Multivariable risk-based patient selection for prostate biopsy in a primary health care setting: referral rate and biopsy results from a urology outpatient clinic
Introduction
Unnecessary testing, overdiagnosis and treatment with accompanying health care costs preclude that prostate-specific antigen (PSA)-based prostate cancer (PCa) screening can be adopted as a public health policy (1,2). The delicate benefit-harm ratio of population-based screening is, however, difficult to translate to the individual patient (3). Therefore, guidelines recommend individualized opportunistic PCa screening along with shared informed decision-making, taking into account the individual potential advantage and damage related to PSA testing (4).
Risk calculators for the prediction of a positive prostate biopsy have been developed to support physicians in this informed decision-making, and to reduce the number of unnecessary biopsies by better identification of those men at risk of PCa (5). The European Randomized Study of Screening for Prostate Cancer (ERSPC)-based Rotterdam Prostate Cancer Risk Calculator (RPCRC), for instance, reduces the percentage of unnecessary, potentially harmful, and costly transrectal ultrasound systematic biopsy (TRUS-Bx) by ±33% when using PSA level ≥3.0 ng/mL and RPCRC risk ≥12.5% as cut-off values in the urology outpatient clinic (6-11). When adopting this strategy, only a small amount of potentially aggressive PCa would be missed.
The guidelines for Dutch general practitioners (GPs) lowered the PSA cut-off value for referral to the urologist from 4.0 to 3.0 ng/mL, under the condition that the urologist uses the RPCRC for patient selection for biopsy (12). This policy results in an increased number of referrals to secondary care. This seems controversial, considering the current demand of the Dutch government to reduce health care costs by keeping more care in the primary care. However, to adhere to government’s request, introduction of the RPCRC into the primary care setting could potentially result in further optimization of the diagnostic pathway by reducing unnecessary referrals to secondary care and, thereby reducing the number of biopsies, costs and workload. The implementation of PCa diagnostic risk models, like the RPCRC, based on PSA, digital rectal examination (DRE) and prostate volume on TRUS and their impact on patient selection in primary care have never been investigated.
As such, the aim of this study was to assess the rate of men referred to the urologist with a PSA level ≥3.0 ng/mL by implementing multivariable risk-stratification with the RPCRC in a primary health care setting. In addition, we assessed adherence of GPs to the RPCRC risk prediction results and of those men biopsied we assessed the PCa detection rates and clinical characteristics.
Methods
Study design and population
In January 2014, this prospective observational study was initiated by the Erasmus MC in collaboration with the primary health care facility of the GP laboratory in Rotterdam (STAR-SHL). GPs were given the possibility of referring men with a suspicion of PCa or a screening wish to this primary care facility. Patients were then offered a so-called ‘prostate consultation’. Inclusion criteria for study participation were prostate biopsy naïve or previously negative biopsied men of 18 years or older of all ethnic backgrounds who were referred by their GP for a prostate consultation and had sufficient understanding of the Dutch language. Men with previously diagnosed PCa were excluded. All men signed informed consent before enrolment. The study was approved by our institutional review board (METC Erasmus MC, number: MEC-2013-572) and conformed to the provisions of the Declaration of Helsinki.
Procedures and data collection
Patients were offered an assessment including DRE and TRUS, regardless of their PSA level, at the primary care facility. DRE was carried out to estimate the prostate volume and search for abnormalities of the prostate. TRUS of the prostate was performed to measure the prostate volume and presence of hypo-echogenic lesions. Study participation included prospective registration of these data in an anonymized database; no additional investigations were done. In case a man did not give his informed consent for data collection, he could still undergo all the examinations and the risk calculation, however, without any data registration for research purposes.
All described examinations are considered routine clinical practice in a urology outpatient clinic to evaluate lower urinary tract symptoms (LUTS) or PCa. The examinations were performed by specially trained Erasmus MC personnel from the department of Urology. With the collected data, the risk of finding any PCa and potentially aggressive PCa in case of performing a prostate biopsy was calculated using the RPCRC calculators 3 or 4 (http://www.prostatecancer-riskcalculator.com/). Based on the outcome of the RPCRC, recommendations on referral to the urologist were formulated as follows:
- Risk of positive prostate biopsy <12.5%: no biopsy;
- Risk of positive prostate biopsy 12.5–20%: consider a biopsy, depending on the comorbidity of the patient and on the risk of a high grade or extended PCa (>4%);
- Risk of positive prostate biopsy >20%: prostate biopsy.
