Stopping screening, when and how?
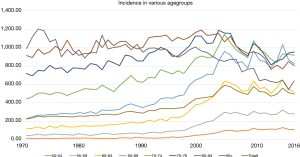
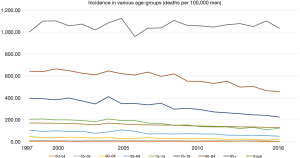
Although very few countries recommend and offer organized screening for prostate cancer (PC) there is globally an ongoing widespread non-organized opportunistic screening. In US and several European countries, a majority of men over age 50 have had a prostate specific antigen (PSA) test (1,2). Despite very few national programs for PC screening the wide-spread use of opportunistic screening has had a large impact on incidence and mortality in PC. The incidence of PC has increased dramatically in younger men but despite frequent testing in elderly men the incidence has decreased in men older than 75 years and even in men 70–74 years there is a slight decrease or at least a stabilization of the incidence peak reached in 2005 (Figure 1). In Sweden the mortality decrease is impressive with >50% mortality decrease in men <60 years, approximately 40% decrease in men aged 60 to 79 years and 30% in men aged 80 to 84 years but no mortality decrease in men 85+ (Figure 2). Several randomized screening trials have been conducted but most of them were not designed and powered to analyse PC mortality as end-point (3-6). The largest study is the European Randomised Study of Screening for Prostate Cancer (ERSPC) which randomized 112,553 men in the age-group 55–69 years to be invited for regular PSA testing and 128,681 as controls. Most of the knowledge we have regarding PSA screening is derived from ERSPC. The last publication in 2014 demonstrated a 21% relative decrease in PC mortality with a FU of 13 years (6).
The major concern and the reason why most health care authorities not recommend PSA screening despite its effectiveness in decreasing PC mortality is the high risk of over-diagnosis. The most common definition of over-diagnosis is diagnosis of a disease in asymptomatic individuals that would not have surfaced as a clinical disease if not detected by means of early detection activities although many other definitions have been proposed (7). Despite the fact that a modeling study on the basis of ERSPC results showed that benefits outweighs the harm, it is the ratio of harms versus benefit that is still the major concern why PC screening not is implemented and generally recommended (8). Even if the quality adjusted life-years (QALYs) favor implementation of a PC screening program on a population level there are probably more men that will be harmed (lowered quality of life due to long-term side effects of a treatment they not needed) than those who will benefit (avoidance of PC death). The high proportion of over-diagnosis is not exclusive for PC screening but exist in many fields of medicine such as treatment of mild hypertension and treatment of hypercholesterolemia but also in cancer screening (9,10). The difference compared with PC is that side-effects from treatment for PC might be more severe and occasionally lead to a miserable life (11,12). This has raised the question also from an ethical point of view, is it acceptable to harm individuals even if the overall goal is achieved on a population level (13). The number needed to diagnose (NND) to avert one PC death was 27 at 13 years of FU in ERSPC and the excess incidence was 57% higher in the screening compared to the control group (6). Even if this number probably will go down with extended FU it is high and expresses the high risk of over-diagnosis (14). In fact, NND is falsely low as this measure is calculated from number needed to invite (the inverse of the absolute difference in PC mortality) multiplied with the excess incidence in the screening arm. As there is a wide-spread testing also in the control arm especially during later years that not has at least yet been translated into decreased mortality the excess incidence is falsely low. In one analysis in the ERSPC Gothenburg it was found that NND at 18 years was 9 if excess incidence was calculated with the control group as reference but 13 if historical incidence data before the PSA era was used (15).
Is over-diagnosis higher in PC compared to other cancer screening programs?
It is well known that over-diagnosis is common also in many other cancer screening programs such as thyroid cancer, breast cancer and lung cancer screening (9). A large reservoir of clinically indolent cancers is a prerequisite for over-diagnosis. It has been known for almost a century that men with increasing age develop small well differentiated cancers in their prostate (16-18). One famous study from Detroit studied traffic victims and found that already from age 30 small tumors were recognizable and their commonness increased with age and after 70 years the majority of men had such cancers (19). In another landmark paper by Stamey and McNeal in 1993 they examined the prostate in 139 men who underwent cystoprostatectomy and 40% had undiagnosed PC but 80% of these cancers were small (volume <0.5 cc) and well differentiated (often called insignificant PC) (20). Similar results were found by Hautmann et al. (21) These criteria have recently been up-dated (22) but the natural course of “insignificant” cancers is mainly unknown but it is obvious that the majority will never develop into clinical cancers.
