Reduction of MRI-targeted biopsies in men with low-risk prostate cancer on active surveillance by stratifying to PI-RADS and PSA-density, with different thresholds for significant disease
Introduction
In the Western world, about one half of all patients diagnosed with prostate cancer (PCa) has low-risk disease (1). In low-risk disease, active treatment hardly yields survival benefit (2). Therefore, active surveillance (AS) is the recommended option for the initial management of low-risk disease (3,4).
Monitoring in AS is based on repeated PSA measurements, clinical T-staging based on digital rectal exams, and repeated random systematic transrectal ultrasound (TRUS)-guided biopsies (3). Repeated biopsies are cumbersome for patients and have severe complication risks. Moreover, sampling errors lead to underestimation of the Gleason grading (5).
The fear of undergrading PCa in men on AS has however led to strict criteria for monitoring, which has resulted in good long-term cancer-specific survival, proving the safety of this approach (6). To reduce the fear of undergrading, MRI and MRI-targeted biopsies are increasingly used in the management of patients with clinically low-risk PCa, despite their role has not yet been established definitively (7). The use of MR imaging in AS has improved the inclusion of true low-grade PCa; targeted biopsies of suspected lesions on MRI may result in excluding those men found with intermediate/high-grade PCa (8).
However, this additional testing by MRI and MRI targeted biopsy may result in an unintended reclassification to perceived higher risk. This is termed as ‘risk inflation’; a cancer that is stable may be more accurately sampled at MRI-targeted biopsy and found to include higher risk features than when it was sampled in a routine systematic manner (9). This lesion targeting results in an increase in risk attribution, if traditional criteria [i.e., Gleason score (GS), cancer core length and the proportion of positive cores on routine sampling] are still applied. It would therefore be wrong to falsely encourage men to cease AS because of an apparent increase in risk (reclassification) rather than a true change in their cancer.
Appropriate risk thresholds are not yet understood when MRI and MRI-targeted biopsies are used. In this study we investigate the upgrading with MRI and MRI-targeted biopsies in men with low-risk disease on AS, and examine the potential reduction of targeted biopsies by stratifying to PI-RADS and PSA-density. We explore the possible risk inflation by MRI and targeted biopsies, and look into the potential extension of GS thresholds for continuing in AS when using MRI in strict monitoring.
Methods
The study was HIPAA compliant and was approved by the institutional ethical review board (NL45884.078.13/A301321). Written informed consent with guarantee of confidentiality was obtained from the participants. Men with low-risk PCa are prospectively enrolled in our in-house database as part of our AS protocol. From November 2013 until October 2017 a total of 347 consecutive men on AS for low-grade (GS 3+3) PCa detected by TRUS guided biopsy received a first multi-parametric MRI and targeted biopsies of visible suspicious MRI lesions at our tertiary referral center.
A total of 331 men were included in the current study. Men were excluded (16/347), as they did not undergo additional targeted biopsy, despite of having a positive MRI (n=8 PI-RADS 3 lesions, n=8 PI-RADS 4 lesions). Results of part of this prospective cohort have been previously published (10).
In 50% (166/331) men were participants of the PRIAS study (www.prias-project.org), an international web-based AS study with strict criteria for inclusion at diagnosis (GS 3+3, T-stage ≤ cT2, PSA ≤10 ng/mL, ≤2 positive cores, PSA density <0.2) and follow-up (11). Within the MRI-PRIAS side study protocol an MRI and targeted biopsies (if indicated) are performed at baseline (3 months after diagnosis) and during every repeat standard TRUS-guided biopsies, scheduled at 1, 4, 7 and 10 years after diagnosis. Inclusion in the MRI-PRIAS side study is also possible after ≥1 repeat TRUS-guided biopsies. The only reclassification criterion in the MRI-PRIAS side study is the presence of high-grade PCa (GS ≥3+4) at MRI-targeted biopsy. A head-to-head comparison of MRI-targeted with standard TRUS-guided biopsies was only available in repeat biopsies, and was not further investigated in this study.
The remaining 165/331 (50%) men in the present study had low-grade PCa based on standard TRUS-guided biopsy findings, but were followed-up outside of the PRIAS protocol as they did not meet the strict PRIAS inclusion criteria or were referred from a center not participating in PRIAS. All men were included in our prospective, institutional review board approved database, which is HIPPA compliant.
