Microfluidic—based sperm sorting & analysis for treatment of male infertility
Introduction
Microfluidics is the science and technology of accurate manipulation of small amounts of fluids, which is typically done in microchannels with dimensions of a few hundred micrometers (1). This sub-millimeter scale has two main benefits: (I) the flows involved are laminar allowing us to take advantage of the fluids deterministic behavior to accurately manipulate the fluids and suspended particles; and (II) channel features have dimensions of the same order as many biological particles (1–100 µm). We refer to the manipulation of fluids at this scale as microfluidics, and to the devices which employ these tools microfluidic devices. Microfluidic devices are typically fabricated by microfabrication techniques, and are the combination of functional microscale parts for fluid delivery, fluid mixing (2), and separation of particles in fluids (3). Furthermore, different types of microfluidic systems can be integrated to produce highly complex systems with small footprints. Thus, microfluidic devices are often called Micro-Total-Analysis Systems (µTAS) or lab-on-a-chip (LOC) devices. Currently, microfluidic devices are utilized in the fields of analytical chemistry, molecular & cellular biology, microbiology, and pharmaceutical drug screening (4). Across these fields, microfluidic devices have been developed to complement or even supplant the existing sample manipulation processes associated with cell culturing, cell separation, and DNA analysis. Utilizing properties unique to the microscale, microfluidic systems demonstrate several advantages when compared to conventional bulk fluid approaches:
- Microfluidic systems are capable of working with small volume samples (mL to nL). Consequently, the operational cost is reduced by reducing the volumes of expensive reagents (4);
- Microfluidic systems have high sensitivity and low response times. The relative enhancement of surface-to-volume ratio, diffusion rate, and heat dissipation rate at the microscale improves the detection limits and sensitivity, and reduces reaction times (4);
- Microfluidic systems enable large-scale parallelization for high throughput operation and multiple parameter assays. Multiple functional structures can be parallelized easily during the fabrication of microfluidic devices. For example, by building a microplate system array in a microfluidic chip, high throughput and high resolution analysis for drug screening and antigen detection has been achieved (5);
- Microfluidic systems are suitable for single cell analysis. The functional structure and forces unique to the microscale enables the precise single cell manipulation or separation of rare cells in highly heterogeneous mixtures. Cell manipulation based on physical trapping, immune capture and optical tweezers can be realized in microfluidic systems (3,6,7);
- Microfluidic systems can achieve automatic sample treatment and analysis. Microfluidic systems provide excellent methods for automatic reagent delivery and condition control (6,8). In addition, portable point of care diagnostic equipment can be developed based on microfluidic systems for onsite rapid sample analysis (9,10).
By relying on these advantages, microfluidic systems have been utilized in the field of assisted reproductive technologies (ART) to assist with sperm sorting (11), oocyte manipulation (12), insemination (13), embryo culturing (14), and for assessing sperm and embryo quality (15). Microfluidics is expected to help to improve the efficiency of sample preparation, enable consistency in cell/embryo culturing and operation environments, and reduce human error. Though proof-of-concept experiments have been performed to demonstrate the capabilities of microfluidic systems in many areas of ART, most of the clinical applications of microfluidic systems have been in sperm purification or sorting.
Selection of qualitatively and quantitatively sufficient sperm is a critical sample preparation process in the treatment of male-factor infertility. Microfluidic system based sperm sorting processes can improve recovery of motile sperm from semen, recovery of sperm from highly heterogeneous mixtures, and possibly reduce clinician skill requirements for sperm purification process (16-19). In this chapter, we will discuss some of the available microfluidic systems for processing sperm samples. We will start with a discussion on semen analysis systems in which sperm are sorted for determination of sperm quantity and/or quality. In the second section, emerging sperm quality-based microfluidic cell sorting technologies are discussed. Then the potential of Raman-microfluidics systems is reviewed for non-invasive single sperm sorting. In the end, we highlight hurdles to commercialization of microfluidic sperm sorting systems and provide suggestions to overcome them.
Microfluidics for semen analysis
The analysis of human semen is essential to the diagnosis and treatment of male-factor infertility (20-30). Several microfluidic devices have shown the ability to analyze the key semen analysis parameters such as sperm count, sperm motility, and sperm morphology. These technologies have proven to be fast, easy to use, and consistently accurate. Furthermore, microfluidics has the potential to fill the need for fertility diagnostics in point-of-care environments and developing countries (31). We review current microfluidic applications that show the potential to revolutionize sperm analysis in research and clinics alike.
An excellent example is Segerink and coworkers (32,33), who developed a microfluidic system that that could accurately quantify sperm count using the electrical impedance method also known as the Coulter Principle—an established method for cell counting and size analysis. This method correctly classified all semen samples in the subfertility zone (<20×106 m/L) and had a strong correlation with conventional methods.
Recently, paper-based microfluidics have emerged as a simple, low cost, and faster alternative to conventional sperm analysis. Similar to pregnancy testing devices, they have the potential to enable effective at-home semen analysis. A promising example is the paper-based microfluidic device developed by Nosrati and coworkers for at-home semen testing (34).
Table 1 summarizes a variety of microfluidic-enabled technologies for semen analysis by providing the sorting principle, a general device description, and types of sperm parameters analyzed. R2 values listed in Table 1 were calculated by comparing the values calculated by the respective microfluidic system and values obtained via conventional methods. Each device had significant accuracy, with sperm concentration and motility range capability comparable to conventional methods.