The findings, the calculated risk of finding PCa and the risk-based advice on referral to the urologist were reported to the GP using the electronic patient chart. He or she subsequently decided whether or not to refer the patient to an urologist for prostate biopsy. If requested, advice on the treatment of LUTS was provided.
The calculated risks and the associated advice were matched with information on actual referral and biopsy rates. Biopsy outcome if applicable was also assessed. In case of a PCa diagnosis initial treatment was recorded together with available follow-up (FU) data. All information was retrieved through direct contact with the different GP practices. GPs were initially not aware of the fact that they would be contacted to provide FU information.
Outcomes
The primary outcome of this study was to assess the rate of men with a PSA level ≥3.0 ng/mL considered at high-risk (i.e., having an elevated calculated PCa risk) on the basis of the RPCRC. The secondary outcomes were the compliance rate of GPs and patients to the RPCRC based advice, the rate of detected (clinically significant) PCa in the urology outpatient clinic and the rate of missed PCa within the available FU time. Clinically significant PCa (csPCa) was defined as any Gleason score (GS) ≥3+4 PCa found in biopsy specimens.
Statistical analysis
Demographic characteristics are presented for the overall group of men. Categorical data are reported as count (percentage). Continuous data are reported as median [interquartile range (IQR)]. Descriptive statistics were used to evaluate the primary and secondary outcomes. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Windows (version 21.0. IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Between January 2014 and September 2017, a total of 243 men were referred by their GP for a prostate consultation at the primary care facility. Median age and PSA level was 64 (IQR, 57–70) years and 2.5 (IQR, 0.9–5.8) ng/mL, respectively. The largest group of men (44%) were referred by their GP because of a PCa screening wish and/or advice for LUTS. Of the 243 men, 46% (n=112) had a PSA level ≥3.0 ng/mL. Other relevant baseline characteristics are presented in Table 1.
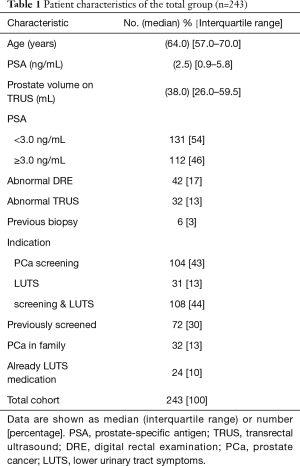
Full table
RPCRC results
A substantial part of men (71%) with PSA level <3.0 ng/mL were referred for LUTS. The majority of men 96% (n=108) with PSA level ≥3.0 ng/mL had a referral related to PCa (Table 2). Of these 108 men, 54% (n=58) were considered high-risk and advised to be referred to the urologist by their GP. Eight men with PSA level <3.0 ng/mL, who according to the guidelines should not be referred, were advised to be referred for prostate biopsy on the basis of the RPCRC results. The rest of the men were considered low-risk and based on the RPCRC would not benefit from a PCa-related visit to the urologist (Figure 1). The median calculated risk of finding any PCa and potentially aggressive PCa in the 66 men considered high-risk was 37% (IQR, 21–59%) and 14% (IQR, 5–39%), respectively.

Full table
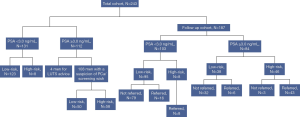
Referral rate, biopsy outcomes and treatment types
Of the total of 243 men, FU data were available of 187 men (FU cohort). Median FU time was 16 (IQR, 9–25) months. Of these 187 men, 45% (n=84) had a PSA level ≥3.0 ng/mL; this was similar to the rate of men with PSA level ≥3.0 ng/mL in the total cohort. In the FU cohort, 54 men (8 men with PSA level <3.0 ng/mL and 46 men with PSA level ≥3.0 ng/mL) were considered high-risk (Figure 1). The median calculated risk of finding any PCa and potentially aggressive PCa in prostate biopsy in these 54 men was also 37% (IQR, 21–59%) and 14% (IQR, 5–39%), respectively. The rates of men considered low- and high-risk in this FU cohort were similar to the rates in the total cohort for both PSA level subgroups. 94% (n=51) of men considered high-risk were actually referred to secondary care and so far 38 (75%) men underwent prostate biopsy. The median calculated risk of finding any PCa and potentially aggressive PCa in these 38 men was 47% (IQR, 32–67%) and 19% (IQR, 9–44%), respectively. Any PCa was detected in 30 men, including 14 (47%) men with GS ≥3+4 PCa. This constitutes an overall positive predictive value (PPV) of 79%. Only 6 of 38 men with PSA level ≥3.0 ng/mL considered low-risk and therefore not advised to be referred were, however, referred to the urologist by their GP. Within the available FU time, in two of these men GS 3+3 PCa was detected, resulting in an overall negative predictive value (NPV) of 96% for any PCa and 100% NPV for csPCa. An overview of treatments applied to the 30 PCa patients is presented in Table 3. The majority of men 80% (n=24) underwent active curative treatment [i.e., radiation therapy or radical prostatectomy (RARP)].