Multi-parametric MRI
The institutional MRI protocol included T2-weighted imaging (T2w), diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) reconstructions, and dynamic contrast enhanced (DCE) imaging, as previously described (12), according to the Prostate Imaging Reporting and Data System (PI-RADS) version 2 guidelines (13). MRI was performed on a 3-T system (Discovery MR750, General Electric Healthcare, USA) using a 32-channel pelvic phased-array coil. All MRIs were reviewed by one urogenital radiologist (IGS) with over 5 years of prostate MRI experience. Individual lesions were scored according to the PI-RADSv2 5-point likelihood scale for high-grade PCa, and the index lesions were annotated and delineated (13). Visible MRI lesions with a PI-RADS score from 3 to 5 were defined as suspicious.
MRI-targeted biopsy
The MRI-TRUS fusion technique was used (UroStation™, Koelis, France) to perform the targeted biopsies of all suspicious lesions, identified on MRI. The suspicious MRI lesions, delineated on DICOM images, were targeted with 2–4 cores under ultrasound guidance. Experienced operators (FHD, DFO, JFV) performed the biopsy procedures.
Pathological review of biopsy specimens
One expert uropathologist (GJvL) reviewed all biopsy specimens according to the International Society of Urological Pathology (ISUP) 2014 modified Gleason Score (14). GS upgrading was defined as any GS ≥3+4 PCa found by MRI-targeted biopsies.
Study objectives
The primary objective of this study was to identify upgrading with MRI and MRI-targeted biopsies (benefit) in men on AS with GS 3+3. In line with this objective, the absence of upgrading (harm) despite of additional testing with MRI and targeted biopsies, was also assessed. The outcome measure is the presence/absence of upgrading to GS ≥3+4.
The secondary objective was to assess the value of risk-stratification based on MRI (PI-RADS) and PSA-density for the presence/absence of additional upgrading. Additional analysis was performed with the outcome measure of GS ≥4+3, focussing on risk inflation with MRI.
Statistical analysis
In accordance with the START recommendations, the outcome measure of clinically significant PCa for MRI and targeted biopsies is the biopsy result of GS 3+4 and higher (15). The PSA density was calculated using the MRI-measured prostate volume. The MRI-measured volume was calculated by the prolate ellipsoid formula (length × width × height × π/6). The PSA density cut-off point of 0.15 and 0.20 ng/mL2 was used for stratification (16-19). Histograms of the stratified PI-RADS and GS biopsy outcomes were constructed to visualize in which men GS upgrading did or did not occur.
Statistical tests were two-sided with the criterion of significance set at P<0.05. Statistically significant differences in continuous non-parametric patient characteristics were assessed with the Mann-Whitney U test, while the χ2 test for trend was used to test for differences in categorical patient characteristics; in case of small numbers the Fischer’s exact test was used. Statistical analyses were performed with SPSS for Windows (Version 21.0. Armonk, NY: IBM Corp.). In addition, R version 3.4.2 and R-package ggplot2 (20) were used for visualization. Gleason scores were dichotomized using cut-off score Gleason ≥3+4, and Gleason ≥4+3, in which a zero indicated a GS below the cut-off and a one indicated a GS above the cut-off score.
Results
In 331 men on AS for GS 3+3 PCa, the median (interquartile range) age and PSA level at diagnosis were respectively 67 (range, 62–72) years and 8.0 (range, 5.6–12.0) ng/mL (Table 1). A total of 66/331 (20%) men had more than two positive systematic biopsy cores at diagnosis. A total of 155/331 (47%) men received their first MRI at baseline, while 176/331 (53%) men received their first MRI at confirmatory biopsy or at surveillance biopsy. In these men no previous MRI was performed. Men included in PRIAS did not significantly differ from men not included in PRIAS, accept for a small PSA and PSA-density difference (Table 1), reflecting PRIAS inclusion criteria.
