The role of salvage brachytherapy for local relapse after external beam radiotherapy for prostate cancer
Introduction
Localized prostate cancer can be treated radically with surgery, external beam radiation (EBRT), brachytherapy or a combination. These therapies can all be effective in the right setting but relapses are not infrequent (1). Biochemical failure after EBRT has been defined as three consecutive rising prostate-specific antigens (PSAs) or, more commonly, a value exceeding the nadir by 2 ng/mL (2). In the past, a higher post-treatment PSA was acceptable after radiation, with the belief that since the prostate was left in-situ, there might be sparing of non-malignant PSA-producing prostate glands. More recent evidence indicates that a higher PSA nadir predicts eventual failure (3-5).
Over the past 3 decades, developments in the delivery of EBRT have moved towards increased conformality using intensity modulation (IMRT) and inverse planning to integrate computer optimization, reducing dose to nearby organs such as the bladder, rectum, and small bowel. Increased precision and image-guidance permit safer dose escalation. Several studies have shown improved biochemical control by increasing conventionally fractionated radiation dose from 64 to 81 Gy (6-11). Hypofractionated radiotherapy, based on radiobiologic data suggesting that the prostate has a low alpha-beta ratio, delivers a larger fraction size over fewer treatments and a shorter overall treatment time, a strategy to achieve higher tumor cell kill while limiting late effects.
Despite these developments, local control remains elusive, with biochemical failures ultimately occurring in 40–60% of patients (12). Many salvage options exist for local recurrence after EBRT, including surgery, brachytherapy, and more recently HIFU, cryotherapy, and stereotactic body radiotherapy (SBRT), but are infrequently used. The previous radical dose of radiation increases toxicity from second line treatment and access to these salvage options is often limited to specialized centers with the most experience (13). Thus, androgen deprivation therapy (ADT) is by far the most widely used treatment for recurrence post-EBRT (13,14). Reports indicate that only 2% of radiation failures are managed with local salvage, the rest being either observed or treated with ADT (13).
ADT
Although initial biochemical response to ADT is almost universal, the median duration is only 24–36 months (15). Furthermore, ADT is associated with significant morbidity and even mortality. In addition to the expected hot flashes, loss of libido, erectile dysfunction, fatigue and mood changes, there is a wide range of metabolic changes leading to osteopenia, decreased muscle mass, obesity, gynecomastia, dyslipidemia, insulin resistance, anemia, and cardiovascular events (16-19). The timing and duration of ADT in the setting of biochemical failure have been investigated to reduce side effects in these patients who have a relatively long life expectancy and are asymptomatic from their local/biochemical recurrence. Two phase III trials, Timing of Androgen Deprivation (TOAD) (20) and Early vs. Late Androgen Ablation Trial (ELAAT) (NCT00439751) have explored the timing of ADT. The TOAD trial randomized 293 patients between immediate or delayed ADT. The hazard ratio (HR) for survival was 0.55 favoring immediate ADT, despite an increase in cardiovascular events, occurring in 9% compared to 6% in the delayed arm. Results of ELAAT trial have not been published yet.
Intermittent ADT has also been investigated to minimize adverse effects of testosterone suppression. The National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) PR7 trial randomized 1,386 men with biochemical failure after radiotherapy and no evidence of distant metastases, to either intermittent ADT (IAD) using 8-month cycles, or continuous ADT (CAD). This was a non-inferiority trial with an acceptable HR for overall survival set at <1.25. At 7 years, the adjusted HR for death was 1.03, showing IAD to be non-inferior to CAD. Furthermore, quality of life was better, with significant improvement in hot flashes, urinary symptoms and libido, and a trend for decreased fatigue. However, the trial also demonstrated that the duration of off-treatment periods decreases over time, from 20 months for the first cycle to 13 months in cycle 2, 9 months in cycle 3 and then 4–5 months for later cycles (21), indicating that PSA doubling time shortens over time. Furthermore, reduced testosterone recovery lessens the potential quality of life (QOL) benefit.
Importance of local control and local salvage
Two decades ago, Fuks et al. evaluated the effect of local recurrence on the incidence of distance metastases in 679 patients. The 15-year distant metastasis free survival with local control was 77% compared to 24% in those with local failure. He concluded that local recurrence is a source for subsequent metastases and recommended complete eradication of local disease (22). Zelefsky et al. also assessed the importance of local tumor control on distant metastases. Biopsies on 339 patients previously treated with conformal radiation showed that those with negative biopsies had 10-year bRFS of 59% compared to 3% for positive biopsies. Multivariate analysis indicated that a positive posttreatment biopsy predicted for both biochemical failure (P<0.001) (23) and distant metastases (P=0.003).
We will review the appropriate work-up when considering a patient for local salvage, and the various options for local salvage, concentrating on brachytherapy and appropriate patient selection.
Baseline imaging
Traditional staging for biochemical recurrence includes a Tc99 bone scan and CT scan of the abdomen and pelvis. However, the sensitivity of traditional imaging is less than adequate at low PSA values. Only 5% of bone scans and 12% of CTs are positive at PSA levels of 4–20 ng/mL (24,25). A review of 23 studies showed that bone metastases were detected in 2%, 5% and 16% for PSA levels <10, 10–20 and 20–50 ng/mL. Hövels et al. assessed the diagnostic accuracy of CT for detecting lymph nodes metastases in patients with prostate cancer in a meta-analysis of 24 studies, and reported a pooled specificity and sensitivity of only 39% and 82% (26). PSMA-PET is much more promising. Pyka et al. compared detection rates of bone metastases in 76 patients using 68Ga-PSMA PET scan and 99m-Tc bone scintigraphy. The sensitivity and specificity of PSMA-PET were 99–100% and 88–100%, while for bone scans, sensitivity and specificity were reduced to 87–89% and 61–96% (27), the range indicating the uncertainty regarding interpretation of equivocal lesions.
There have been significant refinements in imaging for detection of local recurrence, especially in the realm of prostate MRI and multiparametric MRI (mpMRI). The use of 3T magnets, and/or an endorectal coil, permits a higher signal-to-noise ratio which improves structural and functional detail (28). Prostate mpMRI includes both T1-weighted and T2-weighted anatomic imaging as well as functional imaging which distinguishes differences in cell density and diffusion of water molecules between normal and malignant tissue. The prostate imaging and reporting data system (PI-RADS) (29) has been developed to provide a uniform framework for grading the MRI sequences and reporting the likelihood of cancer. In the peripheral zone, the preferred functional sequence is DWI and the calculated apparent diffusion coefficient (ADC) map, whereas in the transition zone it is the T2 weighted image. Dynamic contrast enhancement (DCE) with Gadolinium measures perfusion parameters for rapid wash-in and wash-out on T1 imaging after rapid injection. DCE helps to resolve conflicting results between the T2 and DWI sequences. Incorporation of functional sequences improves the positive predictive value for mpMRI up to 98% compared to 68% for the T2W MRI alone (30). MR spectroscopy, through the detection of choline peaks and choline/creatine ratios, has been used in prostate cancer detection but due its low sensitivity is no longer a required mpMRI sequence (29).