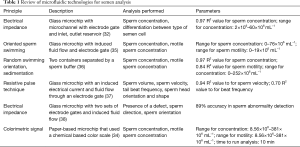
Full table
Commercial adoption
Several commercial microfluidic systems exist to determine semen quality. SpermCheck is a paper based microfluidic device that can identify semen that falls below the WHO reference value for low sperm count (<20×106 mL−1) (21). SpermCheck has demonstrated 100% concurrence in the diagnosis of low quality sperm when compared to cell counting chamber methods (39). Another product, FertilMARQ provides similar information. The commercially available test Fertell can detect semen samples with low motility (40). Though these products are a step in the right direction, they can only identify a single semen parameter and rely on the interpretation of the test result by the consumer. Microfluidics can be used to develop an automated bench-top diagnostic tool that can enable infertility clinicians to quantify multiple semen parameters in single sample run (41), as demonstrated by the technologies listed in Table 1. Furthermore, microfluidic semen analysis systems can help in standardizing semen analysis across multiple infertility clinics, and possibly lower costs for semen analysis.
Despite the proven potential of microfluidics for semen analysis, widespread use is yet to be seen. We believe this is partly due to emergent nature of microfluidic technology over the last two decades. However, microfluidics technology has significantly matured since its inception in the 1990s and may now be ready to make its contributions in ART, as we discuss in later sections.
Microfluidics for sperm sorting
Selection of sperm is important because the quality of selected sperm influences the success rate of ART (42), rate of birth defects (43), and has been linked with potential infertility in male offspring (44). Three conventional sperm sorting techniques have emerged as dominant in Andrology clinics today: density gradient centrifugation, sperm washing, and swim-up (SU). There are essentially two concerns with conventional sorting methods: (I) they bypass the natural barriers that sperm would experience in vivo (45); and (II) many studies have related the centrifugation steps of the sorting process with sperm DNA damage (46,47) that may have long-term effects on embryos’ viability (48).
Microfluidics provides the opportunity to sort sperm cells in a faster, gentler way that more closely mimics the natural selection processes and avoids some of the most detrimental elements of current sperm sorting techniques. Microfluidic sperm sorting approaches can generally be sorted into three categories: (type 1) microfluidic devices that isolate only motile sperm; (type 2) microfluidic devices that isolate sperm cells without relying on sperm motility; (type 3) microfluidic devices for the observation and selection of individual sperm.
Type 1 is the largest group of microfluidic devices and include technologies that improve the swim up method by translating the process of motility screening to a microfluidic system. While some of these systems are able to select enough sperm cells for intrauterine insemination (IUI) (>10 M sperm), the vast majority select a much smaller, and purer, subpopulation fit for in vitro fertilization (IVF) (~100,000 cells). Generally, the subpopulation that these systems select is nearly 100% motile and is of higher quality in terms of morphology and DNA integrity than the unprocessed semen sample. Type 2 microfluidics devices do not utilize sperm motility as a selection factor, but instead use sperm shape, size, or other physical biomarkers as the trapping/selection mechanism. These systems are not necessarily focused on capturing an improved sperm subpopulation, rather these systems have the potential to retain the full fertilization capability of a subfertile semen sample by indiscriminately capturing sperm cells. Type 3 microfluidic devices take advantage of the ability of microfluidics to capture and non-invasively investigate the characteristics of a single sperm cell while maintaining sperm viability. We review and discuss each of these microfluidic systems separately in the following sections.
Type 1: improved selection of high quality sperm subpopulations by targeting highly motile sperm
Sorting sperm cells based on their motility is beneficial for three main reasons: (I) it is a natural way to separate them from somatic cells and debris in semen; (II) dead and damaged sperm cells are also selected against; (III) high motility is required for successful fertilization in IUI or IVF. Sperm separation based on motility is the operating principle behind the SU method which was first explained by Mahadevan and Baker (49) in 1984, and has become the most commonly used sperm separation procedure and the standard technique for patients with normozoospermia and female subfertility (50). The expected advantage of moving the motility selection from wells to a microfluidic chip include the increased surface across which sperm can swim, the ability to fine tune the motility cutoff of the device, and the potential to incorporate sample preparation directly.
The first group to move the motility selection onto a microfluidic chip was Cho and coworkers who used a device that consisted of a single channel with two outlets and two inlets as shown in Figure 1A (51). A sperm sample was introduced to the top inlet, and while immotile sperm and debris maintained their original streamline to exit through the top outlet, motile sperm were able to swim across streamlines to exit through the lower outlet. Their results showed that they could select a subpopulation of sperm that was nearly 100% motile and had a normal morphology of 22.4%±3.3% (compared to 9.5%±1.1% before sorting) and contained approximately 40% of total motile sperm (TMS) from the original sample (51). Their work showed the promise of microfluidic systems to replace traditional clinical procedures as the selected subpopulation was more motile, and contained similar morphological characteristics to the subpopulation selected by traditional clinical procedures. Using a similar device, Shirota and coworkers were able to show that this type of microfluidic separation can select a subpopulation of sperm with very low DNA damage (54). They reported the selection of a subpopulation of sperm cells which was 95% motile, and had a DNA fragmentation index (DFI) that was reduced to 1%.

