Indications for and transitioning to secondary treatment while on active surveillance for prostate cancer
Introduction
Active surveillance (AS) has been increasingly accepted over the last two decades as an option for managing men with localized, low risk prostate cancer (1). Central to the safety of AS is appropriate patient selection and careful disease monitoring to identify early signs of changing risk, or “triggers”, for further intervention with curative intent. Multiple centers have published results with AS and utilize varying monitoring strategies (2-10). In addition to different surveillance strategies, these experiences describe different clinical triggers for recommending definitive local therapy. Understanding this decision to abandon surveillance for more definitive therapy represents an important clinical challenge.
Monitoring low risk prostate cancer for early signs of disease progression
Methods to actively monitor and identify early signs of changing disease risk are central to managing any patient with AS. Although there are no standard guidelines, most published protocols recommend periodic prostate specific antigen (PSA) measurement and repeat prostate biopsy. The American Society for Clinical Oncology (ASCO) has endorsed previously issued AS monitoring guidelines described by Cancer Care Ontario (11) in Canada. This includes PSA every 3–6 months, annual digital rectal exam (DRE), 12-core prostate biopsy every 2–5 years, and may include other “investigatory” measures such as imaging and/or biomarkers. Table 1 describes surveillance strategies of contemporary North American and European AS cohorts. The role for surveillance prostate imaging with either standard ultrasound or mp-MRI remains unclear. While some experiences with stringent inclusion criteria may recommend treatment for any changes in tumor volume (including additional biopsy cores positive for cancer or increased percent core involvement) or changes in Gleason score (GS), others may recommend intervention only after change from low to intermediate risk disease.
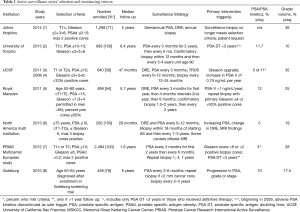
Full table
PSA kinetics in the form of PSA velocity (PSAV) or PSA doubling time (PSA DT) have been utilized and studied for disease monitoring. Much of this is based on the association between PSA kinetics and cancer specific mortality after radiation or surgery (12,13). In these studies, men at highest risk of mortality despite treatment were noted to have an increase in PSA by 2.0 ng/mL the year before diagnosis. In the series from University of Toronto with the longest published median follow up of 15 years, PSA DT of <3 years was initially used to recommend intervention. This cut off was somewhat arbitrarily selected, as it seemed to result in a clinically acceptable treatment rate. Eventually this was abandoned as strict trigger for intervention, however, as it did not correlate with pathologic or more important predictive endpoints. PSA kinetics is currently considered unreliable as a sole trigger to prompt radical treatment (14). Iremashvili et al. reviewed PSA, PSA density, PSAV and PSA DT time in a cohort of 314 men on AS with surveillance biopsy performed at regular intervals (15). PSA metrics did not predict for progression until the 4th biopsy. The authors supported use of PSA kinetics in helping to define indication for repeat biopsy in men who have had regular biopsies for at least 3–4 years. Similar to the experiences from the University of Toronto, the PRIAS trial (16) formerly employed PSA DT <3 years as indication for radical therapy, but since 2009 their protocol was amended for changes in PSA to prompt further workup, including early repeat prostate biopsy. Novel biomarkers or advanced imaging will eventually clarify the role for PSA in following men on AS and may tailor surveillance strategies and timing of tests based on PSA changes.
The greatest clinical predictor of outcome for any man with CaP is GS. Surgical series with pure GS 6 CaP show no evidence of lymph node metastases suggesting that this is the most indolent lesion (17-19). Most protocols therefore utilize confirmatory and repeat biopsy to assess for GS changes over time as the most common trigger for intervention. Biopsy tissues changes in the form of GS upstage, or increasing core number or length are the most common indicator of disease progression and serve as most frequent trigger for intervention. Concerns over the long-term risks of multiple prostate biopsies along with interest in less invasive means of surveillance have prompted ongoing studies with novel imaging techniques and biomarkers for disease progression. Additionally, serial digital rectal exam and TRUS findings may identify disease upstaging (20,21).