Full table
Benefit of additional testing with MRI and targeted biopsies
Upgrading in all men on AS
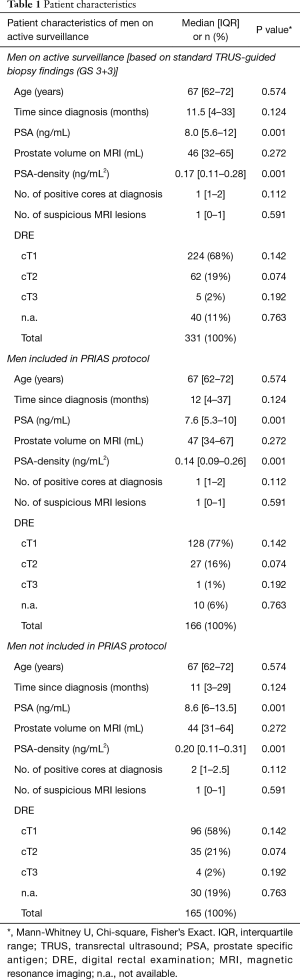
In total, 25% (82/331) of men on AS showed upgrading from GS 3+3, due to additional testing by MRI and if indicated targeted biopsies. The majority (71%) was upgraded to GS 3+4, only 16% (13/82) and 13% (11/82) to GS 4+3 and GS ≥4+4, respectively. Most of the upgraded index lesions (82%) were categorized into PI-RADS 4 and 5. Highest GS’s were associated with PI-RADS 4 and 5.
Upgrading in men with a suspicious MRI index lesion
In 60% (198/331) a suspicious lesion was identified on MRI, and was additionally biopsied (Figure 1). 41% (82/198) of these suspicious lesions showed upgrading from GS 3+3 to GS 3+4 or higher. In PI-RADS 3, 4 and 5 index lesions, the upgrading was 30% (15/50), 36% (36/101) and 66% (31/47), respectively.
Upgrading in men with a PIRADS 3 lesion
Of all suspicious index lesions on MRI, 25% (50/198) was categorized into PI-RADS 3 (Figure 1), meaning that the MRI abnormalities are equivocal to high- or low-grade PCa. An upgrade to GS 3+4 and GS 4+3 was proven in 22% (11/50) and 8% (4/50) respectively, showing the additional value of targeting these PI-RADS 3 lesions. None of these lesions however showed GS ≥4+4.
Harms of additional testing with MRI and targeted biopsies
Abundantly or unnecessary MRI and targeted biopsies
In 40% (133/331) of men, no suspicious lesions on MRI were identified. In these men MRI testing did not result in MRI-targeted biopsies, and no further harm was attributed.
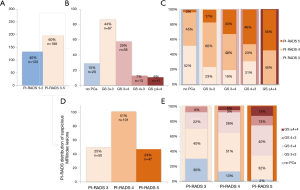
In 59% (116/198) of men with a suspicious MRI, additional MRI-targeted biopsies did not result in upgrading: GS 3+3 PCa was confirmed in 44% (87/198), and no PCa was detected in 15% (29/198). These biopsies can be considered as harm and this was most prominent in the PI-RADS 3 category (70% GS 3+3 or no PCa) (Figure 2). Similar analysis for PI-RADS 4 and PI-RADS 5 category resulted respectively in 64% and 34% unnecessary targeted biopsies.
Abundantly or unnecessary MRI and targeted biopsies adjusted to risk inflation
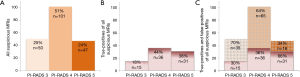
When looking at the detection of GS ≥4+3 PCa additional testing did not result in upgrading in respectively 93% (307/331) of all MRIs, and in 88% (174/198) of all MRI-targeted biopsies (Figure 3).
Potential strategies to reduce further harm
Excluding PI-RADS 3 lesions from targeted biopsies
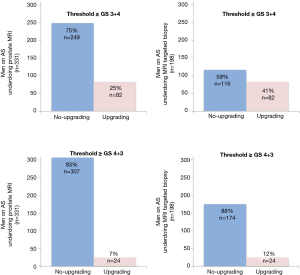
Excluding PI-RADS 3 index lesions from targeted biopsies would result in a reduction of 25% (50/198) targeted biopsies, however, still missing 18% (15/82) of all upgrades to GS ≥3+4. Missed upgrading to GS ≥4+3 would be 5% (4/82).