MpMRI plays an important role in the setting of local recurrence (Figure 1). Although a homogeneously decreased T2 signal is often seen in the peripheral zone after radiotherapy due to gland atrophy and fibrosis, the concomitant decreased vascularity contrasts to the neovascularity of recurrent tumor. While its role in the setting of primary detection may be limited, DCE is particularly useful in the setting of local recurrence (31) with a sensitivity and specificity of 0.77 and 0.89 respectively (32).
PSMA PET is also a very promising tool for detection of recurrent disease. Prostate specific membrane antigen (PSMA) is a type II membrane glycoprotein over-expressed in most localized prostate cancers. 68Ga-PSMA is a PSMA ligand. Eiber et al. assessed its utility in 248 post-prostatectomy patients with a median PSA of 2 ng/mL. Detection rates were 97% for PSA levels of ≥2 ng/mL, decreasing with lower PSA levels but still a respectable 58% for PSA 0.2 to <0.5 ng/mL (33). PSMA-PET has the potential to identify local recurrence at low PSA levels while being a sensitive tool for detection of co-existing systemic disease which would disqualify a patient from definitive local salvage. Hruby et al. (34) assessed the role of PSMA-PET in a cohort of 419 men treated with EBRT 78–82 Gy, 48 of whom had biochemical failure without distant metastases on conventional scans. PSMA PET was positive in all 48, with 52% showing extra prostatic disease and only 17% with isolated local recurrence. PSMA PET scans will revolutionize patient selection after biochemical failure, and can identify those most likely to benefit from local salvage.
Salvage prostatectomy
Although prostatectomy is a standard option in the de novo management of prostate cancer, removing the prostate after full dose radiation is associated with increased risk and toxicity. Many small single institution experiences have been reported, as well as some comprehensive systematic reviews. Overall, biochemical relapse-free survival (BRFS) ranges from 48–82% at 5 years and 28–53% at 10 years. CSS at 5 years is 65–79% and 70–83% at 10 years. In 2011 Chade et al. published a large retrospective series from tertiary care centers including 404 men between 1985 and 2009. With a median follow-up of 4.4 years, the 10-year BRFS, DMFS and CSS were 37%, 77% and 83% (35). Toxicity was not reported. Ward et al. published on 199 patients, reporting 10-year CSS rates of 65% but with rectal injury in 10%, bladder neck contracture in 22%, urinary extravasation in 15% and urinary incontinence in 44%. The most frequent toxicities reported are urinary incontinence in 19–64% and impotence in 70–100%, with anastomotic strictures seen in 7–41%, bladder neck contracture in 15–25%, rectal injury in up to 28%, and occasional rectovesical fistulae (Table 1). Despite the technical advantages and 3D magnification of robotic-assisted salvage prostatectomy, complications are reported in 47%, being major in 35% (38).
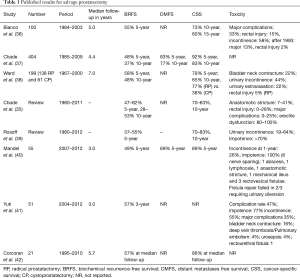
Full table
Salvage therapies based on tissue ablation: high intensity focused ultrasound (HIFU) and cryotherapy
HIFU destroys tissue through the generation of low frequency, high energy ultrasound from a transrectal probe which heats the targeted tissue to 65–85° Celsius and induces coagulative necrosis. Salvage HIFU has demonstrated efficacy but considerable morbidity (Table 2). Crouzet et al. analyzed 418 patients from 9 centers across Europe and the United States. Although the median PSA nadir was 0.19 ng/mL and 5-year BRFS was 67%, 42% and 22% for low, intermediate and high-risk disease, rates of incontinence and bladder outlet obstruction were 30%, with recto-urethral fistulae in up to 10% (43). Jones et al. reported on 100 patients treated with HIFU post EBRT at 16 sites in North America. Of those who had post procedure biopsies, 81% were negative at 12 months but mean PSA nadir at 2 years was quite high at 1.1 ng/mL (44). and 20% developed grade 3 adverse events, including 5 rectal fistulae, 3 osteitis pubis and hematuria.

Full table
Cryotherapy uses freeze-thaw cycles to destroy tissue and has been gaining popularity. Cryoprobes and thermocouple sensors are placed under ultrasound guidance with a warming device in the urethral catheter to limit urinary toxicity. Argon and helium freeze the tumor to minus 40° Celsius. Whole gland cryotherapy is associated with urinary incontinence in 15%, retention in 2%, and recto-urethral fistula in <3% (46) (Table 3). Ismail et al. reported on 100 cases of salvage whole gland cryotherapy. The 5-year BRFS for low, intermediate and high risk groups was very similar to those for HIFU. Focal cryotherapy may reduce toxicity (48). Li et al. reported on 91 patients with a BRFS at 5 years of 47%. Late toxicities included fistulae (3%), urinary retention (7%) and incontinence (6%) (49). In a comparison of hemi gland to whole gland cryoablation, BRFS at 5 years was 54% for hemi-gland and 86% for whole gland, but the latter was associated with worse toxicity.
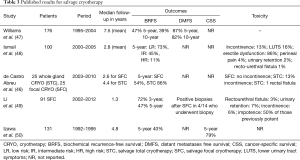
Full table
SBRT
There have also been a few studies assessing the role of SBRT for salvage post EBRT but none have a follow up greater than 2 years (Table 4). Doses range from 30 Gy in 5 fractions to 36 Gy in 6 fractions. PSA nadirs are reported as low as 0.16 ng/mL at 2 years. Early tolerance appears acceptable with grade 3 complications under 10% (51-54). Longer follow up is required.
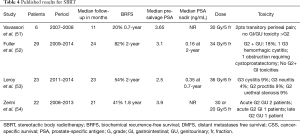
Full table
Brachytherapy
Low dose rate (LDR) or high dose rate (HDR) brachytherapy deliver very high doses of radiation in an ultra-conformal manner. By applying radiation internally, brachytherapy reduces the integral dose in comparison to EBRT, while permitting incomparable dose escalation. Brachytherapy is generally used as monotherapy for low and intermediate risk prostate cancer. In the high risk setting, Level One evidence has established brachytherapy in combination with ADT and pelvic EBRT (3) as the standard of care for upper tier intermediate and high risk disease (55). The role of brachytherapy in the salvage setting is less well defined but multiple small series and two multi-institution phase II trials have shown efficacy. The NCCN guidelines recognize either LDR or HDR as an option for local recurrence after prior radiotherapy (56).
LDR brachytherapy
LDR brachytherapy involves implanting radioactive seeds, generally 125I or 103Pd, directly into the prostate. A pre-operative transrectal ultrasound is generally performed to assess factors such as pubic arch interference, prostate size, and urethral position. Images are taken for planning the ideal needle and seed positions, as well as the number of seeds required. The subsequent procedure is generally performed under spinal or general anesthesia, under ultrasound and fluoroscopic guidance (57). Post procedure imaging is essential for Quality Assurance and is recommended to be performed either day 0 or 1, or at day 30, generally with CT imaging, but the addition of MRI aids contouring accuracy (58).