Molecular markers
While biomarker assays are now commercially available to assess risk beyond pure clinical features and potentially assist in patient selection for AS, investigators are also studying novel biomarkers for surveillance of men with low risk CaP over time. PSA is a serine-protease produced and released by epithelial cells of the prostate gland. It is secreted as an inactive proenzyme (proPSA) into seminal fluid and subsequently activated by multiple enzymes produced by the prostate. Serum PSA itself occurs in several different molecular forms: free PSA (fPSA, composed of several subtypes, proPSA, cleaved PSA and others) and complexed PSA (22). Multiple studies support use of the PSA isoform proPSA as a predictor of significant CaP (23,24). The Prostate Health Index (PHI) combines PSA, fPSA and proPSA and has been shown to improve detection of CaP, particularly clinically significant disease (25). Heidegger et al. evaluated a multi-institutional cohort of men who were considered candidates for AS based on clinical criteria, with proPSA and PHI and found this improved detection of more aggressive disease and therefore may help in patient selection or disease monitoring (26).
The Four-Kallikrein Panel Tissue kallikrein and kallikrein-related enzymes are a family of 15 closely related serine proteases with high homology (27). A serum biomarker test known commercially as the 4Kscore® Test (OPKO Lab, Nashville, TN) incorporates a panel of four kallikrein protein biomarkers (total PSA, free PSA, intact PSA, and human kallikrein-related peptidase 2) and other clinical information in an algorithm that provides a percent risk for presence of high-grade (GS ≥7) cancer on biopsy. Amongst men suspected of having CaP, several studies have found that these markers improve prediction of high grade cancers compared to that of established risk calculator or models using tPSA alone (28,29). The Canary Prostate Active Surveillance Study (PASS) investigators evaluated the utility of 4K panel in predicting presence of high grade CaP in men with GS 6 disease on AS. Men were enrolled as part of a prospective, multi institutional study and the authors found that the 4K panel was significant associated with reclassification at first biopsy (30).
Other biopsy pathologic findings have been investigated as potential biomarkers in men with low risk disease. Serial prostate biopsy and impact on histologic inflammatory cell infiltrate has been described previously (31). The authors concluded that repeated biopsy in an AS population did not appear to be associated with degree of inflammatory cells. Other investigators have evaluated the serum neutrophil to lymphocyte ratio as a marker of cancer-related inflammation. Gokce et al. (32) evaluated 210 prostatectomy specimens of men with clinical low risk disease who would have been candidates for AS and reported that serum neutrophil to lymphocyte ratio predicted upgrading at the time of RP as well as risk of biochemical recurrence after treatment.
Novel imaging
As previously discussed, there are many limitations to standard TRUS for monitoring men on AS and outside of very select centers (20,21) has limited value (33). The utility of multi-parametric MRI (mMRI) in the diagnosis and staging of CaP is rapidly expanding. Accurate identification of those with low risk disease as opposed to clinically significant disease at the time of diagnosis is key to the success and safety of surveillance as a viable treatment strategy. In a study by Ahmed et al. (34), results from the Prostate MRI Imaging Study (PROMIS) trial showed that mMRI when used as a screening tool in men with elevated PSA was more sensitive that TRUS biopsy for detection of clinically significant CaP. Multiparametric MRI demonstrated 88% sensitively (45% specificity) in detection of GS ≥ 3+4 disease.
As mMRI has been shown to primarily identify clinically significant CaP, this is an attractive potential, less invasive modality to follow patients enrolled in AS. In addition, mMRI/US fusion technology has facilitated target lesion biopsy to reduce sampling errors inherent with standard template prostate biopsy. Mullins et al. (35) retrospectively reviewed MRI findings of men on AS and compared with TRUS guided biopsy, and found that men with suspicious MRI lesions were more likely to be reclassified over time. Guo et al. (36) performed a meta-analysis on 7 studies from 2010–2013, studying the diagnostic accuracy of MRI on disease re-classification amongst AS candidates. They found a relatively low positive likelihood ratio of 3.1, high negative likelihood ratio (0.4), along with poor sensitively (0.69) and specificity (0.78). The authors questioned whether the evidence supports use of mMRI for disease reclassification.
