Long-term complications of continent catheterizable channels: a problem for transitional urologists
Introduction
Transitional urology is an emerging area of need given that the life expectancies for patients born with congenital genitourinary conditions is lengthening (1). A majority of the transitional urology population is made up of patients with neurogenic bladder and bowel, and many of these patients have previously undergone creation of continent catheterizable channel (CCC) with or without bladder augmentation during childhood in order to facilitate bladder emptying. There are various types of CCCs, including tunneled channels such as the appendicovesicostomy (APV) and Yang-Monti, or ileocecal valve-dependent channels such as the cutaneous catheterizable ileal cecoplasty (CCIC).
These channels rely on different bowel segments, such as the appendix, the small bowel, and the ileocecal segment. The APV, often referred to using the eponym “Mitrofanoff”, utilizes the appendix on its mesenteric blood supply to create a channel from the bladder to the abdominal wall, most often the umbilicus (2). An anti-reflux submucosal tunnel is typically created at the site of implantation of the appendix into the bladder to attain stomal continence. When the appendix is missing or too short, the Yang-Monti or “Monti” is an alternative channel. The Monti is an ileovesicostomy which utilizes retubularized small bowel on its mesenteric blood supply; it, too, relies on a submucosal bladder tunnel for continence (3,4). In adults, the distance from the bladder to the abdominal wall is longer than in children; thus a single Monti channel (about 6 cm) is usually insufficient. To create a longer channel, a spiral Monti (“Casale” procedure) or double Monti can be created by using a longer piece of bowel in a spiralized retubularization, or using two separate segments of bowel that have been retubularized and joined together, respectively (5). The CCIC is different in that its continence mechanism relies on the ileocecal valve. Creation of the CCIC allows utilization of the ileum for the retubularized channel and cecum for simultaneous bladder augmentation if needed, maintaining blood supply to both the augment and the channel from the ileocecal pedicle (6).
These channels inevitably have complications that lead to difficult catheterization and stomal leakage, and therefore are not expected to last for these patients’ lifetimes. As a result, the transitional urologist may be faced with surgical revision of these channels due to a variety of underlying problems, including stomal stenosis, stomal prolapse, channel stricture, stomal incontinence, and difficult catheterization due to channel redundancy. We aim to review the incidence, timing, and predisposing factors that lead to problems with CCCs; we will also review evaluation strategies and surgical revision techniques and their outcomes.
Incidence of complications requiring revision
Among patients ≤21 years old undergoing CCCs, long-term channel complications and related revisions are common and likely underestimated given a lack of long-term follow-up in many studies. The main complications include stomal prolapse, stomal incontinence, and difficulty catheterizing, typically due to channel stricture/stenosis, channel redundancy, false passages, and diverticuli. The incidence of these complications varies widely based on the series, the type of channel used, and the length of follow-up. In general, the incidence of stomal prolapse ranges from 2–5%, stomal incontinence 1–47%, and difficulty catheterizing 5–32% (7-11). Overall rates of surgical revision range from 18–58%, and these series often vary in terms of what types of revision are included; for example, some series do not count a dilation as a true revision (7-11). For the purposes of this review, “supra-fascial” will include endoscopic or open surgery above the level of the fascia, and “sub-fascial” will include any intra-abdominal revision (i.e., requires a laparotomy). Typically supra-fascial revisions include surgery for stomal-related complications such as stenosis or prolapse, whereas sub-fascial revisions are for long or multi-focal channel stenosis, channel redundancy or channel incontinence, as will be further described below.
Complications by channel type
In the largest series with the longest follow-up to-date, Szymanski et al. report on the results of 510 patients who underwent APV (214, median follow-up 5.7 years) or Monti (296, median follow-up 7.7 years) procedures (11). Overall, 28.2% of the APVs required some kind of revision, while 26.0% of the Montis required revision (Table 1).
