed]
- Klein EA, Yousefi K, Haddad Z, et al. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. Eur Urol 2015;67:778-86. [Crossref] [PubMed]
- Ross AE, Den RB, Yousefi K, et al. Efficacy of post-operative radiation in a prostatectomy cohort adjusted for clinical and genomic risk. Prostate Cancer Prostatic Dis 2016;19:277-82. [Crossref] [PubMed]
- Spratt DE, Yousefi K, Deheshi S, et al. Individual Patient-Level Meta-Analysis of the Performance of the Decipher Genomic Classifier in High-Risk Men After Prostatectomy to Predict Development of Metastatic Disease. J Clin Oncol 2017;35:1991-8. [Crossref] [PubMed]
- Dalela D, Santiago-Jimenez M, Yousefi K, et al. Genomic Classifier Augments the Role of Pathological Features in Identifying Optimal Candidates for Adjuvant Radiation Therapy in Patients With Prostate Cancer: Development and Internal Validation of a Multivariable Prognostic Model. J Clin Oncol 2017;35:1982-90. [Crossref] [PubMed]
- Spratt DE, Dai DLY, Den RB, et al. Performance of a Prostate Cancer Genomic Classifier in Predicting Metastasis in Men with Prostate-specific Antigen Persistence Postprostatectomy. Eur Urol 2018;74:107-14. [Crossref] [PubMed]
- Zhao SG, Chang SL, Spratt DE, et al. Development and validation of a 24-gene predictor of response to postoperative radiotherapy in prostate cancer: a matched, retrospective analysis. Lancet Oncol 2016;17:1612-20. [Crossref] [PubMed]
- Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol 2014;66:550-60. [Crossref] [PubMed]
- Cullen J, Rosner IL, Brand TC, et al. A Biopsy-based 17-gene Genomic Prostate Score Predicts Recurrence After Radical Prostatectomy and Adverse Surgical Pathology in a Racially Diverse Population of Men with Clinically Low- and Intermediate-risk Prostate Cancer. Eur Urol 2015;68:123-31. [Crossref] [PubMed]
- Cuzick J, Berney DM, Fisher G, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer 2012;106:1095-9. [Crossref] [PubMed]
- Bishoff JT, Freedland SJ, Gerber L, et al. Prognostic utility of the cell cycle progression score generated from biopsy in men treated with prostatectomy. J Urol 2014;192:409-14. [Crossref] [PubMed]
- Cooperberg MR, Simko JP, Cowan JE, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol 2013;31:1428-34. [Crossref] [PubMed]
- Zhao SG, Chang SL, Erho N, et al. Associations of Luminal and Basal Subtyping of Prostate Cancer With Prognosis and Response to Androgen Deprivation Therapy. JAMA Oncol 2017;3:1663-72. [Crossref] [PubMed]