Initial reports of salvage brachytherapy date back to the 1990s. Grado et al. (59) and Beyer et al. (60) reported 5-year bRFS of 33% and 53%. Since then much progress has been made on patient selection, dose prescription and volumes (Table 5), resulting in improved efficacy and decreased toxicity. BRFS is as high as 90% at 3 years (62), and 75% at 4–5 years (61,66,67). Burri et al. reported long term outcomes with a 10-year biochemical recurrence free survival rate of 54% (64).
Full table
Given these multiple reports of satisfactory efficacy, the Radiotherapy Oncology Group (RTOG; now NRG) undertook a multi-institution phase 2 trial on salvage LDR brachytherapy to investigate safety with a primary endpoint of late grade 3 or higher treatment-related GI/GU toxicity. The prescribed dose to the whole prostate was 140 Gy with 125I and 120 Gy for 103Pd. Ninety-six men were accrued, the majority treated with 125I. The recently reported primary endpoint based on analysis of 87 eligible patients with a minimum follow up of 23 months showed that treatment-related grade 3 GI/GU adverse events were seen in only 14%, with no grade 4 or 5 toxicity.
Nguyen et al. prospectively treated 25 men with biopsy-proven relapse at least 2 years post EBRT. Patients received salvage MRI-guided 125I LDR brachytherapy to a minimum peripheral dose of 137 Gy. At a median follow-up of 47 months BRFS was 70%. In stark contrast to the recently reported RTOG results, 30% developed grade 3 or 4 GI or GU toxicity and 13% required a colostomy or urostomy due to fistula (67). Potential reasons for this difference will be explored below, under “Patient Selection/Toxicity”. Amongst the many retrospective series, outcomes for both efficacy and toxicity vary greatly and depend largely on patient selection.
Patient selection
Patient selection is important for ensuring successful treatment and minimizing toxicity. As demonstrated through the differences in the various retrospective trials, patient selection can drastically affect outcomes. Grado et al. (59) reported 34% 5-year BRFS but 34% of those treated had high grade disease at initial diagnosis and 55% at salvage. Median baseline PSA was 26 ng/mL and 22% had pre-salvage PSA over 10 ng/mL. Beyer et al. (60) found that only 30% of men with high grade cancers were free of second relapse at 5 years vs. 83% for those with low or moderate grade. Similarly, for PSA <10 ng/mL at time of salvage, the 5-year BRFS was 65% compared to 37% for those with PSA >10 ng/mL This is supported by the more recent experience of Burri et al. (64) who found on multivariate analysis, that a pre-salvage PSA <6 ng/mL was predictive of improved bRFS.
The goal of salvage brachytherapy is to use highly conformal, high-dose radiotherapy to eradicate locally recurrent disease in the absence of co-existing micrometastatic disease. There are several absolute requirements when considering a patient for local salvage, and additional desirable features that should be considered on an individual basis but are not mandatory.
Biopsy proof of recurrent disease
Prostate biopsy must confirm locally recurrent disease, at least 24–30 months after initial radiotherapy. Given the well-established time required for histologic resolution of disease after radiotherapy, earlier biopsies are unreliable (72-74). Furthermore, early biochemical failure (<18 months after radiotherapy) may be indicative of co-existing metastatic spread and correlates with increased prostate-cancer mortality (75,76). Biopsy specimens should be reviewed by an experienced GU pathologist. Radiation induced changes, especially in the first 2–2.5 years, may be interpreted as positive for residual cancer when in reality they represent an indeterminate state evolving towards eventual resolution (Figure 2). The radiation-induced cytoplasmic and nuclear changes have been well characterized and can be recognized by an experienced GU pathologist (77). The identification of residual viable tumour, with no or minimal radiation effect, is critical for demonstrating local recurrence (78).
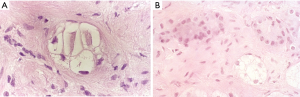
As discussed above, mpMRI with Gadolinium can be useful in detecting sites of recurrence for biopsy. All suspicious areas should be confirmed pathologically.
Systemic staging
The traditional staging including abdominal/pelvic CT scan and a bone scan to rule out distant spread are mandatory. Additional staging with 68Ga PSMA-PET for earlier detection of metastatic disease is desirable if available, and will certainly play an important role in the future. Positivity with PSA values as low as 0.5–1.0 ng/mL is 50–60% and as high as 90% by the time the PSA reaches 2–3 ng/mL (33). The role and utility of PSMA PET has not been reported yet in regards to selection of patients for salvage brachytherapy. Similarly, a higher PSA at the time of salvage is associated with a greater risk of co-existent metastatic disease. Salvage should ideally be undertaken before the PSA reaches 10 ng/mL (67,78,79).
Baseline characteristics
Higher risk features at initial treatment (PSA >20 ng/mL, Gleason score 8–10 and clinical stage ≥T2c) impart a higher risk that biochemical failure is not an isolated local recurrence. However, if other criteria are satisfied, initial high-risk disease does not rule out a role for local salvage. Recent success rates in definitive management of high-risk disease indicate that co-existing metastases may be present in as few as 10% of high risk patients at presentation (3,80,81). If other factors are favourable, including PSA doubling time and interval since prior radiotherapy, then salvage is not unreasonable. Rose et al. reported on 18 patients treated with salvage LDR brachytherapy and found that of 9 patients with high risk disease, only 4 recurred by 3 years, including 2 who had CRPC at time of salvage (82). However, if co-existent with a short PSA doubling time of <6 months, benefit from local salvage is unlikely. For this reason, the recently reported RTOG (NRG) 0526 (NCT00450411) only included patients who had favourable or intermediate risk disease at diagnosis.
Functional criteria
Functional criteria are assessed to limit salvage-related toxicity. RTOG 05-26 specified that eligible patients should have an IPS score <15, with or without alpha-blockers. There should be no residual GI or GU radiation toxicity ≥ grade 2 as per CTCAE criteria. Prostate volume measured on TRUS should be ≤45 cc as higher volumes are associated with decreased tolerance in terms of acute retention and higher rectal doses. Furthermore, although not mandatory, uroflow studies can clarify voiding function in terms of peak flow, post void residual and voided volume, and are more objective than the IPSS questionnaire (83).
Prior EBRT dose
Early experience with salvage LDR brachytherapy involved patients who had been treated with conventional radiation doses of 60–66 Gy. Acknowledging the trend to higher doses, RTOG 0526 specified that the prior EBRT dose to the prostate could be as high as 78–81 Gy in 1.8–2 Gy fractions. There is no published experience on salvage brachytherapy after hypofractionated radiotherapy or SBRT.