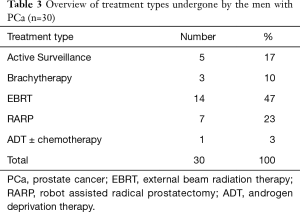
Full table
Discussion
In 2016, the incidence of PCa was 11,064 and the 10-year prevalence of PCa was 79,223 in the Netherlands (13). The number of new PCa cases is expected to increase by 49% in 2030 and consequently the PCa health care costs will rise as well. The importance of an effective diagnostic algorithm in PCa is therefore high. The primary health care could help to further refine PCa detection strategies to become more acceptable to the general population and health care providers (e.g., costs of the prostate consultation at the primary care facility are 85 vs. 592 Euros for a same consultation at a Dutch urologist). However, GPs are still uncertain about managing PCa screening, and for that reason man with PCa suspicion or a screening wish receive different care depending on their GP’s reasoning and practice preferences (14,15). The implementation of validated PCa diagnostic risk models in primary care could be a solution for this problem and may help the GP to facilitate informed decision-making and improve patient selection for referral to secondary care.
The present study is the first study evaluating a risk calculator for patient selection for prostate biopsy in primary care. We show that by implementing multivariable risk-stratification with the RPCRC in a primary health care setting the rate of men referred to the urologist for prostate biopsy with PSA level ≥3.0 ng/mL could be reduced with almost 50%. In more than 75% of men referred for biopsy according to the advice of the RPCRC, the suspicion of PCa has been confirmed and almost half of these men had GS ≥3+4=7 PCa. The vast majority of those men diagnosed with PCa received active treatment with curative intent [only 17% of men were followed-up on active surveillance (AS)]. This seems to indicate a favourable ratio between clinically significant and insignificant PCa after multivariable risk-stratification with the RPCRC in primary care. Within the available FU time, in only 5% of men with PSA level ≥3.0 ng/mL who were considered low-risk based on the RPCRC non-csPCa (GS 3+3=6) has been missed. The RPCRC uses readily available information like PSA and DRE. DRE is already often performed in the GP setting for volume estimation. In addition, abdominal ultrasound instead of TRUS could be used in GP practices for more accurate volume estimation. This is thought to easily be performed by GPs or trained nurses. Our study thereby suggests an important and relevant role for multivariable risk-stratification in primary care to improve patient selection for referral to secondary care.
Health care systems with a strong primary care component are more cost-effective than those that are predominantly led by hospital specialists (16). No previous studies have, however, described the use of PCa diagnostic risk models in the GP setting. Some papers recommend that PSA levels should no longer be referred to as “normal” or “elevated” but should be incorporated into appropriate multivariable risk-based strategies to provide individualized risk information for decision making in primary care practices (17-19). Only a few studies from primary care have examined signs next to an elevated PSA level that could predict PCa and improve patient selection for referral to secondary care this way. In the present study, the RPCRC showed an overall PPV of 79% for PCa. This is significantly higher than the PPV for any PCa (ranging from 12% to 42%) of DRE alone or DRE in combination with weight loss and nycturia in primary care patients found by Walsh et al. and the CAPER studies (20,21).
Several studies have investigated the use of PCa risk calculators, including the RPCRC, in secondary care. Our rates of referrals/biopsies avoided, missed PCa and doctors’ compliance are in line with these previously well-established results of PCa risk calculators in secondary care (5,7,9,22-24). In contrast to these secondary care results, the rate of detected PCa in men considered high-risk was significantly higher in our cohort (79% vs. 15–49% in the other publications). The rate of detected csPCa was, however, similar. The benefits for the PCa diagnostic pathway obtained by using risk calculators for patient selection for biopsy seem similar in both primary and secondary care; probably being even more cost-effective when performing this risk-stratification in the primary care setting.