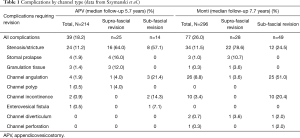
Full table
Complications of adult CCCs
While there are many studies like these that assess outcomes of CCCs among children, there are few data that evaluate the incidence of long-term outcomes among CCCs created in adulthood. What is clear is that adults who undergo channel creation have a high chance of channel-related complications. Based on the three series of adult patients undergoing CCCs, the majority of complications include problems with catheterization (20–46%) and stomal incontinence (8–34%) (12-14). Two studies evaluated complications requiring revision in adults undergoing CCCs, finding an overall revision rate between 31–54% (13,14). Interestingly in the adult population, Redshaw et al. evaluated 61 adults undergoing CCC creation with a mean follow-up of 16 months, and identified much higher revision rates among tunneled channels (such as APV or Monti) than non-tunneled (CCIC) channels (50% vs. 13%) (13).
Timing of complications requiring revision
Among children, two studies have evaluated revision-free survival time of CCCs. Mean revision-free survival time was found to be 99 months in one study and 110 months in another (8,10). These studies tend to report an initial peak in revisions (typically most in the first 5 years), followed by a relatively complication-free period, although note that complications still do occur in longer-term follow-up.
In terms of time to revision by channel type, Szymanski et al. found a median time to stomal revision of 1.3 years among APVs vs. 1.6 years among Montis, and a median time to subfascial revision of 2.3 years among both APVs and Montis (11). Among CCCs created as adults, Redshaw et al. report that the time to revision appears to be shorter, with a median time from CCC creation to revision of 14.3 months (IQR, 11.5–58.5) for CCIC and 4.9 months (IQR, 2.7–6.3) for tunneled channels (13).
Risk factors for complications requiring revision
There is significant variability in the literature about risk factors for CCC complications requiring revision. Some risk factors that are commonly discussed as possible contributors to revision include age, weight, type of channel, and stoma location. Most series do evaluate for difference in revision rate by the type of channel created, with some series showing no differences in revision rates and other finding that Monti channels (compared to APVs) do carry a higher likelihood of revision (8,10,13). This variability is likely related to the fact that these are often small series and even if differences are present, the studies may not be powered to show a statistically significant difference.
Only one study has been able to perform a robust analysis of possible predictors for revision given the large numbers in their series (11). The Indiana group found no difference in suprafascial revision rates but did identify a significant difference in subfascial revision rates between Monti (16.6%) and APV (6.5%) that was present after correcting for differential follow-up time (P=0.0006). In multivariate analysis adjusting for stomal location, concomitant surgeries, gender, age at surgery, date of surgery, and channel type, only the channel type was found to have significant differences in revision rates, with the spiral umbilical Monti having a 4.23 hazards ratio (P<0.001) and all other Monti channels having a 2.09 hazards ratio (P=0.03) compared to the APV.
Management of common channel complications
Stomal prolapse
The incidence of stomal prolapse requiring revision appears to be between 2–5% in most series (7,8). Stomal prolapse typically requires open surgical revision, and patients must be counseled about the possibility of stomal incontinence after stomal revision. Minor mucosal prolapse that leads to staining of the clothing and occasional bleeding can be managed by topical application of silver nitrate. More significant prolapse may require operative revision of the cutaneous end of the stoma (9).
Stomal incontinence
There is no consensus on the definition of incontinence in this population. Some authors have used total absence of involuntary loss of urine day and night between catheterizations (13,15). Most publications however, have considered patients socially continent if they were completely dry or used 1 safety pad between catheterizations every 3 hours. Urinary incontinence is often indicative of a larger problem in CCC and may be related to the reservoir (changes in volume-pressure dynamics) or incompetence of the flap valve or ileocecal valve. It occurs in up to 65% of patients and most commonly within the first 24 months postoperatively. Re-evaluating the urodynamic parameters of the reservoir is the first step, as creation of a CCC or other interventions at the time (augmentation, bladder neck procedures) can change the functional dynamics of the bladder. If incontinence is thought to be related to high reservoir pressures, treatment options include increasing the rate of CIC, maximizing anticholinergic therapy, detrusor injection of onabotulinum toxin, or increasing the volume of the reservoir through bladder augmentation.