The most direct selection of fast swimming sperm was made by Wu and coworkers utilizing a microfluidic system (52). In this system, Wu and coworkers created a triangular flow zone in which the microchannel’s geometry (Figure 1B) essentially created a gradient of mainstream flow speed along the geometry’s length. Due to the velocity distribution, only the fastest swimming sperm were present in the forward part of the device. Using this system, they were able to effectively enrich the percentage of viable sperm to 80% and very effectively select against dead sperm while processing batches of ~200,000 sperm in 5–15 minutes.
It has also been proposed that other biomimicry could be employed to select an even further improved subpopulation of sperm cells (55). For example, microfluidic devices have been made to screen sperm based on their ability to swim up a chemical gradient (chemotaxis) (56) or thermal gradient (thermotaxis) (57). However, these instruments have been used mainly for research purposes, as reviewed extensively by Nosrati and coworkers (11). A microfluidic system capable of mimicking the entire natural selection process of the vaginal canal is currently challenging, but is theoretically possible due to extensive research on organ-on-a-chip (58).
Theoretically, the quality of selected sperm is inversely related to the quantity of selected sperm. Hence, by imposing stricter selection criteria on sperm cells, microfluidic devices are able to select sperm cells with increased fertilization capability. One impressive demonstration of this phenomenon was made by Nosrati and coworkers (53), who used 500 relatively long parallel microchannels to select ~100,000 sperm from 1 mL of raw semen (Figure 1C). The selected sperm cells were shown to have >80% improvement in DNA integrity relative to the heterogeneous population present in the raw semen, and the selection was performed in <20 minutes.
In a more recent device by Asghar and coworkers these small channels were replaced with a membrane with small pores (59). The resulting chip is small, easy to use, and compatible with macroscopic manipulation systems (pipetting). In the optimal configuration, including an 8-µm pore size filter, the device is able to capture a subpopulation of sperm which has ~100% motility, and 60% normal morphology. Chinnasamy et al. tested a similar device, confirming the superiority of the 8 µm pores over larger alternatives (60).
The data generated in the studies mentioned above supports the theory that a subpopulation of sperm that are more motile will be more fit for fertilization based on DNA damage, and the capabilities of microfluidic devices make them optimal for selecting sperm in this way. There is good evidence to suggest that a microfluidic platform could supplant traditional methods as the preferred technology. However, to date, there has been difficulty with moving these devices into the clinic for many reasons. From our perspective, the inertia inherent to the medical field can only be overcome when microfluidic technologies evolve to process raw semen samples, process clinically relevant volumes, and generate clinically relevant data, which ultimately includes pregnancy outcomes.
Type 2: isolation of sperm cells without relying on motility
While motility-based selection methods have many benefits, microfluidic techniques that do not require sperm motility have also been explored. These techniques rely on a sperm cell’s size, shape, or charge for sperm sorting and have been associated with many benefits including speed, high recovery, and a selection of high quality sperm.
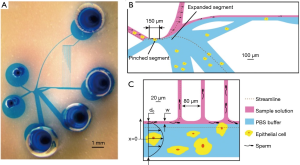
One area where microfluidic devices have particularly excelled is the separation of sperm cells from samples with a low concentration of sperm cells relative to other material, including the separation of sperm from epithelial cells for forensic analysis, from white blood cells in contaminated semen, and from testicular cells after a testicular biopsy. Liu and coworkers developed a microfluidic system (Figure 2) for the separation of sperm and epithelial cells for rapid forensic analysis (61). By designing a microchannel with functional structures, the hydrodynamic interaction between fluid flow and cells led to the separation of different sized cells. The design of the microfluidic device is depicted in Figure 2A,B,C. The separation device is composed of two parts: part I is composed of a pinched segment and an expanded segment; part II is composed of a set of capillary tubes. With a sample flow rate of 2 µL/min, sperm recovery of 41.1% has been demonstrated with this microfluidic chip.
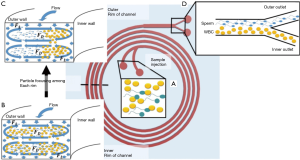
To date, the highest recovery of sperm cells that we are aware of was obtained using a system which was designed for processing pyospermic samples where there is a high concentration of white blood cells present in the semen. By processing the sample through a spiral channel (as shown conceptually in Figure 3), Son and coworkers were able to show that they could remove white blood cells (WBCs) from the sample while still retaining 82% of sperm (18). The mechanism that they use captures such a high percentage of sperm cells that they argue that it could be used to capture extremely rare sperm from microTESE samples (16), which they have demonstrated with a small number of clinical samples (19). In a less extreme implementation, this type of sperm sorting device may be an important tool in retaining all of the sperm present in a subfertile semen sample before IUI. As an example, consider a semen sample which only contains 12 million motile sperm initially. By conventional methods, recovery of about 40% TMS is typical, which would result in the recovery of less than 5 million sperm. Utilizing the microfluidic sperm sorting method developed by Son and coworkers, a clinic would recover almost 9 million (80% TMS), ensuring that the patient’s sample essentially retained the maximum fertilization capability of the original semen (18).
Using density and surface properties instead of size, Horsman et al. was also able to separate sperm cells from epithelial cells based on the physical settling and adherence properties of epithelial cells in a glass microfluidic chamber (62). Approximately 25% of the sperm cells were recovered from the original sample after 70 minutes of treatment.