Serial or surveillance mMRI is attractive as a less invasive means to monitor men over time, however has not been formally validated in AS cohorts. In a single AS series which included men meeting strict inclusion criteria (≤ T1c, GS ≤6, PSA density ≤0.15, no more than 2 cores or 50% disease in single core), 58 men were followed for 16 months (median) with mMRI and mMRI/US fusion biopsy (37). The authors found that one third (17/58) of men experienced evidence of disease progression on mMRI. Fifty-three percent of these men (9/17) demonstrated GS progression (3+3 to 3+4), resulting in predictive values of 53% and 80%, respectively (37). Habibian et al. (38) sought to describe mMRI characteristics of prostate cancers in patients who discontinue AS – specifically for concerns over tumor upgrading. Of 114 men on AS who had mMRI at enrollment and subsequent follow up, 14 (12.3%) discontinued surveillance due to concerning changes seen on MRI including extracapsular extension, new suspicious lesions or increasing size of a known lesion. Re-biopsy of these men found that nearly half had tumor upgrading. Felker et al. (39) described 49 men on AS with GS 6 disease who had mMRI on enrollment and again at 6 months of follow up. Overall, GS progression occurred in 39% of cohort. Ten men experienced MRI progression, 70% (7/10%) of which demonstrated pathological progression, yielding 90% specificity, 37% sensitivity for mMRI (39). Frye et al. (40) followed a cohort of men on AS, including those with 2 or more MRI-fusion guided biopsies (N=166). Targeted biopsy identified 44.9% of patients with progression as compared to 30.6% of men with systematic 12-core biopsy. Progression on mMRI was the sole predictor of pathologic progression during surveillance (P=0.013).
Multiparametric MRI may not be accessible in all centers and in has cost effectiveness implications that remain unanswered. Serial transrectal ultrasound (TRUS) findings in men enrolled in AS have been investigated. Investigators from the University of California San Francisco (20) evaluated the incidence, growth dynamics and clinical significant of changes in prostate lesions of men enrolled in their AS program. They were able to identify 39% of men with progression by TRUS findings including size, number of lesions and stage. TRUS progression was independently associated with biopsy progression. Additionally, investigators from the University of Southern California (21) found that within their AS population over an 11-year period, significant TRUS findings such as blood flow as measures by a Doppler grading scale were associated with pathological progression.
Intervention without clinical progression
Some degree of attrition in AS cohorts, unprompted by any clinical changes, is expected. A 2017 review (41) of prospective trials of AS for low risk CaP reported overall 5- and 10-year treatment free survival rates ranging from 48–76% and 27–63%. Several trials originated with stringent entry criteria, which partly explain such variability in the treatment free survival rates. In addition to eligibility criteria, follow-up strategies, and thresholds for intervention also contributed to decision for radical treatment (41). Sociodemographic factors including race, age, education level and comorbidities have been found to be associated with AS discontinuation (42-44). Kelly et al. (42) found that black men were more likely to switch to active treatment, which has been described in prior studies (44). Additionally, the authors found black men were less likely to undergo serial re-biopsy perhaps explaining the higher rates of eventual treatment. Loeb et al. (43) examined 5-year outcomes of men enrolled in National Prostate Cancer Register of Sweden. After 5 years, about two thirds of men remained on surveillance. Predictors of discontinuation were younger age, less comorbidity, and more education. One fifth of men discontinued due to “patient preference”.