Unexpectedly, the compliance rate of GPs to the RPCRC-based advice on referral to secondary care was very high (94%). Within the short FU time already 75% of the men considered high-risk have actually been biopsied. It must be noted, that despite the very high PPV in those men considered at high-risk and actually biopsied it seems that there is room for improvement. More than half of men had a GS 3+3 PCa and based on only their Gleason grading could be considered as being overdiagnosed. In principle, based on their grading these men are eligible for AS. However, most men (69%) in whom GS 3+3 PCa (without taking into account e.g., tumour volume, MRI characteristics) was detected underwent active treatment, which could be considered as overtreatment. This implies that if we really aim to counterbalance the harms of PSA testing we should not only focus on reducing unnecessary referrals but also aim to uncouple diagnosis from treatment (25).
The strength of the present study is that all the examinations and risk calculation were performed in a true primary care population with prostate-related questions without any pre-selection. Therefore, we were able to test the RPCRC also in men with a PSA level <3.0 ng/mL who were referred mainly for LUTS advice and not specifically for PCa. On the basis of the RPCRC results, eight of these men (PSA levels ranging from 0.7 to 2.8 ng/mL) were advised to be referred for further analysis; in two of these men PCa was detected. This reinforces the argument to implement PCa risk models in primary care since men considered high-risk according to the RPCRC and subsequently diagnosed with PCa are found in both PSA ranges. The fact remains that men with a PSA level <3.0 ng/mL are mainly seen by GPs because the majority of them are not referred to the urologist.
The present study is limited by the fact that the examinations were performed at the primary care facility by specially trained Erasmus MC personnel from the Department of Urology. Given that measuring the prostate volume with TRUS is not common practice for GPs and requires additional training and a TRUS-device, it still remains difficult to translate our study results to the real GP’s office. However, risk-stratification with the RPCRC could also be performed with only PSA and a DRE-based prostate volume. Both information is available in the GP setting. Besides, volume measurement with simple abdominal ultrasound instead of TRUS could be performed by trained GPs or nurses and could help to more accurately estimate the prostate volume. These measures might result in comparable RPCRC results as shown in the present study. The second limitation of this study is the fact that the diagnostic accuracy of the RPCRC in our primary care cohort could not be investigated within the full cohort since not all men considered high-risk underwent prostate biopsy. When additional FU data become available this will give new insight in PCa detection not only in these men but possibly also in those men initially considered as low-risk according to the calculations of the RPCRC.
In conclusion, our study shows that individualized multivariable risk-stratification for prostate biopsy based on PSA, DRE and prostate volume on TRUS in primary care may reduce unnecessary referrals to secondary care and, thereby reduce the number of biopsies, costs and workload in the urology outpatient clinic. Further studies in larger cohorts need to be performed, including the inclusion of GPs or trained nurses to actually perform the DRE and/or (abdominal) ultrasound, to confirm these findings and validate the use of PCa risk calculators in primary care.
Acknowledgements
This work was supported by Stichting Coolsingel.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institutional review board (METC Erasmus MC, number: MEC-2013-572) and conformed to the provisions of the Declaration of Helsinki.
