Clinical significance of subtypes of Gleason pattern 4 prostate cancer
Introduction
In 1966, Donald Gleason, developed a grading scheme for prostatic cancer. His system was a major departure from prior classifications, as he used histologic architectural patterns rather than cytology for assigning the grade. The classification was developed using biopsies, transurethral resections, and radical prostatectomy (RP) specimens from 270 patients. While reviewing these cases, Dr. Gleason realized that most of the tumors had more than one architectural pattern, and he decided to assign two patterns to each case in the order of dominance (1). Subsequently, he evaluated the prognosis of 1,032 prostate cancer patients and concluded that his grading system predicted clinical course (2). Since then, the system has received a worldwide acceptance and is referred to as the Gleason grading system (3).
Although overall the original Gleason grading system still retains great clinical relevance, many problems with this system were discovered over the years. Gleason pattern (GP) 1 and 2 cancers became in disuse. Reporting the two most common patterns both for biopsies and RPs, ignored the presence of a minor component of higher grade. In addition, the original Gleason grading system did not provide guidelines on reporting multiple cores involved by cancer from different sites or on reporting different tumor nodules in RPs. Finally, additional architectural patterns were described after the Gleason era and there was a need to incorporate them into the grading system (4,5).
To address these issues, the International Society of Urological Pathology (ISUP) held two consensus conferences, one in 2005 and a second in 2014. The first conference was attended only by pathologists, but the 2014 conference included experts in urology, oncology, radiation oncology and pathology from 17 countries (6,7). In the 2005 conference the group recommended that any percent of high grade tumor should be mentioned in the biopsy report, and that all large and irregular cribriform glands should be considered as GP 4 (6). In the following years, multiple studies showed that cribriform glands predict poor outcome, concluding that all cribriform glands should be graded as GP 4 (8). Another study found that glomeruloid structures on biopsies are associated with additional higher-grade patterns and concluded that they represent an early stage of cribriform glands and should be graded as pattern 4 (9). In 2013, Pierorazio et al. published a study that evaluated over 7,000 prostate cancer patients graded using the contemporary grading guidelines and concluded that prostate cancer should be divided in five grade groups (GGs) which correlates best with clinical outcomes (10). These findings were later validated in a multi institutional study including five large clinical centers (11). All of these updates were discussed in the 2014 ISUP meeting and the following recommendations were made: (I) all cribriform glands and glomeruloid structures should be considered as pattern 4; (II) the percentage of GP 4 should be reported in cases with Gleason score (GS) 7; (III) the new prognostic GG system should be reported along with the GS in all cases (7).
While many of the problems of the initial Gleason grading system have been addressed over the years, major issues persist including the lack of interobserver reproducibility and the heterogeneity of architectural variants within GP 4. Poorly formed glands, fused glands, glomeruloid structures, and cribriform glands are all considered GP 4 within the current grading system. A growing body of evidence suggests that these architectural variants might have different clinical significance. Herein, we review recently published studies on the clinical significance of the different architectural subtypes of GP 4 prostate cancer.
Methods
A review of the current literature was performed using PubMed with the terms “Gleason pattern 4 and cribriform”, Gleason pattern 4 and poorly formed glands”, “Gleason pattern 4 and fused glands”, and “Gleason pattern 4 and glomeruloid.” Inclusion criteria included: (I) studies published after the issuing of the 2005 ISUP recommendation were reviewed given the significant changes in interpretation of the different morphologies; (II) studies that evaluated the different subtypes of GP 4 and parameters of clinical outcomes; (III) research studies only. Exclusion criteria included: (I) studies without pathology review, (II) studies in languages other than English. A total of 68 studies were reviewed and 16 met all inclusion and exclusion criteria. A summary of the methods outlining literature search is presented in Figure 1.