Stratifying to PSA-density before targeting suspicious MRI lesions
Figures 4 and 5 show the number of men with targeted biopsies, stratifying to PIRADS and to PSA-density.

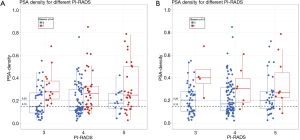
PSA-density cut-off ≥0.15 ng/mL2
Upgrades to GS ≥3+4 in PI-RADS 3 lesions were all identified in men with a PSA-density of ≥0.15 ng/mL2. Hence, when first stratifying according to a PSA-density cut-off ≥0.15 ng/mL2 in men with a PI-RADS 3 lesion, would result in a MRI-targeted biopsy reduction of 36% (18/50) in this category, without missing any upgrade to GS 3+4 or higher. These results are plotted in Figure 5 to visualize the amount of additional MRI testing with targeted biopsies, in men with initially low-risk disease on AS. The PSA-density thresholds of 0.15 and 0.20 ng/mL2 are depicted as dotted lines.
Even for PI-RADS 4 lesions, risk stratification by PSA-density could be beneficial if adjustment to potential risk inflation is performed: only 1% (1/82) of all upgrades would have been missed, reducing 43% (43/101) of targeted biopsies in this category.
PSA-density cut-off ≥0.20 ng/mL2
Stratifying according to the PSA-density cut-off ≥0.20 ng/mL2 would result in a targeted biopsy reduction of 44% (22/50) of all PI-RADS 3 index lesions, at the cost of missing 2% (2/82) upgrades; these 2 index lesions were classified as GS 3+4. When changing outcome to GS ≥4+3, the reduction of 44% in targeted biopsies of PI-RADS 3 lesions, coincides with not missing any upgrades to GS 4+3 or higher (Figures 4 and 5).
Discussion
In our data on men on AS, additional testing with MRI and targeted biopsies could be regarded as beneficial in 25% of men: additional testing resulted in an upgrade to GS ≥3+4 as compared to the GS 3+3 PCa based on systematic TRUS-guided biopsy findings. Our results matches well with upgrading data of other studies on AS, varying from 16% to 29% (21-27). This rate of upgrading was even higher (41%) as we consider only those men who had a positive MRI, defined as PI-RADS 3 to 5. However, the relevance of identifying and acting on this upgrading cannot be adequately interpreted without the presence of long-term data on cancer-specific survival. In fact, a similar cohort of men with low-risk disease, without additional testing by MRI and targeted biopsies, has a 15-year cancer-specific survival of 94.3% (6).
Autopsy data show that many men with intermediate-risk disease (Gleason 7) are never diagnosed and therefore have clinically ‘insignificant’ cancer (28,29). The Prostate Testing for Cancer and Treatment (ProtecT) trial, which compared in a randomized controlled manner three modalities of management (active monitoring, radical prostatectomy, and external beam radiotherapy) on patients with localized PCa (30), demonstrated no significant difference in the 10-year cancer-specific survival or overall survival.
In the ProtecT trial there was a difference in metastasis rate, however, favouring radical treatment at 10 years follow-up. This difference in metastasis rate is considered to result from the 25% of men who had intermediate- or high-risk disease, for whom active monitoring is clearly associated with an increased risk of progression. Nonetheless, the lack of a mortality difference emphasizes that the majority of Gleason 7 patients are not at risk in the 10-year time frame.
In the Sunnybrook surveillance cohort the 15-year metastasis rate was at least 20% in Gleason 7 cancer at initial diagnosis (31). In a recent study, however, no increase in metastasis rate or progression of intermediate risk patients on surveillance was reported compared to low risk, with up to 10 year follow-up (32). This suggests that many intermediate risk patients may still be candidates for AS (33).
The experiences described were from the pre-MRI era: men with Gleason 7 PCa at standard systematic biopsy sampling might have consisted partially of men with higher Gleason grades. Today, such patients will have the benefit of an MRI and targeted biopsies, with a high likelihood of a more accurate biopsy and hence better representative histopathology results (34). This may result in an unintended tightening of inclusion for AS, as schematically depicted (Figure 6).