Other microfluidic technologies are also capable of separating or capturing sperm cells based on their charge/size ratio by employing electrophoresis (63) or dielectrophoresis (64). Among these, electrophoresis has been demonstrated with whole semen samples and shown the ability to select a sperm subpopulation similar to those acquired by traditional clinical methods in less time (5–20 minutes) (63). The presented device was able to process sample in one 400 µL chamber, although it could be scaled or parallelized.
Recently, de Wagenaar and coworkers developed a microfluidic device to detect and sort morphologically normal cells (38). A common sperm defect is the presence of a cytoplasmic droplet attached to the sperm flagellum. Using electrical impedance measurements, droplet presence was detected and the defective sperm cells were flagged. This work is a good example of the concept that microfluidic devices can be used to detect and sort sperm cells with abnormal morphology with considerable precision.
Beyond the current practices for rare sperm cell isolation, many existing microfluidic separation approaches have the potential to be used in the future for sperm isolation. Various tests have been performed for the isolation of rare cells using microfluidics technology (65), though they have not yet been applied to sperm. A comprehensive summary of available rare sperm isolation approaches can be found in tables published by Chen et al. (65) and Samuel et al. (16). In summary, while motility-based selection can lead to the selection of a high quality subpopulation, techniques that do not rely on sperm motility may also play an important role in the clinic.
Type 3: single cell observation and selection
An emerging area of research for single sperm selection involves utilization of Raman spectroscopy in combination with microfluidic sperm sorting systems. Raman spectroscopy is a type of vibrational spectroscopy that relies on inelastic scattering of monochromatic light by the molecular structure of a system to determine the constituents of the system. Raman spectroscopy has been popularly used in material science and analytical chemistry for non-destructive identification of elements and compounds. However, due to significant improvements in optics, semiconductor technologies, and data analysis and processing capabilities in the last 20 years, Raman spectroscopy has a new area of application: single cell analysis.
Unlike a majority of other single cell analysis techniques Raman spectroscopy on a single cell can be non-destructive and is label-free. A Raman spectra of single cell can provide an extensive amount of information on the molecular structure of the cell. This makes Raman spectroscopy very attractive for reproductive medicine where one viable cell (sperm or oocyte) can make a significant difference in the development of a healthy embryo (66). It is likely that viable sperm have Raman spectras with common traits, leading to the realization of biomarkers. With the motivation of identifying such biomarkers, there have been foundational efforts reported in the last 5 years involving Raman spectroscopy of sperm, recently reported by Mallidis and coworkers (66). Many of these works have been focused on measurement of sperm nuclear DNA damage. However, in most of these published works, dead sperm were smeared or fixated on slides. This undermines the non-invasive analytical capability of Raman spectroscopy. The following highlights the contributions from each publication:
- Huser et al. performed preliminary work to quantify the efficiency of DNA packaging within sperm with normal and abnormal head shape. Based on this work, Huser & coworkers conclude that there is evidence of significant inefficient DNA packaging within sperm with normal head shapes (67). Furthermore, this level of inefficient DNA packaging can be similar in both sperm with normal/abnormal head shapes;
- Meister et al. and Mallidis et al. performed studies and were able to distinguish Raman spectral peaks from individual sperm before and after UV exposure, thus quantifying DNA and organelle damage (68,69);
- Liu et al. performed a study in which sperm were sorted in to groups: sperm that bound to human zona pellucida, and those sperm that did not bound. After sorting, Raman spectroscopy was performed on both groups of sperm. Liu & coworkers analyzed the resulting spectras and were able to distinguish between the two groups based on peaks and regions in the spectras. Since sperm bound to zona pellucida are likely to have normal morphology and chromatin DNA, the results of this study suggests that Raman spectroscopy can be used to identify viable sperm (70).
The collection of Raman spectras from live motile cells requires immobilization of the cells. Edengeiser et al. (71)reported a study in which live sperm were immobilized on CaF2 slides using a special wet chemistry protocol. After sperm were immobilized each sperm was analyzed by Raman spectroscopy in an automated fashion. The authors report Raman data acquisition at the speed of 1 sperm/minute. This work is significant, since it reports for the first time Raman spectroscopy of live sperm in near-physiological conditions. The authors suggest that the recovery of sperm after the spectroscopy procedure could be possible by either lowering the pH (within physiological ranges) and/or by exposing the CaF2 substrate with a solution of α-D-mannosyl. In our opinion it is likely that this could lead to sperm loss. Furthermore, it would be important to sort sperm based on their individual spectras, which seems unfeasible based on the mobilization protocol suggested by the authors. However, a microfluidic system could enable researchers to immobilize single sperm, collect Raman spectra, and finally sort the sperm to a well, based on its spectra. Such microfluidic systems have already been adopted for other cell types for Raman spectroscopy in a medium throughput fashion (72-75). But, from the perspective of clinical andrology, medium or high throughput Raman microspectroscopy may not be a requirement in determination of most viable sperm from a patient’s sample since a small number [10–50] of viable sperm should be enough to perform intracytoplasmic sperm injection (ICSI).