Poorly formed glands
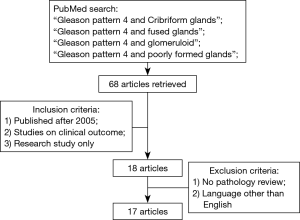
Poorly formed glands include glands with no or rare lumens, elongated compressed glands, and elongated nests. There are no definitive criteria to determine when a small focus of poorly formed glands constitutes GP 4. Some authors suggest that poorly formed glands immediately adjacent to well-formed glands should not be diagnosed as GP 4 regardless of their number. Neither should small foci of up to 5 poorly formed glands regardless of their location (12). Of all morphologic variants of GP 4, poorly formed glands have the highest interobserver variability or poorest reproducibility. Dong et al. reported in a study on 755 RPs that interobserver agreement in assigning GP 4 ranged between 78% and 83% (13). This problem is mainly encountered in biopsies where there is a small focus of poorly formed glands which one might grade as GP 3 while another pathologist might grade as a small component of GP 4. A study by Mckenney et al. compared the diagnoses made by 11 urologic pathologists from seven institutions on a set of prostate cancer biopsies and found that although the overall diagnostic reproducibility was good, the interobserver variability occurred primarily when distinguishing between tangentially sectioned GP 3 glands and the poorly formed glands of GP 4 (14). The ISUP addressed this issue in its 2014 meeting and recommended that the diagnosis of GP 4 should be appreciated at 10× magnification; that occasional/seemingly poorly formed or fused glands between well-formed glands are insufficient for a diagnosis of GP 4; and in borderline cases between GP 4 and GP 3, the latter should be favored (7,15). Figure 2 illustrates an example of GP 4 prostate cancer with poorly formed glands.
Glomeruloid structures
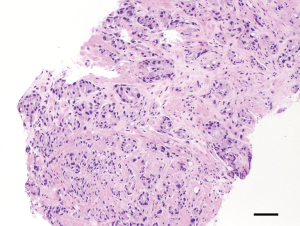
Glomeruloid structures are defined as dilated glands containing intraluminal cribriform structures with a single point of attachment, resembling a renal glomerulus (9). They were first described by Pacelli et al. 20 years ago (5). In 2009, Lotan et al. reported that glomerulations are associated with concurrent GP 4 or higher-grade carcinoma. In that study, the investigators showed that there were transitions between small glomerulations, large glomeruloid structures, and cribriform GP 4 cancer. Their data suggested that glomerulations represent an early stage of cribriform pattern 4 cancer (9).
Overall glomeruloid structures show good interobserver reproducibility and are easily distinguished from GP 3 and GP 5. Choy et al. reported that glomeruloid structures were twice more common when combined with other GP 4 architectures (15). One interesting finding in this study was that glomeruloid structures as the sole GP 4 architecture was only encountered in GS 7 cancers with lesser GP 4 (i.e., 3+4), concluding that glomeruloid morphology represents an early-stage GP 4 (15). The ISUP 2014 meeting recommended that all glomeruloid structures should be reported as GP 4 (7). Figure 3 illustrates prostate cancer glands with glomeruloid structures.
Cribriform glands
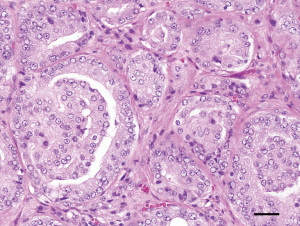
Cribriform glands are defined as a proliferation with multiple punched-out lumina, without intervening stroma (16). The ISUP recommended in the 2005 consensus meeting that cribriform glands in general should be graded as GP 4 but allowed small round cribriform glands to be graded as GP 3 (6). Later, many studies showed that cribriform glands, regardless of the size, correlated best with GP 4. Consequently, in 2014, the ISUP recommended that all cribriform glands, without exceptions, be graded as GP 4 (7). Cribriform glands have excellent interobserver reproducibility when distinguishing them from GP 3 and GP 5. However, distinguishing cribriform glands from intraductal carcinoma (IDC) of prostate can be challenging. IDC of prostate is characterized by the growing of malignant cells into the lumens of prostate ducts and acini. One of the diagnostic criteria for its diagnosis is solid cribriform growth with more than 70% of epithelial component in a gland surrounded by basal cells (17). While this differential diagnosis often requires the use of immunohistochemistry to highlight the presence of basal cells, the clinical utility of this distinction is debatable because IDC, in the great majority of cases, co-exits with higher grade prostate cancer (18). Figure 4 illustrates an example of prostate cancer with cribriform glands.