Most channel-related incontinence is thought to be the result of a short intravesical tunnel of the CCC. Endoscopic approaches using bulking agent injection as well as open revision of the vesical anastomotic site have been reported with varying degrees of success. Bulking agent injection for stoma related incontinence, first described by Kaefer in 1997, involves injection in the submucosa of the vesico-conduit junction and can be performed in an antegrade fashion or retrograde via urethra if not previously closed (16). Welk reported 6/67 incontinent CCC’s, 4 of which underwent endoscopic treatment initially but all of them ultimately failed and required open revision or channel take-down (9). Prieto has reported much higher success rate after endoscopic injection of bulking agents in 14 patients (17). In short-term interval they reported 10/12 dryness after one injection and 11/12 after two injections for a total of 79% success rate after mean follow up of 1 year. Other small series with less than 24-month follow up have published similar results and bulking agent injection is considered routine practice as the first step of treating low leak point pressure incontinence (18-20). Dextranomer/hyaluronic acid is the agent of choice similar to the management of vesicoureteral reflux in children although reports on long-term success of this approach is lacking in the literature.
Open surgical repair of channel incontinence most often involves lengthening of the detrusor tunnel. This can be done by leaving the channel in its exiting tunnel but wrapping additional detrusor muscle around the extravesical portion of the channel. Alternatively, one can take down the existing tunnel and create a new, longer tunnel in a new location. The former approach is preferred when it is possible because it is easier and avoids damage to the existing channel. When an adequate tunnel cannot be created, an alternative is to substitute a CCIC for the tunneled channel. In a large published report of over 500 patients, Szymanski et al. reported a 2% rate of revisions purely for incontinence from an incompetent tunnel—two patients with an APV and ten patients with a Monti (11). Secondary revisions for incontinence were pursued in less than 1% of total population, two who developed incontinence after revision of stomal stenosis and three patients whose initial revision failed. A subsequent update from the same group on 675 patients showed that incontinence was the second most common reason for sub-fascial revision and it was mostly due to an insufficient tunnel length and unrelated to anterior vs. posterior location of vesico-conduit implantation (21). In our experience, these results highlight the fact that in low-pressure reservoirs, incontinence from an incompetent vesico-conduit anastomosis—if it exists—does not typically bother patients enough to warrant open revision.
Difficult catheterization
Difficult catheterization occurs in up to 30% of patients with CCC and over 50% will ultimately require surgical revision (22). However some groups have reported conservative management of channel complications with endoscopic or stomal procedures in up to 82% of cases (23). Based on surgical approach they can be categorized as supra- or sub-fascial revisions. Stomal stenosis, channel angulation, channel redundancy, channel diverticulum/false passage as well as incontinence as described above are common reasons for revision. In most contemporary published reports, over 90% of CCCs with complications are salvageable with a variety of revision operations (11,12,15).
Conservative/endoscopic management
In the literature, up to 50% of channel complications are managed endoscopically, at least initially. In a review of 434 patients with CCC, Casey et al. reported endoscopic intervention on 63 difficult to catheterize CCC obviated the need for an open revision in 43.5% with 3 years of follow-up at a high-volume center (24). However, as expected when managing stenosis/stricture of the channel, dilation and incision of the scar tissue has higher rate of recurrence compared to scar excision and reanastomosis of the efferent segment to the skin (15).
Jacobson et al. reported 80-month follow-up of 110 CCCs (62% Mitrofanoff, 38% MACE) in 81 patients (25). Difficult catheterization was the most common complication and 14 required an intervention under anesthesia, of which 9 were performed endoscopically with or without dilation. The MACE channel complication rate is almost half that of Mitrofanoff channels; this may be due to the fact that Mitrofanoffs are catheterized multiple times a day vs. just once every day or two in MACE’s. Piaggio et al. reported outcome of CCC creation in 41 patients with 33 months of follow-up and showed 29% rate of difficult catheterization of which 40% were managed endoscopically by passive dilation over Foley catheter and 60% needed surgical revision (26). This report lacks data on the durability of the endoscopic treatment given the retrospective design; however, they reported that one procedure was sufficient in 73% of patients and the rest needed at least two surgical revisions. The group from Vancouver, Canada reported 17 complications in 67 patients after median 28 months of follow up (9). Stomal stenosis occurred in four patients: three were managed by endoscopic dilation in combination with steroid lubricant, and one of these patients eventually developed complete channel stricture and required operative revision. Of the four patients with channel stricture in their series, two were managed successfully with endoscopic resection. Other groups have reported endoscopic management of complications in 30–50% of patients although the subsequent success rate of the endoscopic intervention is unknown (27-29).