As possible complements to Raman spectroscopy approaches, other microfluidic-based systems have been developed to trap single or a small number of sperm from semen or samples containing washed sperm in media. Ohta and coworkers have demonstrated that optoelectronic tweezers (OET) can be used to distinguish between live and dead sperm in fresh semen (76). OET generate electric field gradients on specially designed substrates. Live and dead sperm experience different levels of forces from these electric fields, which enables non-invasive selection of live sperm (motile and immotile). Additionally, Ohta and coworkers demonstrated that sperm sorted by OET maintain sperm viability and no DNA damage is induced by OET.
Conclusions and commercialization of microfluidic sperm sorting systems
Based on the technologies reviewed in this work, it can be concluded that there are many relevant applications of microfluidics to ART and male infertility, but the potential of microfluidics has still not been fully realized for human sperm manipulation, since commercialization has not been consistently achieved. Microfluidic systems related to sperm analysis have matured faster and reached commercialization earlier than microfluidic sperm manipulation systems.
The demonstrated results of microfluidic sperm sorting systems are encouraging and based on records available at “clinicaltrials.gov”, clinical studies on some of these systems are being carried out in USA. Review of these records show that these sperm sorting systems can be categorized under systems that allow selection of high quality sperm subpopulations by targeting highly motile sperm (discussed in section “Type 1: improved selection of high quality sperm subpopulations by targeting highly motile sperm”). Hence, it is likely that clinical studies on other microfluidic systems for sperm purification would emerge in the near future.
The slower progress of development of microfluidic sperm sorting systems is likely due to the more recent development of these technologies and the more complex nature of the designs, as well as other factors.
Microfluidics, like any emerging technology, had a period of initial hype from 1990–2009 (77). During this period, the excitement of a new technology, like microfluidics, led to some ineffective problem-solution fits. However, in the past decade after significant research development, microfluidic systems are firmly demonstrating their unique scientific niche for medical applications (4). But commercialization of microfluidic systems still has significant hurdles that are potentially being addressed by the microfluidics research community.
One possible limitation is that currently, most innovative microfluidic systems are being fabricated in polydimethylsiloxane (PDMS). PDMS, though ideal for cost-effective rapid prototyping (ranging from $10 to $500, depending on complexity of the design), has significant cost challenges when it comes to mass production. Hence, it is important that as microfluidic engineers design systems in PDMS, they plan for translating the design to other materials ideal for mass production, e.g., thermoplastics. This design philosophy is beginning to take root based on the increase in number of publications demonstrating thermoplastic-based microfluidic systems (78). Furthermore, rapid prototyping of microfluidic systems by 3D printing technology is being increasingly adopted by drivers of microfluidics innovation that are based in academia (79). Most 3D printers use a variety of low priced thermoplastics or other polymers for printing. Interestingly, a considerable number of these polymers are biocompatible, and in a few cases approved by US Federal Drug Administration (FDA) for medical applications. Hence, 3D printing technology is likely going to facilitate faster commercialization of microfluidic systems, especially since many applications of microfluidics in medicine are limited in numbers and not always amenable to typical mass production techniques. Utilizing thermoplastics for production of microfluidic chips ensures that costs to produce a disposable microfluidic chip remain below $10 for commercialization feasibility.
In the specific case of microfluidic sperm sorting systems, it is important that such systems can handle volumes that are clinically relevant and that are relatively large for microfluidics (1–10 mL). However, such systems are challenging to develop without undermining the unique advantages for processing fluids at the microscale. More recently, microfluidic systems that process large volumes quickly (e.g., inertial microfluidic systems) have been shown that can overcome such technical hurdles (80,81).
In order to facilitate adoption of microfluidic systems in clinical environments, user friendly, simple and robust designs with minimum moving parts need to be pursued. Such designs are in development and will allow a faster path to commercialization; this review of microfluidic-based sperm analysis systems presents many systems with these traits. As an example, microfluidic systems containing embedded microvalves were considerably popular 10 years ago (82), but due to issues with robustness and manufacturability of these microvalves, they are hardly prevalent in current microfluidic systems (4).
Lastly, it is important that miniaturization of a clinical sample treatment to a microfluidic system should have significant advantages when compared to conventional treatments. These advantages are likely to only be found through interdisciplinary research efforts that include experts in both microfluidics and medicine; as such synergistic teams can help discover the right microfluidic tools for solution of an appropriate clinical problem. The last decade has witnessed a growing number of these highly interdisciplinary teams (engineers, medical doctors, biologists, chemists, physiologists, etc.), leading to successful applications of microfluidic systems in the realm of life sciences. Similar collaborations are needed for the development of microfluidics-based sperm sorting systems or microfluidic-based assisted reproductive technologies (µ-ART).
Commercialization of emerging technologies in medicine is best pursued along with publication of peer-reviewed data regarding the effectiveness and use of the technology by end-users. This process, from the conception of the technology to publication of end-user data in any field of medicine, can be considerably arduous. Hence, partnerships between infertility clinics based in universities and startup companies are ideal for the development of µ-ART. These partnerships are mutually beneficial for universities (that are research driven and need to produce scholastic publications), and startups that need to develop a product or intellectual property. These partnerships are highly encouraged by startup-focused investors due to the close initial contact between the development team and the customer, and thus have a higher likelihood of commercial success. When significant numbers of teams of this sort are operating, we anticipate a rapid improvement in the capabilities of ART facilities enabled by microfluidic technologies.