Fused glands
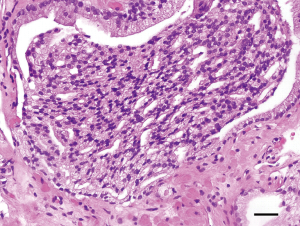
Fused glands are composed of a group of glands that are no longer completely separated by stroma. The edge of a group of fused glands is scalloped and there are occasional thin strands of connective tissue within this group of glands (19). There are no definitive criteria to determine when a small focus of fused glands constitutes GP 4. In a study where 23 genitourinary pathologists that assessed the interobserver reproducibility for grading prostate cancer with GP 4 cases, consensus on fused glands was reached only in 1 case (2%) (20). Similar findings were reported by Egevad et al. who; in a couple of studies; looked at the interobserver reproducibility of GP4 among 15 and 337 pathologists respectively (21,22). One of these studies concluded that the percentage of fused and ill-formed glands inversely correlated with agreement among pathologists (21). The problem arises from the fact that there are no clear criteria on when a small focus of fused glands qualifies for GP 4. The ISUP addressed this issue in the 2014 meeting and recommended that the diagnosis of GP 4 should be appreciated at 10x magnification; that occasional, seemingly poorly-formed or fused glands between well-formed glands are insufficient for a diagnosis of GP 4; and in borderline cases between GP 4 and GP 3, the latter should be favored (7,15). Figure 5 illustrates an example of prostate cancer with fused glands graded as GP 4.
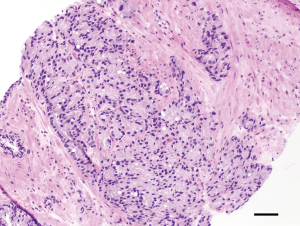
Clinical significance of subtypes GP 4
The clinical outcome of GS 7 prostate cancer is highly variable. Improvement in risk stratification might be of great clinical importance especially to identify patients with GS 7 cancer who might have a more favorable prognosis and be considered for active surveillance protocols (23). There is some evidence in the literature that suggests that discrimination of the different GP 4 morphologies could help stratify patients with different clinical prognoses (18,24,25).
Among all morphologic patterns of GP 4, the presence of cribriform glands appears to be associated with the worst clinical course. Choy et al. found that the presence of cribriform glands was associated with decreased 5-year biochemical recurrence (BCR)-free survival when compared with GS 7 cancers without this architecture. In this study, cribriform was the most prevalent architecture and was more frequent in GS 4+3 than 3+4 tumors (66.7% vs. 38.7%). The endpoint of the study was time to BCR in patients with GS 7 disease including both GS 4+3 and 3+4. The study showed that there was heterogeneity in terms of prognosis within GS 7 by architecture, with cribriform having the strongest positive association with BCR. This same study also reported that prostate cancer with glomeruloid architecture was associated with improved 5-year BCR-free survival when compared with GS 7 cancers without this architecture. Therefore, the investigators concluded that the distinction should be made between glomeruloid and cribriform glands, despite glomeruloid glands being considered an early stage of cribriform glands (15). Kweldam et al. showed similar findings in a study comparing control cases (RP) with prostate cancer metastasis, and found glomeruloid architecture to be present in 9/52 (17%) of metastasis compared to 31/109 (28%) of controls, a finding that was not statistically significant. In the same study cribriform glands were present in 42/52 (81%) of metastasis compared to 41/109 (38%) of controls, with a P value of 0.001. This leads the authors to conclude that cribriform growth in GP 4 is a strong prognostic marker for distant metastasis (16). In the same study, the investigator studied the predictive value of cribriform pattern separately in GS 3+4=7 (n=115) and GS 4+3=7 (n=46). In GS 3+4=7, cribriform pattern was an independent predictor for both distant metastasis-free survival and disease-specific survival in multivariable analysis (16). When evaluating distant metastasis-free survival for GS 4+3=7, no model could fit due to the limited number of events and number of covariates. However, cribriform pattern was an independent predictor for disease-specific survival in GS 4+3=7 (16).