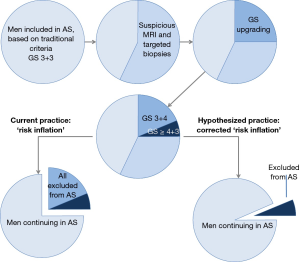
We detected 25% upgrading to Gleason 7 and higher, however, only 3% was Gleason 8 and higher, identical to other published reports (26,37). It is likely that only these men with Gleason 8 and higher disease significantly influenced cancer-specific survival in men classified as having low-risk disease, in which the overall prognosis showed to be excellent in the pre-MRI era (6). We may argue that low-risk patients upgraded with targeted biopsies to intermediate-risk disease should not be excluded from AS based on the Gleason criterion alone. The higher precision of MRI and targeted biopsies may create the opportunity to specify new risk thresholds that potentially could open AS to a larger group of patients. Treatment decisions should be based on multiple parameters next to patient age and co-morbidity, including percentage of Gleason 4, growth patterns (e.g., cribriform), PSA-density, and MRI findings.
Therefore, the major challenge is accurate patient selection for AS, without the burden of intensively additional testing. The additional testing by MRI and targeted biopsies, recommended in recent reviews on MRI in AS (8,36), comes with an extra invasive procedure, increasing the burden for patients staying in AS.
In this study, MRIs were abundantly or unnecessary performed in 75% of men in AS. Furthermore, unnecessary targeted biopsies were performed in 59% in men with a suspicious MRI (Figure 3), most prominent in PI-RADS 3 and 4 assessment category (Figure 2). Unnecessary testing would even be 93% for MRI and 88% for targeted biopsies, if we accept GS 3+4 as less significant disease. This critical evaluation of additional invasive testing is not to devaluate MRI, instead, this evaluation supports exploring refinements in the role of MRI at primary diagnosis and in AS, as proposed in Figure 6.
The PI-RADS steering committee recommended to biopsy lesions with PI-RADS category 4 and 5, and not lesions with PI-RADS category 1 and 2 (13). For findings with PI-RADS category 3, biopsy may or may not be appropriate, depending on factors other than MRI alone. In our study we biopsied all PI-RADS category 3 lesions, and detected high volume Gleason 4 pattern in 8% PI-RADS 3 lesions (all GS 4+3, no GS ≥4+4). We may argue that in some circumstances this might be acceptable, reducing the additional harms of additional biopsies. However, further stratification by PI-RADS, to biopsy only PI-RADS category 4 and 5 would results in missing high volume Gleason 4 pattern, as also confirmed by other studies (23,38).
We therefore additionally investigated the combination of PSA-density and MRI to further tailor the patient risk stratification in reducing unnecessary biopsies and improving the balance between the benefit and harms of additional testing. Using the PSA-density cut-off ≥0.15 ng/mL2 in men with a PI-RADS 3 lesion would result in a targeted biopsy reduction of 36% in this category, without missing any upgrade to GS 3+4 or higher (Figures 4 and 5). Even for PI-RADS 4 lesions, tailored risk stratification by PSA-density could be beneficial if adjustment to risk inflation is performed (Figures 4 and 5, Table 2).

Full table
Others have confirmed this correlation, however, data in men on AS is limited (37,39-41). In a multivariate cox-regression analysis, the PSA-density was shown to be a positive predictor to detect upgrading in men on AS, with a hazard ratio of 1.72 (40). In a receiver operating characteristic (ROC) analysis by Lai et al. the optimal PSA-density cut-off point was 0.18 ng/mL2 with an AUC of 0.77 (39). The optimal cut-off in men on AS should be further determined in larger cohorts. In a cohort of men with initially diagnosed low-risk disease by MRI/US fusion biopsy and monitoring with serial fusion biopsies, still PSA-density was an important predictor of subsequent upgrading (41). Our study clearly suggests that men on AS with a PI-RADS 3 index lesion and a PSA-density of <0.15 ng/mL2 may not benefit from a follow-up biopsy.