Conclusions
The oncologic safety of AS for appropriately selected men with CaP is well supported by early and intermediate outcomes described by large centers. With promising survival outcomes as well as avoided morbidity of radical treatment, this strategy should be offered to men with low risk disease. Key to the success of surveillance is accurate and timely monitoring for cancer progression. While traditionally this included PSA changes or DRE findings, the ever-growing number of available biologic molecular markers, is now revealing potentially greater ability to detect clinically significant disease. Additionally, the emergence of advanced MRI technology has shown improved detection of high-grade cancer in AS populations. Despite these advances, we face an ongoing dilemma as to how best to incorporate these novel technologies into a feasible, cost effective and efficacious monitoring strategy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990-2013. JAMA 2015;314:80-2. [Crossref] [PubMed]
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272-7. [Crossref] [PubMed]
- Dall'Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer 2008;112:2664-70. [Crossref] [PubMed]
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol 2015;33:3379-85. [Crossref] [PubMed]
- Soloway MS, Soloway CT, Eldefrawy A, et al. Careful selection and close monitoring of low-risk prostate cancer patients on active surveillance minimizes the need for treatment. Eur Urol 2010;58:831-5. [Crossref] [PubMed]
- Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013;63:597-603. [Crossref] [PubMed]
- Kakehi Y, Kamoto T, Shiraishi T, et al. Prospective evaluation of selection criteria for active surveillance in Japanese patients with stage T1cN0M0 prostate cancer. Jpn J Clin Oncol 2008;38:122-8. [Crossref] [PubMed]
- Godtman RA, Holmberg E, Khatami A, et al. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Goteborg randomised population-based prostate cancer screening trial. Eur Urol 2013;63:101-7. [Crossref] [PubMed]
- Roemeling S, Roobol MJ, de Vries SH, et al. Active surveillance for prostate cancers detected in three subsequent rounds of a screening trial: characteristics, PSA doubling times, and outcome. Eur Urol 2007;51:1244-50; discussion 1251. [Crossref] [PubMed]
- Selvadurai ED, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol 2013;64:981-7. [Crossref] [PubMed]
- Chen RC, Rumble RB, Loblaw DA, et al. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol 2016;34:2182-90. [Crossref] [PubMed]
- D'Amico AV, Chen MH, Roehl KA, et al. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med 2004;351:125-35. [Crossref] [PubMed]
- D'Amico AV, Renshaw AA, Sussman B, et al. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. JAMA 2005;294:440-7. [Crossref] [PubMed]
- Ross AE, Loeb S, Landis P, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol 2010;28:2810-6. [Crossref] [PubMed]
- Iremashvili V, Kava BR, Manoharan M, et al. Is It Time to Revisit the Role of Prostate-specific Antigen Kinetics in Active Surveillance for Prostate Cancer? Urology 2016;95:139-44. [Crossref] [PubMed]
- Bokhorst LP, Valdagni R, Rannikko A, et al. A Decade of Active Surveillance in the PRIAS Study: An Update and Evaluation of the Criteria Used to Recommend a Switch to Active Treatment. Eur Urol 2016;70:954-60. [Crossref] [PubMed]
- Ross HM, Kryvenko ON, Cowan JE, et al. Do adenocarcinomas of the prostate with Gleason score (GS) ≤6 have the potential to metastasize to lymph nodes? Am J Surg Pathol 2012;36:1346-52. [Crossref] [PubMed]
- Donin NM, Laze J, Zhou M, et al. Gleason 6 prostate tumors diagnosed in the PSA era do not demonstrate the capacity for metastatic spread at the time of radical prostatectomy. Urology 2013;82:148-52. [Crossref] [PubMed]
- Wenger H, Weiner AB, Razmaria A, et al. Risk of lymph node metastases in pathological gleason score ≤6 prostate adenocarcinoma: Analysis of institutional and population-based databases. Urol Oncol 2017;35:31.e1-6. [Crossref] [PubMed]
- Eltemamy MM, Leapman MS, Cowan JE, et al. Serial Anatomical Prostate Ultrasound during Prostate Cancer Active Surveillance. J Urol 2016;196:727-33. [Crossref] [PubMed]
- Shoji S, Ukimura O, de Castro Abreu AL, et al. Image-based monitoring of targeted biopsy-proven prostate cancer on active surveillance: 11-year experience. World J Urol 2016;34:221-7. [Crossref] [PubMed]
- Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 1987;317:909-16. [Crossref] [PubMed]
- Hori S, Blanchet JS, McLoughlin J. From prostate-specific antigen (PSA) to precursor PSA (proPSA) isoforms: a review of the emerging role of proPSAs in the detection and management of early prostate cancer. BJU Int 2013;112:717-28. [Crossref] [PubMed]
- Sokoll LJ, Sanda MG, Feng Z, et al. A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [-2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness. Cancer Epidemiol Biomarkers Prev 2010;19:1193-200. [Crossref] [PubMed]
- Loeb S, Catalona WJ. The Prostate Health Index: a new test for the detection of prostate cancer. Ther Adv Urol 2014;6:74-7. [Crossref] [PubMed]
- Heidegger I, Klocker H, Pichler R, et al. ProPSA and the Prostate Health Index as predictive markers for aggressiveness in low-risk prostate cancer-results from an international multicenter study. Prostate Cancer Prostatic Dis 2017;20:271-5. [Crossref] [PubMed]
- Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer 2008;8:268-78. [Crossref] [PubMed]
- Parekh DJ, Punnen S, Sjoberg DD, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol 2015;68:464-70. [Crossref] [PubMed]
- Bryant RJ, Sjoberg DD, Vickers AJ, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst 2015.107. [PubMed]
- Lin DW, Newcomb LF, Brown MD, et al. Evaluating the Four Kallikrein Panel of the 4Kscore for Prediction of High-grade Prostate Cancer in Men in the Canary Prostate Active Surveillance Study. Eur Urol 2017;72:448-54. [Crossref] [PubMed]
- Glass AS, Porten SP, Bonham M, et al. Active surveillance: does serial prostate biopsy increase histological inflammation? Prostate Cancer Prostatic Dis 2013;16:165-9. [Crossref] [PubMed]
- Gokce MI, Tangal S, Hamidi N, et al. Role of neutrophil-to-lymphocyte ratio in prediction of Gleason score upgrading and disease upstaging in low-risk prostate cancer patients eligible for active surveillance. Can Urol Assoc J 2016;10:E383-7. [Crossref] [PubMed]
- Hruby G, Choo R, Klotz L, et al. The role of serial transrectal ultrasonography in a 'watchful waiting' protocol for men with localized prostate cancer. BJU Int 2001;87:643-7. [Crossref] [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Mullins JK, Bonekamp D, Landis P, et al. Multiparametric magnetic resonance imaging findings in men with low-risk prostate cancer followed using active surveillance. BJU Int 2013;111:1037-45. [Crossref] [PubMed]
- Guo R, Cai L, Fan Y, et al. Magnetic resonance imaging on disease reclassification among active surveillance candidates with low-risk prostate cancer: a diagnostic meta-analysis. Prostate Cancer Prostatic Dis 2015;18:221-8. [Crossref] [PubMed]
- Walton Diaz A, Shakir NA, George AK, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol 2015;33:202.e1-7. [Crossref] [PubMed]
- Habibian DJ, Liu CC, Dao A, et al. Imaging Characteristics of Prostate Cancer Patients Who Discontinued Active Surveillance on 3-T Multiparametric Prostate MRI. AJR Am J Roentgenol 2017;208:564-9. [Crossref] [PubMed]
- Felker ER, Wu J, Natarajan S, et al. Serial Magnetic Resonance Imaging in Active Surveillance of Prostate Cancer: Incremental Value. J Urol 2016;195:1421-7. [Crossref] [PubMed]
- Frye TP, George AK, Kilchevsky A, et al. Magnetic Resonance Imaging-Transrectal Ultrasound Guided Fusion Biopsy to Detect Progression in Patients with Existing Lesions on Active Surveillance for Low and Intermediate Risk Prostate Cancer. J Urol 2017;197:640-6. [Crossref] [PubMed]
- Moschini M, Carroll PR, Eggener SE, et al. Low-risk Prostate Cancer: Identification, Management, and Outcomes. Eur Urol 2017;72:238-49. [Crossref] [PubMed]
- Kelly SP, Van Den Eeden SK, Hoffman RM, et al. Sociodemographic and Clinical Predictors of Switching to Active Treatment among a Large, Ethnically Diverse Cohort of Men with Low Risk Prostate Cancer on Observational Management. J Urol 2016;196:734-40. [Crossref] [PubMed]
- Loeb S, Folkvaljon Y, Makarov DV, et al. Five-year nationwide follow-up study of active surveillance for prostate cancer. Eur Urol 2015;67:233-8. [Crossref] [PubMed]
- Abern MR, Bassett MR, Tsivian M, et al. Race is associated with discontinuation of active surveillance of low-risk prostate cancer: results from the Duke Prostate Center. Prostate Cancer Prostatic Dis 2013;16:85-90. [Crossref] [PubMed]