However, it is not only small well differentiated cancers that might be over-diagnosed. Also moderately and poorly differentiated PC have a rather slow tumor growth and if detected in an early detection program may have a long period before becoming symptomatic (lead-time). During this long lead-time there is always a risk that the patient dies from something else, the older he is the higher the risk. Also these patients are by definition over-diagnosed. In one publication from the Swedish National Prostate Cancer Registry (NPCR) it was found that the majority of men with low and intermediate risk cancer died from causes other than PC even if treated conservatively (23). Presence of comorbidity also decreased the risk of dying from PC especially in men <65 years of age at diagnosis. The more we move forward the diagnosis, the older the man is and the more pre-existing co-morbidities the higher is the risk of over-diagnosis.
Mechanisms driving the high risk of over-diagnosis in PC
The most important factor that influence the risk of over-diagnosis is the biological aggressiveness of the cancer. Size and grade are still the two most important factors characterizing the oncological feature of PC but current clinical work-up is far from optimal in selective diagnosis of clinically relevant PC (24). A major problem is the diagnostic pathway which until now has implied systematic “blind” biopsies of the prostate. The diagnosis of PC typically starts with an elevated PSA test followed by systematic biopsies aiming to cover the peripheral zone of the prostate where 80% of PC are found (25). As PSA give no information of where a possible cancer is located this has become a standard procedure and guidelines recommend 10–14 spread core biopsies (25). Even if small insignificant cancers not give rise to a PSA increase these may still be diagnosed in a PSA driven screening program. PSA has a very low specificity and in ERSPC the positive predictive value (PPV) of finding PC in men with PSA ≥3 ng/mL was only 25% (26). As at least one third of the cancers diagnosed were small and insignificant the conclusion is that only 15% to 20% of men who have an elevated PSA has that caused by PC (27), in the remaining 80–85% of men other causes such as benign prostatic hyperplasia (BPH), inflammatory diseases or unknown reasons are the cause of the PSA elevation. In such cases it is of course very likely that men also have an insignificant PC that is “hit” by accident with present random biopsy technique. As other causes to PSA elevations such as BPH increases with age it is reasonable to believe that the risk of detecting insignificant cancers is higher in elderly men and as the remaining life-time also is shorter it is very important to establish an optimal age where screening for PC should no longer be recommended as benefits will no longer outweigh the harms. Also considerations on costs and cost-effectiveness must be taken into account as PC is such a frequent disease in elderly men and screening men in a non-optimal age group will become very costly.
Age as a risk factor for PC
No other cancer is so correlated to age as PC. For every 5-year increase in age the risk of PC increase more than 50%. This holds for both for incidence and mortality (Figures 1,2). The median age for those men dying from PC is 82 years in Sweden (www.socialstyrelsen.se). When considering a screening program for PC it is not the age to start that is influencing the risk of over-diagnosis but the stop age (28). In that study it was found that the cumulative incidence of PC at age 60 was around 6–8%, 13–15% at age 65 and 20–23% at age 70 irrespective of starting age. One important finding is that the rate of high risk cancer is increasing with increasing age (29). In this Swedish study by Assel et al. frozen serum was used to retrospectively assess a so-called base-line PSA level which was subsequently correlated to PC risk and grade at diagnosis many years later. The longer time it took to clinical diagnosis from a given PSA the higher the risk of having high risk PC at diagnosis. Their interpretation was that upgrading occurred slowly during the non-symptomatic pre-diagnostic phase. Similar results were reported in men aged 72–74 years from ERSPC Rotterdam where high risk PC were more likely to be diagnosed in the fifth and last screening round in men who previously not had been biopsied while men who had a previous biopsy had a much lower rate (30). Men diagnosed in earlier rounds had a much lower rate of high risk PC. This is in harmony with a Danish study that showed that men with a previous benign biopsy had a very low risk of dying from PC up to 20 years after the biopsy (31). Also in ERSPC Gothenburg it was found that the risk of being diagnosed with high risk and advanced PC was steeply increasing after age 65 both in the screening and control arm (32). It thus seems as if higher age is a risk factor for developing more aggressive PC. But even when taking cancer stage and grade into account it seems as if age is an independent risk factor for PC death except for Gleason 8–10 where age did not affect outcome (33). This suggest that men in Rotterdam ERSPC who had their first time biopsy in the fifth round despite a normal PSA in previous rounds had PC for many years but grade progression is more common at higher age as compared to younger age. Similar results were found in the ERSPC Gothenburg where post-screening cases often were high risk in men who previously not were biopsied (32), typically in men with a PSA between 1.5 and 3 in the last screening round.