Salvage brachytherapy dose
Dose and coverage are two quintessentially important factors for treatment success. If insufficient dose is delivered, toxicity may be reduced, but so will efficacy. Lee et al. (63) prescribed only 90 Gy using 103Pd and had no grade 3 toxicity but 5-year biochemical control was only 38%. Wong et al. (61) had excellent bRFS of 75% at 4 years using either 125I or 103Pd. Although there was no statistically significant association between BRFS and D90, all patients with D90 >140 Gy for 125I or >125 Gy for Pd-103 achieved biochemical control, whereas 4 of 11 patients with a lower D90 experienced failure. Over the decades, a range of prescription doses has been used for salvage, 120–145 Gy for 125I, 90–113 Gy for 103Pd. Although D90 is not always reported, it is an essential dosimetric quantifier to compare efficacy and evaluate toxicities. For example, Moman et al. (65) treated 31 patients with salvage 125I brachytherapy with a prescribed dose of 145 Gy. However, the median delivered D90 was much higher, at 196 Gy, with a median V150 of 74% and V200 33%. As one could now predict, toxicity was high, with late G3 complications seen in 26%. Rose et al. reported increased late toxicity with a higher D90 (P=0.04) (82).
Reports by Nguyen et al. (67) and Aaronson et al. (62) with 125I doses of 137 and 144 Gy treating appropriately selected patients have shown promising BRFS rates of 70% at 4 years and 88% at 3 years respectively. In RTOG 0526, the prescribed doses for 125I and 103Pd were respectively 140 and 120 Gy. RTOG 0526 also used several dosimetric parameters to try to avoid overly “hot” implants. Preplans were designed to keep V150 <45% for 125I and <55% for 103Pd and V200 <10% for 125I and <15% for 103Pd. Compliance with these recommendations was high in the 20 participating centers. In the postplan evaluations, the median V150 was 50% with an interquartile range (IQR) of 43–59% and for V200 20% with an IQR of 17–27%. The RTOG doses are widely accepted as standard for salvage LDR brachytherapy.
Planning algorithm and volume
Initial reports on salvage brachytherapy by Grado et al. (59) and Beyer et al. (60) have concerning rates of toxicity. Grado et al. (59) reported post-salvage transurethral resection of the prostate (TURP) in 15% and colostomies in 2%, whereas Beyer et al noted incontinence in 24%. The high urinary toxicity is likely related to the use of uniform seed loading, placing the seeds 1 cm apart, center to center throughout the prostate, resulting in a higher urethral dose (84). The Association of Physicists in Medicine (AAPM) recommends a modified peripheral loading approach placing seeds peripherally, and removing periurethral central seeds, to improve dose homogeneity and spare the urethra. Another approach to reduce toxicity, involves maintaining high dose to the site of recurrence but reducing dose to the remainder of the gland. Aaronson et al. used MRI and MR spectroscopy to identify the dominant lesion and delivered 144 Gy to this site and 108 Gy to the remaining gland. At a median follow-up of 30 months, 24 patients had a 3-year BRFS of 90%. Toxicities included 1 urethral stricture, 1 grade 3 rectal haemorrhage, and 5 patients with grade 2 gross haematuria that resolved with conservative management. They concluded that salvage LDR brachytherapy provided excellent local control with acceptable toxicity (62).
More recently, the focus has shifted to partial gland brachytherapy to further reduce toxicity (Figure 3) (85-87). A few reports provide promising results. Hsu et al. treated 15 patients with 125I partial salvage using MRI/magnetic resonance spectroscopy (MRS) planning. At a median follow-up of 2 years, the 3-year BRFS was 71%. Toxicity was very acceptable with only one third developing G2 GU toxicity, no G3 or higher GU toxicity, and no G2 or higher GI toxicity. Following salvage, 87% maintained erections (2/3 with PDE5 inhibitors) (68). Peters et al. analyzed 20 patients treated with focal salvage 125I brachytherapy using mpMRI and choline PET CT (69). Prescription dose to the tumor was 144 Gy. At a median follow-up of 3 years, biochemical control was 70%. Grade 3 GU toxicity occurred in only one patient with a urethral stricture.

Toxicity
The potential for increased severity of toxicity in the salvage setting is real, but efforts to control rectal and urethral doses have reduced the incidence, and focal salvage may further minimize toxicity. In the long term, patients are at risk for incontinence, retention, frequency, urethral stricture, proctitis, rectal ulcers and recto-urethral fistula (Figure 4). Late G3 or higher GU toxicity ranges from 5–25% and late grade 3 or higher GI toxicity from 5–10%. The primary endpoint of RTOG 0526 was evaluation of late treatment–related grade 3 or higher GI/GU toxicity. The hypothesis was that a frequency of ≤10% would be acceptable in a salvage situation but if over 20%, salvage brachytherapy would be considered too morbid. With a median follow-up of 4.5 years, there was only 14% late grade G3 GI/GU toxicity. Of 87 analyzable patients with a minimum of 23 months follow up, there was only 1 proctitis, 1 urethral fistula, and the remainder were grade 3 urinary frequency, incontinence and retention. Notably, there was no grade 4 or 5 toxicity reported. Nguyen et al.’s (67) prospective trial of salvage brachytherapy noted a much higher rate of 4-year grade 3 or 4 GI or GU toxicity at 30%, with 13% colostomy or urostomy. He reported that an interval of <4.5 years from EBRT to salvage brachytherapy was associated with a hazard ratio of 12 for grade 3 or 4 toxicity, and a hazard ratio of 25 for colostomy or urostomy. Interestingly, the median time from EBRT to salvage in the RTOG 0526 trial was 7 years (IQR, 5–9 years) which may explain the markedly lower grade 3 toxicity rate of 14% and the absence of any grade 4 or 5 toxicity in this trial.
Peters et al. (88) published on rectal dose constraints for salvage prostate brachytherapy. He compared 30-day CT dosimetry of 20 focal salvage plans with 28 total gland implants and assessed GI toxicity through CTCAE criteria. He found that focal salvage reduced rectal dose significantly at his institution. Median reduction in rectal dose was 46 Gy for D1cc and D2cc. Severe GI toxicity in the whole gland salvage group reported by this author is by far the highest in the literature, being 41%. For comparison, grade 3 or higher late GI toxicity in the RTOG trial was <2%. Fortunately, the focal approach adopted by Peters et al. (88) corrected this at his institution.
HDR brachytherapy
HDR brachytherapy is performed by implanting a scaffolding of catheters into the prostate, through which a high activity 192Ir source navigates to deliver the required dose. Once the catheters are positioned, images are obtained for catheter reconstruction and inverse planning. Traditionally CT-based planning has been used but currently US-based planning is making in-roads as it eliminates the risk of catheter shift with patient movement, and ensures precise dose delivery. Dosimetric coverage and constraints are reviewed before treatment is started and duration of the source transiting through the catheters is roughly 15–20 minutes. Multiple fractions can be given per implant but require catheter position re-verification prior to each dose delivery (89,90). HDR salvage brachytherapy appears to have comparable results to LDR. Several retrospective studies and one phase II study on whole gland treatment have been reported to guide treatment (Table 6).
Full table
HDR selection
Selection criteria for salvage HDR are the same as for LDR brachytherapy. HDR has the advantage of being able to deliver dose to the seminal vesicles, or areas of extracapsular extension, but whether such patients are candidates for salvage is arguable.