The relative importance of cribriform glands within GP 4 was also emphasized in a study by Kweldam et al. that found that patients with GS 7 who lacked large cribriform glands and IDC had an overall good clinical outcome similar to patients with GS 6 (23). In this study, separating each GS group for Cribriform/IDC status, the disease specific-survival probabilities were significantly lower in Cribriform/IDC+ patients within each GS 3+4=7, 8, and 9–10 (23). In the same study, although there was some evidence of lower survival probabilities in Cribriform/IDC+ GS 4+3=7, the differences between groups were not statistically significant (P=0.054) (23). Furthermore, another study found that cribriform patterns have a recurrence free survival (RFS) that is worse than that of “poorly formed glands” (25,26). Other studies have also found that the presence of cribriform pattern is an independent predictor of BCR and metastasis in GS 7 (3+4 and 4+3) tumors after RP (13,15). A study by Dong et al., using a Cox regression model taking into account the GS, architectural pattern, preoperative PSA, patient age, pathologic stage, surgical margin, and prostate weight, found that the presence of cribriform architecture and preoperative PSA were independent predictors of biochemical failure after RP (P=0.003) (13). Using another Cox regression model only taking into account the GS and architectural pattern, the study found that the presence of cribriform architecture predicted biochemical failure independently of the primary Gleason grade (P=0.01) (13). In a third Cox regression model taking into account the GS, architectural pattern, preoperative PSA, patient age, pathologic stage, surgical margin, and prostate weight, the only independent predictors of metastasis after RP were cribriform architecture and GS (P=0.02) (13).
A study based on RP findings reported that cribriform pattern is a major predictive factor for distant metastasis and disease-specific death (16). In this study, cribriform morphology was the strongest predictor for adverse clinical events in multivariate analysis, where they adjusted for both established clinicopathologic parameters (age, PSA, GS, pathologic tumor stage, and surgical margins) and for contemporary additional pathologic parameters such as IDC and tertiary GP 5. Another study found an association between cribriform glands at RP and rate of metastasis (27). Finally, similar results were seen in a study on “latent” prostatic adenocarcinomas in cystoprostatectomy and autopsy. This study examined 320 autopsy specimens and 248 cystoprostatectomy specimens and found that cribriform architecture was significantly associated with increased tumor volume (P<0.001) and extraprostatic extension (EPE) (P=0.003). Small fused glands had a strong negative association with EPE in the autopsy series (P=0.015) (28).
Overall, the results of the studies presented here suggest that prostate cancer with cribriform morphology bears a worse prognosis and supports a move towards specifically reporting its presence in the surgical pathology report.
Conclusions
Histologic grading of prostate cancer remains an important prognostic factor. Recently published studies suggest that the optimal way to report GP 4 should include a description of the morphologic patterns, especially the presence of cribriform glands. Poorly formed glands and fused glands have the poorest reproducibility among pathologists. Further research is needed to improve interobserver reproducibility and to better establish the clinical significance of the different subtypes of GP 4.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep 1966;50:125-8. [PubMed]
- Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64. [Crossref] [PubMed]
- Matoso A, Epstein JI. Grading of Prostate Cancer: Past, Present, and Future. Curr Urol Rep 2016;17:25. [Crossref] [PubMed]
- Epstein JI. Prostatic ductal adenocarcinoma: a mini review. Med Princ Pract 2010;19:82-5. [Crossref] [PubMed]
- Pacelli A, Lopez-Beltram A, Egan AJ, et al. Prostatic adenocarcinoma with glomeruloid features. Hum Pathol 1998;29:543-6. [Crossref] [PubMed]