Acknowledgements
Funding: This work was supported by the National Science Foundation (1747505 to A. J.).
Footnote
Conflicts of Interest: Raheel Samuel, Timothy Jenkins, and Bruce Gale hold equity in NanoNC Inc. NanoNC is developing innovative solutions for reproductive medicine by utilizing microfluidics technology. The other authors have no conflicts of interest to declare.
References
- Whitesides GM. The origins and the future of microfluidics. Nature 2006;442:368-73. [Crossref] [PubMed]
- Lee CY, Chang CL, Wang YN, et al. Microfluidic Mixing: A Review. Int J Mol Sci 2011;12:3263-87. [Crossref] [PubMed]
- Murphy TW, Zhang Q, Naler LB, et al. Recent advances in the use of microfluidic technologies for single cell analysis. Analyst 2017;143:60-80. [Crossref] [PubMed]
- Chiu DT, deMello AJ, Di Carlo D, et al. Small but Perfectly Formed? Successes, Challenges, and Opportunities for Microfluidics in the Chemical and Biological Sciences. Chem 2017;2:201-23. [Crossref]
- Chi CW, Ahmed AR, Dereli-Korkut Z, et al. Microfluidic cell chips for high-throughput drug screening. Bioanalysis 2016;8:921-37. [Crossref] [PubMed]
- Jin D, Deng B, Li JX, et al. A microfluidic device enabling high-efficiency single cell trapping. Biomicrofluidics 2015;9. [Crossref] [PubMed]
- Menachery A, Kumawat N, Qasaimeh M. Label-free microfluidic stem cell isolation technologies. Trends Analyt Chem 2017;89:1-12. [Crossref]
- Mondal S, Hegarty E, Martin C, et al. Large-scale microfluidics providing high-resolution and high-throughput screening of Caenorhabditis elegans poly-glutamine aggregation model. Nat Commun 2016;7:13023. [Crossref] [PubMed]
- Jung W, Han J, Choi JW, et al. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron Eng 2015;132:46-57. [Crossref]
- Sharma S, Zapatero-Rodríguez J, Estrela P, et al. Point-of-Care Diagnostics in Low Resource Settings: Present Status and Future Role of Microfluidics. Biosensors 2015;5:577-601. [Crossref] [PubMed]
- Nosrati R, Graham PJ, Zhang B, et al. Microfluidics for sperm analysis and selection. Nat Rev Urol 2017;14:707-30. [Crossref] [PubMed]
- Sadani Z, Wacogne B, Pieralli C, et al. Microsystems and microfluidic device for single oocyte transportation and trapping: Toward the automation of in vitro fertilising. Sensors Actuators A Phys 2005;121:364-72. [Crossref]
- Matsuura K, Uozumi T, Furuichi T, et al. A microfluidic device to reduce treatment time of intracytoplasmic sperm injection. Fertil Steril 2013;99:400-7. [Crossref] [PubMed]
- Krisher RL, Wheeler MB. Towards the use of microfluidics for individual embryo culture. Reprod Fertil Dev 2010;22:32. [Crossref] [PubMed]
- Yanez LZ, Camarillo DB. Microfluidic analysis of oocyte and embryo biomechanical properties to improve outcomes in assisted reproductive technologies. Mol Hum Reprod 2017;23:235-47. [Crossref] [PubMed]
- Samuel R, Badamjav O, Murphy KE, et al. Microfluidics: The future of microdissection TESE? Syst Biol Reprod Med 2016;62:161-70. [Crossref] [PubMed]
- Son J, Murphy K, Samuel R, et al. Non-motile sperm cell separation using a spiral channel. Anal. Methods 2015;7:8041-7. [Crossref]
- Son J, Samuel R, Gale BK, et al. Separation of sperm cells from samples containing high concentrations of white blood cells using a spiral channel. Biomicrofluidics 2017;11. [Crossref] [PubMed]
- Jenkins T, Samuel R, Jafek A, et al. Rapid microfluidic sperm isolation from microtese samples in men with non-obstructive azoospermia. Fertil Steril 2017;108. [Crossref]
- Franken DR, Oehninger S. Semen analysis and sperm function testing. Asian J Androl 2012;14:6-13. [Crossref] [PubMed]
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231-45. [Crossref] [PubMed]
- Park JY, Kricka LJ. Male Infertility and Microchips. Clin Chem 2013;59:457-8. [Crossref] [PubMed]
- Murray KS, James A, McGeady JB, et al. The effect of the new 2010 World Health Organization criteria for semen analyses on male infertility. Fertil Steril 2012;98:1428-31. [Crossref] [PubMed]