HDR dose
As is the case for LDR salvage, dose for HDR salvage has varied in the literature. Yamada et al. (90) used 32 Gy in 4 fractions in a single implant. At a median follow-up of 3 years, the 5-year BRFS and DMFS were 69% and 82% for 42 patients. Other fractionation schedules are 36 Gy in 6 fractions with two implants 1 week apart (63,92); as well as 10 Gy per fraction ×3 fractions with weekly implants (95-97). If multiple fractions are delivered per implant, a minimal interval of 6 hours between fractions is recommended. Kukiełka et al. treated 25 patients with combined interstitial hyperthermia and HDR brachytherapy, delivering 30 Gy in 3 fractions at 21-day intervals. Hyperthermia heated the prostate to 41–43° Celsius for sixty minutes. At a median follow-up of 13 months, the 2-year biochemical control was 74% (94). Wojcieszek et al. assessed 83 patients treated with 10 Gy ×3, once every 2 weeks to a total dose of 30 Gy. At a median follow-up of 41 months, the 5-year BRFS was 67% (95). Jiang et al. achieved 5-year biochemical control of 45% for 29 patients Treated with 3 weekly fractions of 10 Gy to the peripheral zone and PET positive area (97).
HDR volume
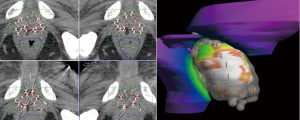
HDR salvage brachytherapy can be delivered to the whole gland but if the site of recurrence can be identified on mpMRI or PSMA PET, the dose can be escalated with relative ease. While most series have focused on whole gland treatments, partial salvage brachytherapy is readily achievable and is currently being investigated (NCT03246802, NCT01583920). By treating the recurrence and sparing part of the gland, toxicity may be reduced. Transrectal ultrasound and multiparametric MRI can be done prior to the procedure for off-line fusion using either rigid or deformable registration. The site of recurrence is identified on mpMRI in each of the sequences: T2, ADC and dynamic contrast enhanced (DCE) and then combined through a Boolean addition of the abnormalities seen on each sequence, areas of low-intensity on T2W MRI, low ADC map values and high DCE map values (Figure 5) (99). Manual rigid registration can be used if required to align the sequences to account for variations in prostate position during sequence acquisition. This contour becomes the focal GTV (F-GTV) as previously described by Mason et al. (99).
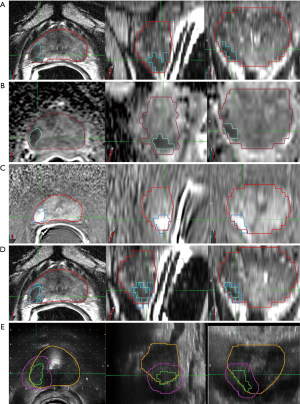
To account for uncertainties in image registration and tumor delineation, a margin is added to create a F-PTV, cropped at the urethra and posteriorly at the prostate-rectal interface. Mason et al. (99) estimated the uncertainty margin in tumor delineation and registration by repeating the contours 4 times on 5 cases and found the required margin to be 2.6 to 5.2 mm with a mean of 4.2 mm. For simplification, a 4.5 mm isotropic margin was chosen for the purposes of focal dose escalation to the dominant lesion. For purely focal treatment, a 6-mm margin is recommended (100).
During the HDR procedure, the number and placement of treatment catheters are determined using a standard grid spacing of 0.5 cm and manual catheter steering. After HDR needles are placed under TRUS guidance and locked in place, a set of continuously-acquired images is obtained. Prostate, urethral and rectal volumes are contoured. The F-PTV is then transposed from the pre-procedure TRUS (101). The catheters are reconstructed and inverse treatment planning is used for dose optimization.
HDR toxicity
Toxicities in HDR brachytherapy are similar in nature to LDR brachytherapy and rates vary with fractionation as well as whole vs. partial gland salvage. Yamada et al. noted late grade 2 GU toxicity in 48% and one grade 3 incontinence. Late Grade 2 GI toxicity was noted in 8%, with no G3 or higher GI toxicities (98). Jiang et al. used 3 weekly fractions of 10 Gy to PET positive target volumes in the peripheral zone with 9% each late G2 GI and grade 3 GU toxicity. By sparing the urethra and respecting dose constraints, the urethral stricture rate is acceptable and should be in single digits (63,70,94,98). Yamada et al. reported a grade 2 stricture rate of 7% (98). Kollmeier et al. reviewed experience with both LDR and HDR brachytherapy and found no significant differences in toxicity. However, there were more patients with acute urinary retention and late urethral strictures (8:1) with HDR compared to LDR. After analysis of IPS scores, a higher peak in urinary symptoms was noted with LDR. However, for most patients, urinary scores returned to baseline by 24–36 months (71).
Conclusions
There is no consensus on the best salvage treatment for failure after radiotherapy. Androgen deprivation therapy is currently the most common and most conservative approach. Although at times, it may be appropriate, it is purely palliative and exposes the patient to the many side effects of testosterone ablation. Given that a significant proportion of biochemical failures after EBRT may be attributable to local recurrence, robust local therapies are important. By eradicating residual disease, in the absence of micrometastases, a second chance at cure is possible. Several local treatments, such as salvage prostatectomy, SBRT, cryotherapy, HIFU and brachytherapy have all been used with varying degrees of success. However, all are associated with higher toxicity than when used in the primary setting.
Amongst these options, brachytherapy is an ideal salvage treatment for localized disease, delivering a high dose in a very conformal manner, minimizing dose to adjacent organs. Although most published experience concerns whole gland salvage, focal salvage has been made possible by improved imaging and is being explored, particularly with HDR, with the potential advantage of further reduced toxicity.
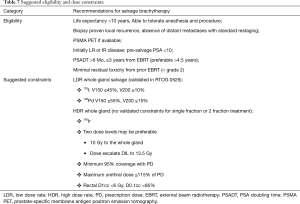
Appropriate patient selection and advances in treatment planning have benefited both HDR and LDR salvage brachytherapy. Eligibility and dose constraints are summarized in Table 7. We await the efficacy results of the RTOG 0526 trial but in the meantime, improved imaging and image registration are changing the landscape, permitting more precise tumour targeting and treatment delivery.