References
- Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 2012;366:981-90. [Crossref] [PubMed]
- Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384:2027-35. [Crossref] [PubMed]
- Roobol MJ, Schroder FH. The rate of overdiagnosis inextricably linked to prostate-specific antigen-based screening for prostate cancer can be quantified in several ways, but what is the practicable message? Eur Urol 2014;65:1056-7. [Crossref] [PubMed]
- Roobol MJ, Carlsson SV. Risk stratification in prostate cancer screening. Nat Rev Urol 2013;10:38-48. [Crossref] [PubMed]
- Louie KS, Seigneurin A, Cathcart P, et al. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann Oncol 2015;26:848-64. [Crossref] [PubMed]
- Roobol MJ, Schroder FH, Hugosson J, et al. Importance of prostate volume in the European Randomised Study of Screening for Prostate Cancer (ERSPC) risk calculators: results from the prostate biopsy collaborative group. World J Urol 2012;30:149-55. [Crossref] [PubMed]
- Roobol MJ, Steyerberg EW, Kranse R, et al. A risk-based strategy improves prostate-specific antigen-driven detection of prostate cancer. Eur Urol 2010;57:79-85. [Crossref] [PubMed]
- van Vugt HA, Kranse R, Steyerberg EW, et al. Prospective validation of a risk calculator which calculates the probability of a positive prostate biopsy in a contemporary clinical cohort. Eur J Cancer 2012;48:1809-15. [Crossref] [PubMed]
- van Vugt HA, Roobol MJ, Busstra M, et al. Compliance with biopsy recommendations of a prostate cancer risk calculator. BJU Int 2012;109:1480-8. [Crossref] [PubMed]
- van Vugt HA, Roobol MJ, Kranse R, et al. Prediction of prostate cancer in unscreened men: external validation of a risk calculator. Eur J Cancer 2011;47:903-9. [Crossref] [PubMed]
- Trottier G, Roobol MJ, Lawrentschuk N, et al. Comparison of risk calculators from the Prostate Cancer Prevention Trial and the European Randomized Study of Screening for Prostate Cancer in a contemporary Canadian cohort. BJU Int 2011;108:E237-44. [Crossref] [PubMed]
- De Reijke T, Van Moorselaar RJA, Van Vulpen M, et al. Prostaatcarcinoom Landelijke richtlijn, Versie: 2.0. IKNL, 2014.
- IKNL: Integraal Kankercentrum Nederland-Nederlandse Kankerregistratie. 2016. Available online: http://www.iknl.nl
- Pickles K, Carter SM, Rychetnik L. Doctors' approaches to PSA testing and overdiagnosis in primary healthcare: a qualitative study. BMJ Open 2015;5:e006367. [Crossref] [PubMed]
- Pickles K, Carter SM, Rychetnik L, et al. General Practitioners' Experiences of, and Responses to, Uncertainty in Prostate Cancer Screening: Insights from a Qualitative Study. PLoS One 2016;11:e0153299. [Crossref] [PubMed]
- Macinko J, Starfield B, Shi L. The contribution of primary care systems to health outcomes within Organization for Economic Cooperation and Development (OECD) countries, 1970-1998. Health Serv Res 2003;38:831-65. [Crossref] [PubMed]
- Misra-Hebert AD, Hu B, Klein EA, et al. Prostate cancer screening practices in a large, integrated health system: 2007-2014. BJU Int 2017;120:257-64. [Crossref] [PubMed]
- Thompson IM Jr, Leach RJ, Ankerst DP. Focusing PSA testing on detection of high-risk prostate cancers by incorporating patient preferences into decision making. JAMA 2014;312:995-6. [Crossref] [PubMed]
- Emery JD, Shaw K, Williams B, et al. The role of primary care in early detection and follow-up of cancer. Nat Rev Clin Oncol 2014;11:38-48. [Crossref] [PubMed]
- Walsh AL, Considine SW, Thomas AZ, et al. Digital rectal examination in primary care is important for early detection of prostate cancer: a retrospective cohort analysis study. Br J Gen Pract 2014;64:e783-7. [Crossref] [PubMed]
- Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer 2009;101 Suppl 2:S80-6. [Crossref] [PubMed]
- Gayet M, Mannaerts CK, Nieboer D, et al. Prediction of Prostate Cancer: External Validation of the ERSPC Risk Calculator in a Contemporary Dutch Clinical Cohort. Eur Urol Focus 2016:S2405-4569(16)30107-9.
- Poyet C, Nieboer D, Bhindi B, et al. Prostate cancer risk prediction using the novel versions of the European Randomised Study for Screening of Prostate Cancer (ERSPC) and Prostate Cancer Prevention Trial (PCPT) risk calculators: independent validation and comparison in a contemporary European cohort. BJU Int 2016;117:401-8. [Crossref] [PubMed]
- Ankerst DP, Hoefler J, Bock S, et al. Prostate Cancer Prevention Trial risk calculator 2.0 for the prediction of low- vs high-grade prostate cancer. Urology 2014;83:1362-7. [Crossref] [PubMed]
- Murphy DG, Ahlering T, Catalona WJ, et al. The Melbourne Consensus Statement on the early detection of prostate cancer. BJU Int 2014;113:186-8. [Crossref] [PubMed]