Supra-fascial revision
Stomal stenosis is the most common complication that requires revision. Treatment of mucocutaneous junction stenosis can include serial dilations, L-stent or Malone antegrade continence enema (MACE) stopper placement with or without a short course of steroid ointment (30-32). Most superficial stenosis can be managed by excision of all scar tissue and marsupialization of healthy channel mucosa to the surrounding skin edges (9). This can mean using techniques such as a tubularized skin flap, Y-V plasty or VQZ plasty; success rates are similar with these various procedures but the case series are small (33,34). A potential advantage for using multiple skin flaps (VQZ or Y-V plasty) as opposed to single V flap or direct anastomosis to the skin is to minimize the risk of stomal stenosis (28). However, given the risk of stomal stenosis, others prefer to create a rosebud-type stoma, which likely further decreases the risk of recurrent stomal stenosis.
Faure et al. reported stomal stenosis in 17/34 patients with CCC, of which 75% underwent V-Y plasty and the rest underwent dilation (23). They did not report long-term outcomes of revision; however, 2 patients developed new urinary leakage after stomal dilation and 3 developed new conduit stricture after dilation or Y-V plasty of stenosis. In a long-term outcome report of the original Mitrofanoff group from France, Liard reported outcomes of CCC creation in 169 patients with mean 5.8 (range, 1–15) years follow up (35). Skin level stenosis that required revision occurred in 17% but they did not provide insight to the treatment approach and their eventual outcome except that 96% of CCCs were functional. McAndrew and Malone reported stenosis in 35/112 stomas at a median 34 months (28). A single dilation was attempted in 17 (49%) of which 9 ultimately required a surgical revision. Five patients needed serial dilations. 18/35 (51%) needed an open surgical revision for stenosis and 9 of them needed a second revision.
Injection of triamcinolone parastomally at the time of surgical revision was introduced in 1999 by Snodgrass. The rational is that, as most stenosis occurs at the most distal few centimeters of the conduit, the pathophysiology is though to be excessive local collagen formation rather than tissue ischemia (36). Reddy reported a retrospective experience of 22 steroid injections (1 mL of triamcinolone acetonide, 40 mg/mL) in 18 stenotic CCCs with difficult catheterizations who had failed other conservative measures such as dilation or stoma stoppers (37). They reported overall 82% success rate with median 11 months of follow up with significant reduction in cost of care compared to alternative invasive treatments. These results must be interpreted with caution as the case series are small and follow-up is short.
Sub-fascial revision
Major intrabdominal revisions are required if supra-fascial salvage procedures fail and/or a multi-segment or long stenosis occurs proximally in the catheterizable limb. Channel redundancy is another common reason for sub-fascial revision. The surgeon may not know the extent of the stricture before surgical exploration. Often, all that is visible in the examination room is stenosis of the stoma. Only after dissecting out the stoma and finding that the stricture extends deep to the fascia does it become clear that a sub-fascial revision will be required. Thus, it is important to consent the patient or his/her guardian for all indicated procedures.
After opening the abdominal fascia, the channel is dissected free from surrounding adhesions. There can often be significant scarring around the channel from prior channel dilations and/or false passages; one must take care not to injure bowel that is adherent to the channel. Once the channel is resected back to a healthy segment that catheterizes easily, the length is assessed and it becomes clear whether the best options is to (I) mature the channel in its original location on the abdominal wall; (II) mature the channel in a new location that is closer to the shortened channel; or (III) harvest a new segment of bowel for a new stoma.
In a retrospective series from a high volume center, Szymanski et al. reported a 12% rate of subfascial revision in 510 CCCs with a median of 7 years of follow-up (11). In this series, 14/214 patients with APV underwent subfascial revision due to channel stricture (3.7%), angulation (1.4%), or incontinence (0.9%). Interestingly, 2/14 underwent a second sub-fascial revision due to new-onset incontinence at 9 months and 2.1 years after initial revision. On the other hand, 49/296 patients who had a Monti underwent a subfascial revision for channel stricture (4.1%), angulation (8.4%), or incontinence (3.4%). Of this group 6/49 needed a second revision for incontinence [3], channel angulation [2], or channel diverticulum [1] at a median 3.5 years after primary sub-fascial revision (range: 7 months to 8.6 years). Overall, APV is associated with the lowest rate of subfascial revision compared to other catheterizable channels. When the CCC is implanted in a new segment of the bladder with adequate technique the rate of complications does not seem to be significantly different than initial anastomosis (23).