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591-7. [Crossref] [PubMed]
- Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965-74. [Crossref] [PubMed]
- Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys 2017;98:275-85. [Crossref] [PubMed]
- Helou J, D’Alimonte L, Loblaw A, et al. High dose-rate brachytherapy boost for intermediate risk prostate cancer: Long-term outcomes of two different treatment schedules and early biochemical predictors of success. Radiother Oncol 2015;115:84-9. [Crossref] [PubMed]
- Thompson A, Keyes M, Pickles T, et al. Evaluating the Phoenix Definition of Biochemical Failure After 125I Prostate Brachytherapy: Can PSA Kinetics Distinguish PSA Failures From PSA Bounces? Int J Radiat Oncol Biol Phys 2010;78:415-21. [Crossref] [PubMed]
- Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys 1998;41:491-500. [Crossref] [PubMed]
- Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 2014;15:464-73. [Crossref] [PubMed]
- Kuban DA, Tucker SL, Dong L, et al. Long-Term Results of the M. D. Anderson Randomized Dose-Escalation Trial for Prostate Cancer. Int J Radiat Oncol Biol Phys 2008;70:67-74. [Crossref] [PubMed]
- Zietman AL, Bae K, Slater JD, et al. Randomized Trial Comparing Conventional-Dose With High-Dose Conformal Radiation Therapy in Early-Stage Adenocarcinoma of the Prostate: Long-Term Results From Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol 2010;28:1106-11. [Crossref] [PubMed]
- Al-Mamgani A, van Putten WL, Heemsbergen WD, et al. Update of Dutch Multicenter Dose-Escalation Trial of Radiotherapy for Localized Prostate Cancer. Int J Radiat Oncol Biol Phys 2008;72:980-8. [Crossref] [PubMed]
- Shipley WU, Verhey LJ, Munzenrider JE, et al. Advanced prostate cancer: The results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys 1995;32:3-12. [Crossref] [PubMed]
- Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol 2013;11:14-23. [PubMed]
- Tran H, Kwok J, Pickles T, et al. Underutilization of local salvage therapy after radiation therapy for prostate cancer. Urol Oncol 2014;32:701-6. [Crossref] [PubMed]
- Agarwal PK, Sadetsky N, Konety BR, et al. Treatment failure after primary and salvage therapy for prostate cancer. Cancer 2008;112:307-14. [Crossref] [PubMed]
- Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of neoplastic diseases of the haematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Histopathology 2000;36:69-86. [Crossref] [PubMed]
- Nguyen PL, Alibhai SM, Basaria S, et al. Adverse Effects of Androgen Deprivation Therapy and Strategies to Mitigate Them. Eur Urol 2015;67:825-36. [Crossref] [PubMed]
- Ahmadi H, Daneshmand S. Androgen deprivation therapy: evidence-based management of side effects. BJU Int 2013;111:543-8. [Crossref] [PubMed]
- Higano CS. Side effects of androgen deprivation therapy: monitoring and minimizing toxicity. Urology 2003;61:32-8. [Crossref] [PubMed]
- Gomella LG. Contemporary use of hormonal therapy in prostate cancer: managing complications and addressing quality-of-life issues. BJU Int 2007;99:25-9. [Crossref] [PubMed]
- Duchesne GM, Woo HH, King M, et al. Health-related quality of life for immediate versus delayed androgen-deprivation therapy in patients with asymptomatic, non-curable prostate cancer (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol 2017;18:1192-201. [Crossref] [PubMed]
- Crook J. The role of intermittent androgen suppression in biochemically recurrent or newly diagnosed metastatic prostate cancer. Curr Opin Support Palliat Care 2013;7:258-64. [PubMed]
- Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys 1991;21:537-47. [Crossref] [PubMed]
- Zelefsky MJ, Reuter VE, Fuks Z, et al. Influence of Local Tumor Control on Distant Metastases and Cancer Related Mortality After External Beam Radiotherapy for Prostate Cancer. J Urol 2008;179:1368-73. [Crossref] [PubMed]
- Albertsen PC, Hanley JA, Harlan LC, et al. The positive yield of imaging studies in the evaluation of men with newly diagnosed prostate cancer: a population based analysis. J Urol 2000;163:1138-43. [Crossref] [PubMed]
- Abuzallouf S, Dayes I, Lukka H. Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol 2004;171:2122-7. [Crossref] [PubMed]
- Hövels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 2008;63:387-95. [Crossref] [PubMed]
- Pyka T, Okamoto S, Dahlbender M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging 2016;43:2114-21. [Crossref] [PubMed]
- Rouvière O, Hartman RP, Lyonnet D. Prostate MR imaging at high-field strength: evolution or revolution? Eur Radiol 2006;16:276-84. [Crossref] [PubMed]
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40. [Crossref] [PubMed]
- Turkbey B, Mani H, Shah V, et al. Multiparametric 3T Prostate Magnetic Resonance Imaging to Detect Cancer: Histopathological Correlation Using Prostatectomy Specimens Processed in Customized Magnetic Resonance Imaging Based Molds. J Urol 2011;186:1818-24. [Crossref] [PubMed]
- Oppenheimer DC, Weinberg EP, Hollenberg GM, et al. Multiparametric Magnetic Resonance Imaging of Recurrent Prostate Cancer. J Clin Imaging Sci 2016;6:18. [Crossref] [PubMed]
- Arumainayagam N, Kumaar S, Ahmed HU, et al. Accuracy of multiparametric magnetic resonance imaging in detecting recurrent prostate cancer after radiotherapy. BJU Int 2010;106:991-7. [Crossref] [PubMed]
- Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med 2015;56:668-74. [Crossref] [PubMed]
- Hruby G, Eade T, Kneebone A, et al. Delineating biochemical failure with 68Ga-PSMA-PET following definitive external beam radiation treatment for prostate cancer. Radiother Oncol 2017;122:99-102. [Crossref] [PubMed]
- Chade DC, Eastham J, Graefen M, et al. Cancer Control and Functional Outcomes of Salvage Radical Prostatectomy for Radiation-recurrent Prostate Cancer: A Systematic Review of the Literature. Eur Urol 2012;61:961-71. [Crossref] [PubMed]
- Bianco FJ, Scardino PT, Stephenson AJ, et al. Long-term oncologic results of salvage radical prostatectomy for locally recurrent prostate cancer after radiotherapy. Int J Radiat Oncol Biol Phys 2005;62:448-53. [Crossref] [PubMed]
- Chade DC, Shariat SF, Cronin AM, et al. Salvage Radical Prostatectomy for Radiation-recurrent Prostate Cancer: A Multi-institutional Collaboration. Eur Urol 2011;60:205-10. [Crossref] [PubMed]
- Ward JF, Sebo TJ, Blute ML, et al. Salvage surgery for radiorecurrent prostate cancer: contemporary outcomes. J Urol 2005;173:1156-60. [Crossref] [PubMed]