So, on the basis of the results described above one could hypothesize that many clinically important PC cases are present in the PSA range below 3 ng/mL at the time when screening is stopped and if then left undiagnosed these cancers might progress and surface as lethal cancers in the post screening period. There are several studies supporting this. The Stockholm 3 study used the so-called Stockholm 3 test to select men in the PSA range 1–3 ng/mL for biopsy. They found a high proportion of significant cancers especially in the PSA range 2–3 ng/mL (34). Similar results were found in the ERSPC Gothenburg where men in the tenth and last screening invitation were offered MRI if PSA were ≥1.8 ng/mL. Also in this study several important cancers were diagnosed in men with PSA <3 ng/mL (35). In the study by Hautmann based on cystoprostatectomy specimen again found that PSA was below 3 ng/mL in the majority of significant cancers (21).
In conclusion, there are several indications that men in the age group >70 years, an age where most guidelines recommend to stop PSA testing, with a PSA below 3 ng/mL actually have clinically important cancers which if left undiagnosed may progress to lethal cancer. On the other hand, continued screening of men in this age-group might lead to a non-acceptable high rate of over-diagnosis.
At what age should screening for PC be stopped?
Within ERSPC different centers applied different age for stopping screening. Most centers stopped screening at age >74 years but Finland and Sweden stopped at age >71 years. Median age at last visit in ERSPC Rotterdam was 73 years and in ERSPC Gothenburg was 69 years. There are some studies who have tried to analyse at what age harms of screening will outweighs the benefits. All these studies are based upon simulation studies with the weaknesses such studies have. One major concern is how to incorporate in the model the independent effect of higher age have on the risk for progression and cancer death. Another problem is evaluating quality of life effects within various age groups. The main harm by screening is over-diagnosis and over-treatment reducing quality of life. In the paper by Heijnsdijk and co-workers it was clearly demonstrated in their sensitivity analysis that the main negative effects on quality of life were long-term side-effects from treatment (36). The negative influence of such side-effects as incontinence and erectile dysfunction (ED) on quality of life is probably much less in elderly compared to younger men although large individual variations can be anticipated and unfortunately studies are lacking. Severe urinary as well as fecal incontinence has become a rare side-effect from treatment at least in high volume centers but the risk of ED is a frequent permanent damage most patients have to live with both after surgery and radiation (37,38). It is likely that an older population would grade the effect of ED on quality of life differently as compared to a younger population.
Etzioni and co-workers simulated the effects of 35 different screening programs. A PSA based program (PSA cut off at 4 ng/mL) that started at age 40 and stopped at age 74 rendered an absolute mortality reduction of 0.72% while a program that stopped at 69 would have saved 0.54% of dying from PC but the NND was 4.79 if stopping at age 74 compared to 3.66 if stopping at age 69 (39).
In another modelling study by Heijnsdijk et al. QALYs, costs and cost-effectiveness were analysed in various screening models and with various stopping ages (40). This study showed that stopping screening at age 59 resulted in that 32% of cancers were over-diagnosed, while with stopping at age 67, 41% were over-diagnosed and stopping screening at 75 years resulted in that 48% of PC cases were over-diagnosed. When analyzing QALYs gained per 1,000 men there was a plateau between 60 and 66 years around 12 years but a steep decrease in higher age groups and reaching to only 6 life-years gained if screening was continued up to 75 years. The explanation of these differences is a high frequency of side-effects in over-diagnosed men, while on the other hand the mortality reduction if continuing screening up to 75 (applying a 1-year interval) was estimated to be 40%. Stopping at 69 resulted in a 30% mortality reduction and stopping at age 59 resulted in only a 13% PC mortality reduction. The total costs with a 2-year interval increased from 1 million dollars if screening was stopped at age 65 to 1.2 million dollars if stopped at age 69 and reaching 1.6 million dollar if continuing screening up to 75 years. The most cost-effective screening algorithm was one time screening at age 55 but this resulted in only a 5% decrease in PC mortality. Strengths with this study is that the model is build from “real” empirical ERSPC data. Weaknesses are that the rate of over-treatment is calculated from ERSPC Rotterdam data only and that during the early phases of the ERSPC, active surveillance as a treatment option for low-risk PC was not that common and accepted. This is especially important as the impact on the quality of life is mainly associated with the long-term side-effects of active treatment (36). If fewer men, especially in higher age groups, will be less often actively treated this will have a major impact on the reported reduction of reduction of QALY. Another important aspect as discussed above is how quality of life in elderly men is actually affected by side-effects such as ED, a condition many elderly men already suffer from.