Outcomes of channel revisions for adults
While most of the data on channel revision comes from the pediatric literature, one recent study evaluated channel revision among adults ≥18 years of age who underwent channel revision or replacement at three academic centers (38). A total of 51 patients aged 18–82 were identified as having undergone CCC revision or replacement for stomal stenosis, channel obstruction, or difficulty with catheterization, with a total of 68 revision surgeries performed in this population. 66% of patients attained channel patency at a median of 19 months (range, 0.5–81 months), with channel replacement being the most successful (89% success for replacement, 62% for supra-fascial revisions, and 52% for sub-fascial revisions, P=0.046). Of note, 40% of cases developed new incontinence after surgical revision, with 12% categorized as moderate to severe. Surgical complications occurred in nearly 30% of surgeries, the majority of which were grade 1 complications and occurred in channel replacement surgeries.
Discussion
As children with continent catheterizable channels age into adulthood, adult reconstructive urologists will be increasingly faced with troubleshooting and managing the complications that will inevitably arise. Managing these complications can be quite difficult in the adult population, given that patients have previously undergone abdominal surgery, may have had a long-term ventriculoperitoneal shunt with associated inflammatory bowel adhesions, have fewer intestinal segments available for reconstruction, and have more abdominal obesity compared to when their channels were originally created. Furthermore, the diseased bladder often shrinks with age whereas the abdominal wall grows, meaning a longer distance for the channel to traverse. Surgical revision and creation of new channels in these cases can be particularly challenging in light of this, given that channels may not easily reach to previous stoma locations and the length of a channel segment that may have been successful in a pediatric patient may not be sufficient in an adult patient.
Given these difficulties, it is vital to attempt to maintain an existing channel when possible. Conservative measures are often successful and can avoid a trip to the operating room for a formal revision. These conservative measures include channel dilation, channel rest for 1–2 weeks with an indwelling channel catheter, long-term nocturnal channel dilation with an indwelling overnight catheter, or leaving an “L-stent” in overnight or even at all times. The authors do not support dilation to supraphysiologic sizes (e.g., >16 F) or endoscopic incision of channel stenosis because we have witnessed complications of entero-stomal fistula after these procedures.
If surgery is required for stomal stenosis or channel stricture, we attempt to salvage the existing channel if possible, sequentially cutting back on the channel until the channel is patent. When the channel is short, we prefer to mature the channel to a new location when we can, even one that may be not as convenient for the patient as the umbilical location, rather than harvest a new segment of bowel. When one must harvest a new segment of bowel, it is often possible to add a single Monti to the cutaneous end of the old channel in a spatulated end-to-end fashion, akin to a double Monti (Figure 1). This preserves the original continence mechanism. When the entire channel is compromised then one must resect it and create an entirely new channel.
Clearly, patient counseling is paramount in these circumstances, and patients often have very strong feelings about the location and appearance of their stomas given that they have had them sometimes for very long periods of time and they may have become part of their identity. Patients must understand the possibility going into surgery for channel revision that an entirely new channel may need to be created, that the stoma location may need to be moved, and that the stoma appearance may be different. There are various methods of creating new stomas, whether it is performing a Y-V plasty approach as is typical among pediatric urologists, or a brooke rosebud stoma if there is enough channel length, which may decrease the rates of stomal stenosis (Figure 2).
One other consideration if sub-fascial revision is required is the type of incision that is used. Data from colorectal surgery shows that incisional hernias are more common in vertical midline incisions than transverse or Pfannenstiel incisions (39). Furthermore, these patients may be at even higher risk of hernia due to having had a prior umbilical stoma that can create fascial weakness. As a result, some advocate the use of a Pfannenstiel incision to decrease the risk of ventral and parastomal hernia repair (Figure 2).