- Rosoff JS, Savage SJ, Prasad SM. Salvage radical prostatectomy as management of locally recurrent prostate cancer: outcomes and complications. World J Urol 2013;31:1347-52. [Crossref] [PubMed]
- Mandel P, Steuber T, Ahyai S, et al. Salvage radical prostatectomy for recurrent prostate cancer: verification of European Association of Urology guideline criteria. BJU Int 2016;117:55-61. [Crossref] [PubMed]
- Yuh B, Ruel N, Muldrew S, et al. Complications and outcomes of salvage robot-assisted radical prostatectomy: a single-institution experience. BJU Int 2014;113:769-76. [Crossref] [PubMed]
- Corcoran NM, Godoy G, Studd RC, et al. Salvage prostatectomy post-definitive radiation therapy: The Vancouver experience. Can Urol Assoc J 2013;7:87-92. [Crossref] [PubMed]
- Crouzet S, Blana A, Murat FJ, et al. Salvage high-intensity focused ultrasound (HIFU) for locally recurrent prostate cancer after failed radiation therapy: Multi-institutional analysis of 418 patients. BJU Int 2017;119:896-904. [Crossref] [PubMed]
- Jones TA, Chin J, Mcleod D, et al. High Intensity Focused Ultrasound for Radiorecurrent Prostate Cancer: A North American Clinical Trial. J Urol 2018;199:133-9. [Crossref] [PubMed]
- Murat F-J, Poissonnier L, Rabilloud M, et al. Mid-term Results Demonstrate Salvage High-Intensity Focused Ultrasound (HIFU) as an Effective and Acceptably Morbid Salvage Treatment Option for Locally Radiorecurrent Prostate Cancer. Eur Urol 2009;55:640-7. [Crossref] [PubMed]
- de Castro Abreu AL, Bahn D, Leslie S, et al. Salvage focal and salvage total cryoablation for locally recurrent prostate cancer after primary radiation therapy. BJU Int 2013;112:298-307. [Crossref] [PubMed]
- Williams AK, Martínez CH, Lu C, et al. Disease-Free Survival Following Salvage Cryotherapy for Biopsy-Proven Radio-Recurrent Prostate Cancer. Eur Urol 2011;60:405-10. [Crossref] [PubMed]
- Ismail M, Ahmed S, Kastner C, et al. Salvage cryotherapy for recurrent prostate cancer after radiation failure: a prospective case series of the first 100 patients. BJU Int 2007;100:760-4. [Crossref] [PubMed]
- Li YH, Elshafei A, Agarwal G, et al. Salvage focal prostate cryoablation for locally recurrent prostate cancer after radiotherapy: Initial results from the cryo on-line data registry. Prostate 2015;75:1-7. [Crossref] [PubMed]
- Izawa JI, Madsen LT, Scott SM, et al. Salvage cryotherapy for recurrent prostate cancer after radiotherapy: variables affecting patient outcome. J Clin Oncol 2002;20:2664-71. [Crossref] [PubMed]
- Vavassori A, Jereczek-Fossa BA, Beltramo G, et al. Image-guided robotic radiosurgery as salvage therapy for locally recurrent prostate cancer after external beam irradiation: retrospective feasibility study on six cases. Tumori 2010;96:71-5. [Crossref] [PubMed]
- Fuller DB, Wurzer J, Shirazi R, et al. High-dose-rate stereotactic body radiation therapy for postradiation therapy locally recurrent prostatic carcinoma: Preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract Radiat Oncol 2015;5:e615-23. [Crossref] [PubMed]
- Leroy T, Lacornerie T, Bogart E, et al. Salvage robotic SBRT for local prostate cancer recurrence after radiotherapy: preliminary results of the Oscar Lambret Center. Radiat Oncol 2017;12:95. [Crossref] [PubMed]
- Zerini D, Jereczek-Fossa BA, Fodor C, et al. Salvage image-guided intensity modulated or stereotactic body reirradiation of local recurrence of prostate cancer. Br J Radiol 2015;88. [Crossref] [PubMed]
- Chin J, Rumble RB, Kollmeier M, et al. Brachytherapy for Patients With Prostate Cancer: American Society of Clinical Oncology/Cancer Care Ontario Joint Guideline Update. J Clin Oncol 2017;35:1737-43. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. NCCN Guidelines ® Prostate Cancer 2017.
- Sylvester JE, Grimm PD, Eulau SM, et al. Permanent prostate brachytherapy preplanned technique: The modern Seattle method step-by-step and dosimetric outcomes. Brachytherapy 2009;8:197-206. [Crossref] [PubMed]
- Davis BJ, Horwitz EM, Lee WR, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy 2012;11:6-19. [Crossref] [PubMed]
- Grado GL, Collins JM, Kriegshauser JS, et al. Salvage brachytherapy for localized prostate cancer after radiotherapy failure. Urology 1999;53:2-10. [Crossref] [PubMed]
- Beyer DC. Permanent brachytherapy as salvage treatment for recurrent prostate cancer. Urology 1999;54:880-3. [Crossref] [PubMed]
- Wong WW, Buskirk SJ, Schild SE, et al. Combined prostate brachytherapy and short-term androgen deprivation therapy as salvage therapy for locally recurrent prostate cancer after external beam irradiation. J Urol 2006;176:2020-4. [Crossref] [PubMed]
- Aaronson DS, Yamasaki I, Gottschalk A, et al. Salvage permanent perineal radioactive-seed implantation for treating recurrence of localized prostate adenocarcinoma after external beam radiotherapy. BJU Int 2009;104:600-4. [Crossref] [PubMed]
- Lee HK, Adams MT, Motta J. Salvage prostate brachytherapy for localized prostate cancer failure after external beam radiation therapy. Brachytherapy 2008;7:17-21. [Crossref] [PubMed]
- Burri RJ, Stone NN, Unger P, et al. Long-term outcome and toxicity of salvage brachytherapy for local failure after initial radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010;77:1338-44. [Crossref] [PubMed]
- Moman MR, van der Poel HG, Battermann JJ, et al. Treatment outcome and toxicity after salvage 125-I implantation for prostate cancer recurrences after primary 125-I implantation and external beam radiotherapy. Brachytherapy 2010;9:119-25. [Crossref] [PubMed]
- Vargas C, Swartz D, Vashi A, et al. Salvage brachytherapy for recurrent prostate cancer. Brachytherapy 2014;13:53-8. [Crossref] [PubMed]
- Nguyen PL, Chen MH, D’Amico AV, et al. Magnetic resonance image-guided salvage brachytherapy after radiation in select men who initially presented with favorable-risk prostate cancer. Cancer 2007;110:1485-92. [Crossref] [PubMed]
- Hsu CC, Hsu H, Pickett B, et al. Feasibility of MR Imaging/MR Spectroscopy-Planned Focal Partial Salvage Permanent Prostate Implant (PPI) for Localized Recurrence After Initial PPI for Prostate Cancer. Int J Radiat Oncol Biol Phys 2013;85:370-7. [Crossref] [PubMed]
- Peters M, Maenhout M, van der Voort van Zyp JRN, et al. Focal salvage iodine-125 brachytherapy for prostate cancer recurrences after primary radiotherapy: A retrospective study regarding toxicity, biochemical outcome and quality of life. Radiother Oncol 2014;112:77-82. [Crossref] [PubMed]
- Henríquez I, Sancho G, Hervás A, et al. Salvage brachytherapy in prostate local recurrence after radiation therapy: predicting factors for control and toxicity. Radiat Oncol 2014;9:102. [Crossref] [PubMed]