In a third study from ERSPC Gothenburg the incidence and mortality after termination of screening was analysed. In ERSPC Gotehnburg men were invited biennially up to a mean age of 69 years. After termination of screening the incidence of PC and especially high risk and advanced PC were very low during the first 3 years in the screening compared to the control group but then gradually increased and 9 years after discontinuing screening there was no difference in the incidence of high risk cancer between the two groups. In addition, the effect of lowered PC mortality seemed to vanish already after 10 years (32). The strength of this study is that it is based on empirical data. Weakness is the small sample size resulting in a low number of events for PC mortality. However, this study corroborates with the first publication of ERSPC that reported a significant mortality difference of 21% already 8.8 years after randomization in favor of men randomized to screening (26). These data suggest that a majority of lethal cancers have a rather rapid disease course much faster than what has previously been estimated (41). Calculations of lead-time do not account for differences in lethal and non-lethal cancers. As only a minority of cancers detected at PSA screening are potentially lethal cancers we cannot use the average lead-time for all cancers when designing the best possible screening program for PC.
The new data from ERSPC Rotterdam and ERSPC Gothenburg and supported by the simulation studies thus indicate that stopping already at age 70 will only save a small proportion of all preventable PC deaths at least with current screening strategy based on PSA with a cut off at 3 ng/mL. The average age for dying from PC is 82 years in Sweden and the average life length is steadily increasing around the world. The big challenge is to find the optimal screening program in men aged 70 to 80 years where probably most preventable PC deaths are to be diagnosed but also where the risk of over-diagnosis and harms is most likely the highest.
New screening methodologies are underway. The big problem with PSA based screening as conducted within the ERSPC trial is the low specificity of PSA and current applied “blind” biopsy technique. Better markers and imaging looks promising. Changing from “blind” systematic biopsies to targeted-directed biopsies decrease the risk of detecting small non-significant cancers (42). One large MRI based screening study is on-going in Gothenburg (the Gothenburg2 study) and another one is soon to start in Finland. If these studies can validate the value of MRI and targeted–directed biopsies the risk of harm with screening elderly men will become much less and the benefit to harm balance will change.
How to stop screening?
Stopping screening for PC is difficult mostly due to the fact that a PSA test is easily accessible and its strong positive re-assurance value. For a man who for many years repeatedly has had a PSA test it is not easy to convince him to stop testing. Shared decision making is usually proposed but the effect on the final decision is obscure (43). It is evident that older age and comorbidities are important factors increasing the risk of over-diagnosis and a personalized decision making approach would be the best but will be difficult to implement in a nationwide screening program (44) in which usually only start and stop age is included. Selective and more intense screening of risk-groups such as men with family history is another suggestion to decrease harms but in one study this is not always improving the harm-benefit balance (45). Currently we are in a situation where it is difficult to guide especially men over age 70 when to stop PSA testing. The most common practice today is probably a shared decision making process where most men will despite information of benefits and harms continue PSA testing but where urologists are trying to avoid unnecessary biopsies in men at low risk for clinically important disease even if their PSA is slightly elevated (46). A man with stable or only slowly increasing PSA has a low risk of dying from PC and with PSA surveillance progressive cancers could usually be diagnosed in time (47). Incorporating PSA density and other risk factors in a nomogram may also help to select men for biopsies. New and better markers and imaging have already changed the clinical situation and is already in use for selecting men at high risk for biopsy (48). In men with PC diagnosed active surveillance should be the treatment of choice in men with low risk disease although many men will switch to active treatment during follow-up.
The overall aim is not to stop screening but to stop the harms with screening in elderly men. To achieve that we need better selection of men who should be biopsied, better precision in biopsy guidance, better selection of men who need active treatment and offering treatment in highly specialized quality-assured centers where the risk of permanent side-effects is minimal.