- Epstein JI, Allsbrook WC Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2005;29:1228-42. [Crossref] [PubMed]
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 2016;40:244-52. [PubMed]
- Epstein JI. An update of the Gleason grading system. J Urol 2010;183:433-40. [Crossref] [PubMed]
- Lotan TL, Epstein JI. Gleason grading of prostatic adenocarcinoma with glomeruloid features on needle biopsy. Hum Pathol 2009;40:471-7. [Crossref] [PubMed]
- Pierorazio PM, Walsh PC, Partin AW, et al. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 2013;111:753-60. [Crossref] [PubMed]
- Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol 2016;69:428-35. [Crossref] [PubMed]
- Zhou M, Li J, Cheng L, et al. Diagnosis of "Poorly Formed Glands" Gleason Pattern 4 Prostatic Adenocarcinoma on Needle Biopsy: An Interobserver Reproducibility Study Among Urologic Pathologists With Recommendations. Am J Surg Pathol 2015;39:1331-9. [Crossref] [PubMed]
- Dong F, Yang P, Wang C, et al. Architectural heterogeneity and cribriform pattern predict adverse clinical outcome for Gleason grade 4 prostatic adenocarcinoma. Am J Surg Pathol 2013;37:1855-61. [Crossref] [PubMed]
- McKenney JK, Simko J, Bonham M, et al. The potential impact of reproducibility of Gleason grading in men with early stage prostate cancer managed by active surveillance: a multi-institutional study. J Urol 2011;186:465-9. [Crossref] [PubMed]
- Choy B, Pearce SM, Anderson BB, et al. Prognostic Significance of Percentage and Architectural Types of Contemporary Gleason Pattern 4 Prostate Cancer in Radical Prostatectomy. Am J Surg Pathol 2016;40:1400-6. [Crossref] [PubMed]
- Kweldam CF, Wildhagen MF, Steyerberg EW, et al. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod Pathol 2015;28:457-64. [Crossref] [PubMed]
- Xu W, Zhou M. A concise update on prostate pathology. Cesk Patol 2014;50:120-8. [PubMed]
- Trudel D, Downes MR, Sykes J, et al. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur J Cancer 2014;50:1610-6. [Crossref] [PubMed]
- Montironi R, Mazzuccheli R, Scarpelli M, et al. Gleason grading of prostate cancer in needle biopsies or radical prostatectomy specimens: contemporary approach, current clinical significance and sources of pathology discrepancies. BJU Int 2005;95:1146-52. [Crossref] [PubMed]
- Kweldam CF, Nieboer D, Algaba F, et al. Gleason grade 4 prostate adenocarcinoma patterns: an interobserver agreement study among genitourinary pathologists. Histopathology 2016;69:441-9. [Crossref] [PubMed]
- Egevad L, Ahmad AS, Algaba F, et al. Standardization of Gleason grading among 337 European pathologists. Histopathology 2013;62:247-56. [Crossref] [PubMed]
- Egevad L, Algaba F, Berney DM, et al. Interactive digital slides with heat maps: a novel method to improve the reproducibility of Gleason grading. Virchows Arch 2011;459:175-82. [Crossref] [PubMed]
- Kweldam CF, Kummerlin IP, Nieboer D, et al. Disease-specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsy. Mod Pathol 2016;29:630-6. [Crossref] [PubMed]
- Delahunt B, Lamb DS, Srigley JR, et al. Gleason scoring: a comparison of classical and modified (international society of urological pathology) criteria using nadir PSA as a clinical end point. Pathology 2010;42:339-43. [Crossref] [PubMed]
- McKenney JK, Wei W, Hawley S, et al. Histologic Grading of Prostatic Adenocarcinoma Can Be Further Optimized: Analysis of the Relative Prognostic Strength of Individual Architectural Patterns in 1275 Patients From the Canary Retrospective Cohort. Am J Surg Pathol 2016;40:1439-56. [Crossref] [PubMed]
- Iczkowski KA, Torkko KC, Kotnis GR, et al. Digital quantification of five high-grade prostate cancer patterns, including the cribriform pattern, and their association with adverse outcome. Am J Clin Pathol 2011;136:98-107. [Crossref] [PubMed]
- Ross HM, Kryvenko ON, Cowan JE, et al. Do adenocarcinomas of the prostate with Gleason score (GS)</=6 have the potential to metastasize to lymph nodes? Am J Surg Pathol 2012;36:1346-52. [Crossref] [PubMed]
- Siadat F, Sykes J, Ziotta AR, et al. Not all gleason pattern 4 prostate cancers are created equal: A study of latent prostatic carcinomas in cystoprostatectomy and autopsy series. Prostate 2015;75:1277-84. [Crossref] [PubMed]