- Park YS. Influence of motility on the outcome of in vitro fertilization/intracytoplasmic sperm injection with fresh vs. frozen testicular sperm from men with obstructive azoospermia. Fertil Steril 2003;80:526-30. [Crossref] [PubMed]
- Repping S, van Weert JM, Mol BW, et al. Use of the total motile sperm count to predict total fertilization failure in in vitro fertilization. Fertil Steril 2002;78:22-8. [Crossref] [PubMed]
- Kime DE, Van Look KJ, McAllister BG, et al. Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comp Biochem Physiol C Toxicol Pharmacol 2001;130:425-33. [Crossref] [PubMed]
- Brazil C. Practical semen analysis: from A to Z. Asian J Androl 2010;12:14-20. [Crossref] [PubMed]
- Amann RP, Katz DF. Andrology Lab Corner*: Reflections on CASA After 25 Years. J Androl 2004;25:317-25. [Crossref] [PubMed]
- Mortimer ST, van der Horst G, Mortimer D. The future of computer-aided sperm analysis. Asian J Androl 2015;17:545-53. [Crossref] [PubMed]
- Maatman TJ, Aldrin L, Carothers GG. Patient noncompliance after vasectomy. Fertil Steril 1997;68:552-5. [Crossref] [PubMed]
- Smith S, Mager D, Perebikovsky A, et al. CD-Based Microfluidics for Primary Care in Extreme Point-of-Care Settings. Micromachines (Basel) 2016;7:22. [Crossref]
- Segerink LI, Sprenkels AJ, ter Braak PM, et al. On-chip determination of spermatozoa concentration using electrical impedance measurements. Lab Chip 2010;10:1018-24. [Crossref] [PubMed]
- Segerink LI, Sprenkels AJ, Oosterhuis GJE, et al. Microfluidic Chips for Semen Analysis. EJIFCC 2012;23:66-9. [PubMed]
- Nosrati R, Gong MM, San Gabriel MC, et al. Paper-Based Quantification of Male Fertility Potential. Clin Chem 2016;62:458-65. [Crossref] [PubMed]
- Chen YA, Huang ZW, Tsai FS, et al. Analysis of sperm concentration and motility in a microfluidic device. Microfluid Nanofluidics 2011;10:59-67. [Crossref]
- Chen CY, Chiang TC, Lin CM, et al. Sperm quality assessment via separation and sedimentation in a microfluidic device. Analyst 2013;138:4967-74. [Crossref] [PubMed]
- Chen YA, Chen KC, Tsai VFS, et al. Direct Characterization of Motion-Dependent Parameters of Sperm in a Microfluidic Device: Proof of Principle. Clin Chem 2013;59:493-501. [Crossref] [PubMed]
- de Wagenaar B, Dekker S, de Boer HL, et al. Towards microfluidic sperm refinement: impedance-based analysis and sorting of sperm cells. Lab Chip 2016;16:1514-22. [Crossref] [PubMed]
- Coppola MA, Klotz KL, Kim KA, et al. SpermCheck® Fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum Reprod 2010;25:853-61. [Crossref] [PubMed]
- Björndahl L, Kirkman-Brown J, Hart G, et al. Development of a novel home sperm test. Hum Reprod 2006;21:145-9. [Crossref] [PubMed]
- Swain JE, Lai D, Takayama S, et al. Thinking big by thinking small: application of microfluidic technology to improve ART. Lab Chip 2013;13:1213-24. [Crossref] [PubMed]
- Schieve LA. Live-Birth Rates and Multiple-Birth Risk Using In Vitro Fertilization. JAMA 1999;282:1832-8. [Crossref] [PubMed]
- Boulet SL, Kirby RS, Reefhuis J, et al. Assisted Reproductive Technology and Birth Defects Among Liveborn Infants in Florida, Massachusetts, and Michigan, 2000-2010. JAMA Pediatr 2016;170. [Crossref] [PubMed]
- Belva F, Bonduelle M, Roelants M, et al. Semen quality of young adult ICSI offspring: the first results. Hum Reprod 2016;31:2811-20. [Crossref] [PubMed]
- Schultz RM, Williams CJ. The Science of ART. Science 2002;296:2188-90. [Crossref] [PubMed]
- Rappa KL, Rodriguez HF, Hakkarainen GC, et al. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnol Adv 2016;34:578-87. [Crossref] [PubMed]
- Aitken RJ, De Iuliis GN, Finnie JM, et al. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod 2010;25:2415-26. [Crossref] [PubMed]
- Smith GD, Takayama S. Application of microfluidic technologies to human assisted reproduction. Mol Hum Reprod 2017;23:257-68. [PubMed]
- Mahadevan M, Baker G. Assessment and Preparation of Semen for In Vitro Fertilization. In: Wood C, Trounson A. editors. Clinical In Vitro Fertilization. London: Springer, 1984:83-97.