Acknowledgements
The author would like to thank the Swedish Cancer Society, the County Council for West Sweden (ALF-project), the Swedish Research Council, Lions West and the Swedish Prostate Cancer Federation for financial support.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Nordstrom T, Aly M, Clements MS, et al. Prostate-specific antigen (PSA) testing is prevalent and increasing in Stockholm County, Sweden, Despite no recommendations for PSA screening: results from a population-based study, 2003-2011. Eur Urol 2013;63:419-25. [Crossref] [PubMed]
- Drazer MW, Huo D, Schonberg MA, et al. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol 2011;29:1736-43. [Crossref] [PubMed]
- Kjellman A, Akre O, Norming U, et al. 15-year followup of a population based prostate cancer screening study. J Urol 2009;181:1615-21; discussion 1621. [Crossref] [PubMed]
- Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20 year follow-up. BMJ 2011;342:d1539. [Crossref] [PubMed]
- Labrie F, Candas B, Cusan L, et al. Screening decreases prostate cancer mortality: 11-year follow-up of the 1988 Quebec prospective randomized controlled trial. Prostate 2004;59:311-8. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384:2027-35. [Crossref] [PubMed]
- Marcus PM, Prorok PC, Miller AB, et al. Conceptualizing overdiagnosis in cancer screening. J Natl Cancer Inst 2015;107:djv014. [Crossref] [PubMed]
- Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120-34. [Crossref] [PubMed]
- Shieh Y, Eklund M, Sawaya GF, et al. Population-based screening for cancer: hope and hype. Nat Rev Clin Oncol 2016;13:550-65. [Crossref] [PubMed]
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst 2010;102:605-13. [Crossref] [PubMed]
- Steentjes L, Siesling S, Drummond FJ, et al. Factors associated with current and severe physical side-effects after prostate cancer treatment: What men report. Eur J Cancer Care (Engl) 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Holm HV, Fossa SD, Hedlund H, et al. How should continence and incontinence after radical prostatectomy be evaluated? A prospective study of patient ratings and changes with time. J Urol 2014;192:1155-61. [Crossref] [PubMed]
- Adami HO, Baron JA, Rothman KJ. Ethics of a prostate cancer screening trial. Lancet 1994;343:958-60. [Crossref] [PubMed]
- Hugosson J, Godtman RA, Carlsson SV, et al. Eighteen-year follow-up of the Goteborg Randomized Population-based Prostate Cancer Screening Trial: effect of sociodemographic variables on participation, prostate cancer incidence and mortality. Scand J Urol 2017.1-11. [Crossref] [PubMed]
- Arnsrud Godtman R, Holmberg E, Lilja H, et al. Opportunistic testing versus organized prostate-specific antigen screening: outcome after 18 years in the Goteborg randomized population-based prostate cancer screening trial. Eur Urol 2015;68:354-60. [Crossref] [PubMed]
- Bell KJ, Del Mar C, Wright G, et al. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int J Cancer 2015;137:1749-57. [Crossref] [PubMed]
- Franks LM. Latent carcinoma of the prostate. J Pathol Bacteriol 1954;68:603-16. [Crossref] [PubMed]
- Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst 2013;105:1050-8. [Crossref] [PubMed]
- Sakr WA, Grignon DJ, Crissman JD, et al. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20-69: an autopsy study of 249 cases. In Vivo 1994;8:439-43. [PubMed]
- Stamey TA, Freiha FS, McNeal JE, et al. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 1993;71:933-8. [Crossref] [PubMed]
- Hautmann SH, Conrad S, Henke RP, et al. Detection rate of histologically insignificant prostate cancer with systematic sextant biopsies and fine needle aspiration cytology. J Urol 2000;163:1734-8. [Crossref] [PubMed]
- Van der Kwast TH, Roobol MJ. Defining the threshold for significant versus insignificant prostate cancer. Nat Rev Urol 2013;10:473-82. [Crossref] [PubMed]
- Rider JR, Sandin F, Andrén O, et al. Long-term outcomes among noncuratively treated men according to prostate cancer risk category in a nationwide, population-based study. Eur Urol 2013;63:88-96. [Crossref] [PubMed]
- McNeal JE. Cancer volume and site of origin of adenocarcinoma in the prostate: relationship to local and distant spread. Hum Pathol 1992;23:258-66. [Crossref] [PubMed]
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014;65:124-37. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. [Crossref] [PubMed]
- Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010;11:725-32. [Crossref] [PubMed]
- Godtman RA, Carlsson S, Holmberg E, et al. The Effect of Start and Stop Age at Screening on the Risk of Being Diagnosed with Prostate Cancer. J Urol 2016;195:1390-6. [Crossref] [PubMed]
- Assel M, Dahlin A, Ulmert D, et al. Association between lead time and prostate cancer grade: evidence of grade progression from long-term follow-up of large population-based cohorts not subject to prostate-specific antigen screening. Eur Urol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Alberts AR, Schoots IG, Bokhorst LP, et al. Characteristics of Prostate Cancer Found at Fifth Screening in the European Randomized Study of Screening for Prostate Cancer Rotterdam: Can We Selectively Detect High-grade Prostate Cancer with Upfront Multivariable Risk Stratification and Magnetic Resonance Imaging? Eur Urol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Klemann N, Røder MA, Helgstrand JT, et al. Risk of prostate cancer diagnosis and mortality in men with a benign initial transrectal ultrasound-guided biopsy set: a population-based study. Lancet Oncol 2017;18:221-9. [Crossref] [PubMed]
- Grenabo Bergdahl A, Holmberg E, Moss S, et al. Incidence of prostate cancer after termination of screening in a population-based randomised screening trial. Eur Urol 2013;64:703-9. [Crossref] [PubMed]
- Gandaglia G, Karakiewicz PI, Abdollah F, et al. The effect of age at diagnosis on prostate cancer mortality: a grade-for-grade and stage-for-stage analysis. Eur J Surg Oncol 2014;40:1706-15. [Crossref] [PubMed]
- Grönberg H, Adolfsson J, Aly M, Nordstrom T, et al. Prostate cancer screening in men aged 50-69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol 2015;16:1667-76. [Crossref] [PubMed]
- Grenabo Bergdahl A, Wilderäng U, Aus G, et al. Role of magnetic resonance imaging in prostate cancer screening: a pilot study within the goteborg randomised screening trial. Eur Urol 2016;70:566-73. [Crossref] [PubMed]
- Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med 2012;367:595-605. [Crossref] [PubMed]
- Hjälm-Eriksson M, Lennernäs B, Ullén A, et al. Long-term health-related quality of life after curative treatment for prostate cancer: a regional cross-sectional comparison of two standard treatment modalities. Int J Oncol 2015;46:381-8. [Crossref] [PubMed]
- Haglind E, Carlsson S, Stranne J, et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. European urology 2015;68:216-25. [Crossref] [PubMed]
- Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen--based prostate cancer screening strategies: model estimates of potential benefits and harms. Ann Intern Med 2013;158:145-53. [Crossref] [PubMed]
- Heijnsdijk EA, de Carvalho TM, Auvinen A, et al. Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data. J Natl Cancer Inst 2014;107:366. [PubMed]
- Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst 2009;101:374-83. [Crossref] [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Lillie SE, Partin MR, Rice K, et al. The Effects of shared decision making on cancer screening - a systematic review. Washington (DC): Department of Veterans Affairs (US); 2014.
- Gulati R, Inoue LY, Gore JL, et al. Individualized estimates of overdiagnosis in screen-detected prostate cancer. J Natl Cancer Inst 2014;106:djt367. [Crossref] [PubMed]
- Gulati R, Cheng HH, Lange PH, et al. Screening Men at Increased Risk for Prostate Cancer Diagnosis: Model Estimates of Benefits and Harms. Cancer Epidemiol Biomarkers Prev 2017;26:222-7. [Crossref] [PubMed]
- Roobol MJ, Verbeek JF, van der Kwast T, et al. Improving the Rotterdam European randomized study of screening for prostate cancer risk calculator for initial prostate biopsy by incorporating the 2014 international society of urological pathology gleason grading and cribriform growth. Eur Urol 2017;72:45-51. [Crossref] [PubMed]
- Kettermann AE, Ferrucci L, Trock BJ, et al. Interpretation of the prostate-specific antigen history in assessing life-threatening prostate cancer. BJU Int 2010;106:1284-90; discussion 1290-2. [Crossref] [PubMed]
- van Leeuwen PJ, Hayen A, Thompson JE, et al. A multiparametric magnetic resonance imaging-based risk model to determine the risk of significant prostate cancer prior to biopsy. BJU Int 2017;120:774-81. [Crossref] [PubMed]