Conclusions
Ultimately the transitional urologist will be faced with complications of CCCs in his or her practice, given the high incidence of channels requiring revision. While some of these patients may require supravesical diversion in the future, data show that revision is feasible with good outcomes. These salvage procedures allow patients to maintain their CCC for at least an extended period of time, giving them additional years of continence and use of their lower urinary tract. We will need more data with longer follow-up to understand the life-span and best practices of new CCCs created among the transitional population.
Acknowledgements
Funding: LA Hampson received funding from NIH/NIDDK 4K12DK083021-09.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Woodhouse C. Adolescent urology and transitional care. Eur Urol 2015;68:745-6. [Crossref] [PubMed]
- Mitrofanoff P. Trans-appendicular continent cystostomy in the management of the neurogenic bladder. Chir Pediatr 1980;21:297-305. [PubMed]
- Yang WH. Yang needle tunneling technique in creating antireflux and continent mechanisms. J Urol 1993;150:830-4. [Crossref] [PubMed]
- Monti PR, Lara RC, Dutra MA, et al. New techniques for construction of efferent conduits based on the Mitrofanoff principle. Urology 1997;49:112-5. [Crossref] [PubMed]
- Casale AJ. A long continent ileovesicostomy using a single piece of bowel. J Urol 1999;162:1743-5. [Crossref] [PubMed]
- Levy ME, Elliott SP. Reconstructive techniques for creation of catheterizable channels: tunneled and nipple valve channels. Transl Androl Urol 2016;5:136-44. [PubMed]
- Süzer O, Vates TS, Freedman AL, et al. Results of the Mitrofanoff procedure in urinary tract reconstruction in children. Br J Urol 1997;79:279-82. [Crossref] [PubMed]
- Leslie B, Lorenzo AJ, Moore K, et al. Long-term followup and time to event outcome analysis of continent catheterizable channels. J Urol 2011;185:2298-302. [Crossref] [PubMed]
- Welk BK, Afshar K, Rapoport D, et al. Complications of the catheterizable channel following continent urinary diversion: their nature and timing. J Urol 2008;180:1856-60. [Crossref] [PubMed]
- Polm PD, de Kort LMO, de Jong TPVM, et al. Techniques used to create continent catheterizable channels: a comparison of long-term results in children. Urology 2017;110:192-5. [Crossref] [PubMed]
- Szymanski KM, Whittam B, Misseri R, et al. Long-term outcomes of catheterizable continent urinary channels: What do you use, where you put it, and does it matter? J Pediatr Urol 2015;11:210.e1-7. [Crossref] [PubMed]
- Gowda BDR, Agrawal V, Harrison SCW. The continent, catheterizable abdominal conduit in adult urological practice. BJU Int 2008;102:1688-92. [Crossref] [PubMed]
- Redshaw JD, Elliott SP, Rosenstein DI, et al. Procedures needed to maintain functionality of adult continent catheterizable channels: a comparison of continent cutaneous ileal cecocystoplasty with tunneled catheterizable channels. J Urol 2014;192:821-6. [Crossref] [PubMed]
- Van der Aa F, Joniau S, De Baets K, et al. Continent catheterizable vesicostomy in an adult population: success at high costs. Neurourol Urodyn 2009;28:487-91. [Crossref] [PubMed]
- Deuker M, Roos FC, Großmann A, et al. Long-term outcome after urinary diversion using the ileocecal segment in children and adolescents: Complications of the efferent segment. J Pediatr Urol 2016;12:247.e1-7. [Crossref] [PubMed]
- Kaefer M, Tobin MS, Hendren WH, et al. Continent urinary diversion: the Children's Hospital experience. J Urol 1997;157:1394-9. [Crossref] [PubMed]
- Prieto JC, Perez-Brayfield M, Kirsch AJ, et al. The treatment of catheterizable stomal incontinence with endoscopic implantation of dextranomer/hyaluronic acid. J Urol 2006;175:709-11. [Crossref] [PubMed]
- Guys JM, Fakhro A, Haddad M, et al. Endoscopic cure of stomal leaks in continent diversion. BJU Int 2002;89:628-9. [Crossref] [PubMed]
- Halachmi S, Farhat W, Metcalfe P, et al. Efficacy of polydimethylsiloxane injection to the bladder neck and leaking diverting stoma for urinary continence. J Urol 2004;171:1287-90. [Crossref] [PubMed]
- Roth CC, Donovan BO, Tonkin JB, et al. Endoscopic injection of submucosal bulking agents for the management of incontinent catheterizable channels. J Pediatr Urol 2009;5:265-8. [Crossref] [PubMed]
- Szymanski KM, Lopez PJ, Corbetta JP, et al. Do anterior catheterizable urinary channels have fewer complications than posterior channels? An international cohort study. J Pediatr Urol 2018;14:48.e1-48.e7. [Crossref] [PubMed]
- Thomas JC, Dietrich MS, Trusler L, et al. Continent catheterizable channels and the timing of their complications. J Urol 2006;176:1816-20; discussion1820.