- Kollmeier MA, McBride S, Taggar A, et al. Salvage brachytherapy for recurrent prostate cancer after definitive radiation therapy: A comparison of low-dose-rate and high-dose-rate brachytherapy and the importance of prostate-specific antigen doubling time. Brachytherapy 2017;16:1091-8. [Crossref] [PubMed]
- Crook JM, Malone S, Perry G, et al. Twenty-four-month postradiation prostate biopsies are strongly predictive of 7-year disease-free survival. Cancer 2009;115:673-9. [Crossref] [PubMed]
- Pollack A, Zagars GK, Antolak JA, et al. Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys 2002;54:677-85. [Crossref] [PubMed]
- Vance W, Tucker SL, de Crevoisier R, et al. The predictive value of 2-year posttreatment biopsy after prostate cancer radiotherapy for eventual biochemical outcome. Int J Radiat Oncol Biol Phys 2007;67:828-33. [Crossref] [PubMed]
- Shilkrut M, McLaughlin PW, Merrick GS, et al. Interval to Biochemical Failure Predicts Clinical Outcomes in Patients With High-Risk Prostate Cancer Treated by Combined-Modality Radiation Therapy. Int J Radiat Oncol Biol Phys 2013;86:721-8. [Crossref] [PubMed]
- Buyyounouski MK, Hanlon AL, Horwitz EM, et al. Interval to Biochemical Failure Highly Prognostic for Distant Metastasis and Prostate Cancer-Specific Mortality After Radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:59-66. [Crossref] [PubMed]
- Crook JM, Bahadur YA, Robertson SJ, et al. Evaluation of radiation effect, tumor differentiation, and prostate specific antigen staining in sequential prostate biopsies after external beam radiotherapy for patients with prostate carcinoma. Cancer 1997;79:81-9. [Crossref] [PubMed]
- Tetreault-Laflamme A, Crook J. Options for Salvage of Radiation Failures for Prostate Cancer. Semin Radiat Oncol 2017;27:67-78. [Crossref] [PubMed]
- Nguyen PL, D’Amico AV, Lee AK, et al. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure. Cancer 2007;110:1417-28. [Crossref] [PubMed]
- Stock RG, Ho A, Cesaretti JA, et al. Changing the patterns of failure for high-risk prostate cancer patients by optimizing local control. Int J Radiat Oncol Biol Phys 2006;66:389-94. [Crossref] [PubMed]
- Martinez AA, Gonzalez J, Ye H, et al. Dose Escalation Improves Cancer-Related Events at 10 Years for Intermediate- and High-Risk Prostate Cancer Patients Treated With Hypofractionated High-Dose-Rate Boost and External Beam Radiotherapy. Int J Radiat Oncol Biol Phys 2011;79:363-70. [Crossref] [PubMed]
- Rose JN, Crook JM, Pickles T, et al. Salvage low-dose-rate permanent seed brachytherapy for locally recurrent prostate cancer: Association between dose and late toxicity. Brachytherapy 2015;14:342-9. [Crossref] [PubMed]
- Martens C, Pond G, Webster D, et al. Relationship of the International Prostate Symptom score with urinary flow studies, and catheterization rates following 125I prostate brachytherapy. Brachytherapy 2006;5:9-13. [Crossref] [PubMed]
- Butler WM, Merrick GS, Lief JH, et al. Comparison of seed loading approaches in prostate brachytherapy. Med Phys 2000;27:381-92. [Crossref] [PubMed]
- Cosset JM, Cathelineau X, Wakil G, et al. Focal brachytherapy for selected low-risk prostate cancers: A pilot study. Brachytherapy 2013;12:331-7. [Crossref] [PubMed]
- Langley S, Ahmed HU, Al-Qaisieh B, et al. Report of a consensus meeting on focal low dose rate brachytherapy for prostate cancer. BJU Int 2012;109:7-16. [Crossref] [PubMed]
- Valerio M, Ahmed HU, Emberton M, et al. The Role of Focal Therapy in the Management of Localised Prostate Cancer: A Systematic Review. Eur Urol 2014;66:732-51. [Crossref] [PubMed]
- Peters M, Hoekstra CJ, van der Voort van Zyp JR, et al. Rectal dose constraints for salvage iodine-125 prostate brachytherapy. Brachytherapy 2016;15:85-93. [Crossref] [PubMed]
- Morton GC. The emerging role of high-dose-rate brachytherapy for prostate cancer. Clin Oncol (R Coll Radiol) 2005;17:219-27. [Crossref] [PubMed]
- Yamada Y, Rogers L, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy 2012;11:20-32. [Crossref] [PubMed]
- Tharp M, Hardacre M, Bennett R, et al. Prostate high-dose-rate brachytherapy as salvage treatment of local failure after previous external or permanent seed irradiation for prostate cancer. Brachytherapy 2008;7:231-6. [Crossref] [PubMed]
- Chen CP, Weinberg V, Shinohara K, et al. Salvage HDR Brachytherapy for Recurrent Prostate Cancer After Previous Definitive Radiation Therapy: 5-Year Outcomes. Int J Radiat Oncol Biol Phys 2013;86:324-9. [Crossref] [PubMed]
- Lee B, Shinohara K, Weinberg V, et al. Feasibility of high-dose-rate brachytherapy salvage for local prostate cancer recurrence after radiotherapy: The University of California–San Francisco experience. Int J Radiat Oncol Biol Phys 2007;67:1106-12. [Crossref] [PubMed]
- Kukiełka AM, Hetnał M, Dąbrowski T, et al. Salvage prostate HDR brachytherapy combined with interstitial hyperthermia for local recurrence after radiation therapy failure. Strahlenther Onkol 2014;190:165-70. [Crossref] [PubMed]
- Wojcieszek P, Szlag M, Głowacki G, et al. Salvage high-dose-rate brachytherapy for locally recurrent prostate cancer after primary radiotherapy failure. Radiother Oncol 2016;119:405-10. [Crossref] [PubMed]
- Łyczek J, Kawczyńska MM, Garmol D, et al. HDR brachytherapy as a solution in recurrences of locally advanced prostate cancer. J Contemp Brachytherapy 2009;1:105-8. [PubMed]
- Jiang P, van der Horst C, Kimmig B, et al. Interstitial high-dose-rate brachytherapy as salvage treatment for locally recurrent prostate cancer after definitive radiation therapy: Toxicity and 5-year outcome. Brachytherapy 2017;16:186-92. [Crossref] [PubMed]
- Yamada Y, Kollmeier MA, Pei X, et al. A Phase II study of salvage high-dose-rate brachytherapy for the treatment of locally recurrent prostate cancer after definitive external beam radiotherapy. Brachytherapy 2014;13:111-6. [Crossref] [PubMed]
- Mason J, Al-Qaisieh B, Bownes P, et al. Multi-parametric MRI-guided focal tumor boost using HDR prostate brachytherapy: A feasibility study. Brachytherapy 2014;13:137-45. [Crossref] [PubMed]
- Mason J, Al-Qaisieh B, Bownes P, et al. Dosimetry modeling for focal high-dose-rate prostate brachytherapy. Brachytherapy 2014;13:611-7. [Crossref] [PubMed]
- Crook J, Ots A, Gaztañaga M, et al. Ultrasound-planned high-dose-rate prostate brachytherapy: Dose painting to the dominant intraprostatic lesion. Brachytherapy 2014;13:433-41. [Crossref] [PubMed]