- Jameel T. Sperm swim-up: A simple and effective technique of semen processing for intrauterine insemination. J Pak Med Assoc 2008;58:71-4. [PubMed]
- Cho BS, Schuster TG, Zhu X, et al. Passively driven integrated microfluidic system for separation of motile sperm. Anal Chem 2003;75:1671-5. [Crossref] [PubMed]
- Wu JK, Chen PC, Lin YN, et al. High-throughput flowing upstream sperm sorting in a retarding flow field for human semen analysis. Analyst 2017;142:938-44. [Crossref] [PubMed]
- Nosrati R, Vollmer M, Eamer L, et al. Rapid selection of sperm with high DNA integrity. Lab Chip 2014;14:1142. [Crossref] [PubMed]
- Shirota K, Yotsumoto F, Itoh H, et al. Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil Steril 2016;105:315-21.e1. [Crossref] [PubMed]
- Sakkas D, Ramalingam M, Garrido N, et al. Sperm selection in natural conception: what can we learn from Mother Nature to improve assisted reproduction outcomes? Hum Reprod Update 2015;21:711-26. [Crossref] [PubMed]
- Xie L, Ma R, Han C, et al. Integration of sperm motility and chemotaxis screening with a microchannel-based device. Clin Chem 2010;56:1270-8. [Crossref] [PubMed]
- Li Z, Liu W, Qiu T, et al. The construction of an interfacial valve-based microfluidic chip for thermotaxis evaluation of human sperm. Biomicrofluidics 2014;8. [Crossref] [PubMed]
- Zhang B, Radisic M. Organ-on-a-chip devices advance to market. Lab Chip 2017;17:2395-420. [Crossref] [PubMed]
- Asghar W, Velasco V, Kingsley JL, et al. Selection of Functional Human Sperm with Higher DNA Integrity and Fewer Reactive Oxygen Species. Adv Healthc Mater 2014;3:1671-9. [Crossref] [PubMed]
- Chinnasamy T, Behr B, Demirci U. Microfluidic sperm sorting device for selection of functional human sperm for IUI application. Fertil Steril 2016;105:e17-8. [Crossref]
- Liu W, Chen W, Liu R, et al. Separation of sperm and epithelial cells based on the hydrodynamic effect for forensic analysis. Biomicrofluidics 2015;9:44127. [Crossref] [PubMed]
- Horsman KM, Barker SLR, Ferrance JP, et al. Separation of sperm and epithelial cells in a microfabricated device: Potential application to forensic analysis of sexual assault evidence. Anal Chem 2005;77:742-9. [Crossref] [PubMed]
- Ainsworth C, Nixon B, Aitken RJ. Development of a novel electrophoretic system for the isolation of human spermatozoa. Hum Reprod 2005;20:2261-70. [Crossref] [PubMed]
- Rosales-Cruzaley E, Cota-Elizondo PA, Sánchez D, et al. Sperm cells manipulation employing dielectrophoresis. Bioprocess Biosyst Eng 2013;36:1353-62. [Crossref] [PubMed]
- Chen Y, Li P, Huang PH, et al. Rare cell isolation and analysis in microfluidics. Lab Chip 2014;14:626-45. [Crossref] [PubMed]
- Mallidis C, Sanchez V, Wistuba J, et al. Raman microspectroscopy: Shining a new light on reproductive medicine. Hum Reprod Update 2014;20:403-14. [Crossref] [PubMed]
- Huser T, Orme CA, Hollars CW, et al. Raman spectroscopy of DNA packaging in individual human sperm cells distinguishes normal from abnormal cells. J Biophotonics 2009;2:322-32. [Crossref] [PubMed]
- Mallidis C, Wistuba J, Bleisteiner B, et al. In situ visualization of damaged DNA in human sperm by Raman microspectroscopy. Hum Reprod 2011;26:1641-9. [Crossref] [PubMed]
- Meister K, Schmidt DA, Bründermann E, et al. Confocal Raman microspectroscopy as an analytical tool to assess the mitochondrial status in human spermatozoa. Analyst 2010;135:1370-4. [Crossref] [PubMed]
- Liu F, Zhu Y, Liu Y, et al. Real-time Raman microspectroscopy scanning of the single live sperm bound to human zona pellucida. Fertil Steril 2013;99:684-9.e4. [Crossref] [PubMed]
- Edengeiser E, Meister K, Bründermann E, et al. Non-invasive chemical assessment of living human spermatozoa. RSC Adv 2015;5:10424-9. [Crossref]
- Song Y, Yin H, Huang WE. Raman activated cell sorting. Curr Opin Chem Biol 2016;33:1-8. [Crossref] [PubMed]
- Huang WE, Ward AD, Whiteley AS. Raman tweezers sorting of single microbial cells. Environ Microbiol Rep 2009;1:44-9. [Crossref] [PubMed]
- Kuzmin AN, Pliss A, Prasad PN. Ramanomics: New Omics Disciplines Using Micro Raman Spectrometry with Biomolecular Component Analysis for Molecular Profiling of Biological Structures. Biosensors (Basel) 2017.7. [PubMed]
- Zhang Q, Zhang P, Gou H, et al. Towards high-throughput microfluidic Raman-activated cell sorting. Analyst 2015;140:6163-74. [Crossref] [PubMed]
- Ohta AT, Garcia M, Valley JK, et al. Motile and non-motile sperm diagnostic manipulation using optoelectronic tweezers. Lab Chip 2010;10:3213-7. [Crossref] [PubMed]
- Mukhopadhyay R. Microfluidics: On the Slope of Enlightenment. Anal Chem 2009;81:4169-73. [Crossref] [PubMed]
- Gencturk E, Mutlu S, Ulgen KO. Advances in microfluidic devices made from thermoplastics used in cell biology and analyses. Biomicrofluidics 2017;11. [Crossref] [PubMed]
- Bhattacharjee N, Urrios A, Kang S, et al. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016;16:1720-42. [Crossref] [PubMed]
- Zhang J, Yan S, Yuan D, et al. Fundamentals and Applications of Inertial Microfluidics: A Review. Lab Chip 2016;16:10-34. [Crossref] [PubMed]
- Strohmeier O, Keller M, Schwemmer F, et al. Centrifugal microfluidic platforms: advanced unit operations and applications. Chem Soc Rev 2015;44:6187-229. [Crossref] [PubMed]
- Au AK, Lai H, Utela BR, et al. Microvalves and Micropumps for BioMEMS. Micromachines 2011;2:179-220. [Crossref]