- Faure A, Cooksey R, Bouty A, et al. Bladder continent catheterizable conduit (the Mitrofanoff procedure): Long-term issues that should not be underestimated. J Pediatr Surg 2017;52:469-72. [Crossref] [PubMed]
- Casey JT, Zhang M, Chan KH, et al. Does endoscopy of difficult to catheterize channels spare some patients from formal open revision? J Pediatr Urol 2016;12:248.e1-6. [Crossref] [PubMed]
- Jacobson DL, Thomas JC, Pope J, et al. Update on continent catheterizable channels and the timing of their complications. J Urol 2017;197:871-6. [Crossref] [PubMed]
- Piaggio L, Myers S, Figueroa TE, et al. Influence of type of conduit and site of implantation on the outcome of continent catheterizable channels. J Pediatr Urol 2007;3:230-4. [Crossref] [PubMed]
- Chulamorkodt NN, Estrada CR, Chaviano AH. Continent urinary diversion: 10-year experience of Shriners Hospitals for Children in Chicago. J Spinal Cord Med 2004;27 Suppl 1:S84-7. [Crossref] [PubMed]
- McAndrew HF, Malone PSJ. Continent catheterizable conduits: which stoma, which conduit and which reservoir? BJU Int 2002;89:86-9. [Crossref] [PubMed]
- Narayanaswamy B, Wilcox DT, Cuckow PM, et al. The Yang-Monti ileovesicostomy: a problematic channel? BJU Int 2001;87:861-5. [Crossref] [PubMed]
- Mickelson JJ, Yerkes EB, Meyer T, et al. L stent for stomal stenosis in catheterizable channels. J Urol 2009;182:1786-91. [Crossref] [PubMed]
- Subramaniam R, Taylor C. The use of an antegrade continence enema stopper in catheterizable channels virtually eliminates the incidence of stomal stenosis: preliminary experience. J Urol 2009;181:299-301. [Crossref] [PubMed]
- Kaefer M, Retik AB. The Mitrofanoff principle in continent urinary reconstruction. Urol Clin North Am 1997;24:795-811. [Crossref] [PubMed]
- Landau EH, Gofrit ON, Cipele H, et al. Superiority of the VQZ over the tubularized skin flap and the umbilicus for continent abdominal stoma in children. J Urol 2008;180:1761-5; discussion1765-6.
- Van Savage JG, Khoury AE, McLorie GA, et al. Outcome analysis of Mitrofanoff principle applications using appendix and ureter to umbilical and lower quadrant stomal sites. J Urol 1996;156:1794-7. [Crossref] [PubMed]
- Liard A, Séguier-Lipszyc E, Mathiot A, et al. The Mitrofanoff procedure: 20 years later. J Urol 2001;165:2394-8. [Crossref] [PubMed]
- Snodgrass W. Triamcinolone to prevent stenosis in Mitrofanoff stomas. J Urol 1999;161:928. [Crossref] [PubMed]
- Reddy PP, Strine AC, Reddy T, et al. Triamcinolone injection for treatment of Mitrofanoff stomal stenosis: Optimizing results and reducing cost of care. J Pediatr Urol 2017;13:375.e1-375.e5. [Crossref] [PubMed]
- Gor RA. Outcomes of revision surgery for difficult to catheterize continent channels in a multi-institutional cohort of adults. Can Urol Assoc J 2018;12:E126-31. [Crossref]
- Lee L, Abou-Khalil M, Liberman S, et al. Incidence of incisional hernia in the specimen extraction site for laparoscopic colorectal surgery: systematic review and meta-analysis. Surg Endosc 2017;31:5083-93. [Crossref] [PubMed]