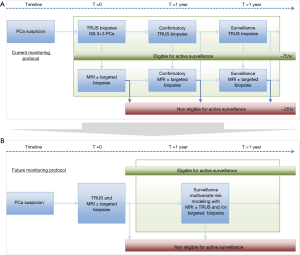
Incorporating prostate MRI at primary PCa diagnosis will result in better discrimination between true low-risk disease and intermediate-/high-risk disease. If TRUS-guided biopsies combined with MRI and targeted biopsies are able to minimise misclassification of PCa, we may abandon the currently used confirmation biopsy testing at 1 year in AS management, as depicted in Figure 7. MRI and targeted biopsies are increasingly used in the surveillance management of patients with clinically low-risk PCa; however, their role has not yet been established definitively (7). We may hypothesize that surveillance management of men with low-risk PCa will incorporate results from MRI and targeted biopsies into multivariate risk models in nearby future (Figure 7B) (42).
Our study comes with some limitations. First, our study is a retrospective design. Retrospective studies are known for their risk of selection bias. However, our study represents a prospectively monitored cohort of consecutive men on AS, with strict monitoring (within or without the PRIAS protocol). Second, we did not perform co-reading of MRIs, which likely would have increased detection sensitivity. However, even without co-reading the MRI detection rate in our cohort is comparable to those reported in recent publications on MRI in AS (21-27). Third, clinicians involved were not blinded to clinical data and MRI results. Hence, this process is daily clinical practice and therefore can be extrapolated to other hospitals. Fourth, the presence of standard systematic biopsy results in this study next to MRI-targeted biopsies would have shown the imperfection of MRI in detection all clinically significant PCa. Studies evaluating this added value are reporting up to 11% additionally found clinically significant PCa (8). As part of the monitoring protocol, the majority of men received their initial MRI without additional systematic biopsies. However, this group of men will decrease as a result of the increased introduction of MRI and targeted biopsies at the primary diagnostic work-up.
We acknowledge that the outcome measurement of our analysis was upgrading, based on MRI-revealed Gleason grading as recommended by the START consortium (15). Instead, the cancer-specific survival rate in long-term follow-up would have been more appropriate, especially in disputing the relevance of this high upgrading rate due to MRI targeted biopsies. However, this outcome may be debatable in a cohort of men with mostly low-risk disease that exhibits excellent long-term cancer-specific survival (6,35), and that furthermore experiences most shifts from AS to active treatment during the first 2 years of follow-up (43).
Conclusions
In this study on AS, we detected by MRI-targeted biopsies an upgrading to Gleason 7 and higher in 25%, however, only 3% was Gleason 8 and higher. This rate of upgrading was even higher (41%) as we consider only those men who had a suspicious finding on prostate MRI, defined as PI-RADS 3 to 5. Further stratification to PI-RADS 4–5 would have missed a small number of primary Gleason 4 PCa in the PI-RADS 3 category. Stratification with the combination of PI-RADS and PSA-density may reduce unnecessary additional MRI biopsy testing. We showed that men on AS with a PI-RADS 3 index lesion and a PSA-density of <0.15 ng/mL2 will not benefit from a follow-up targeted biopsy.
The high rate of detected upgrading may result in an unintended tightening of continuing in AS. Since patients, included under current surveillance criteria showed extremely favorable outcome, there might be no need to further restrict continuing on AS with MRI and targeted biopsies. The higher precision of MRI and targeted biopsies may create the opportunity to specify new risk thresholds that potentially could open AS to a larger group of patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was HIPAA compliant and was approved by the institutional ethical review board (NL45884.078.13/A301321). Written informed consent with guarantee of confidentiality was obtained from the participants.
References
- Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr 2012;2012:152-6. [Crossref] [PubMed]
- Heijnsdijk EA, de Carvalho TM, Auvinen A, et al. Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data. J Natl Cancer Inst 2014;107:366. [PubMed]
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Chen RC, Rumble RB, Loblaw DA, et al. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol 2016;34:2182-90. [Crossref] [PubMed]
- Kvåle R, Moller B, Wahlqvist R, et al. Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: a population-based study. BJU Int 2009;103:1647-54. [Crossref] [PubMed]
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272-7. [Crossref] [PubMed]
- Moore CM, Giganti F, Albertsen P, et al. Reporting Magnetic Resonance Imaging in Men on Active Surveillance for Prostate Cancer: The PRECISE Recommendations-A Report of a European School of Oncology Task Force. Eur Urol 2017;71:648-55. [Crossref] [PubMed]
- Schoots IG, Moore CM, Rouviere O. Role of MRI in low-risk prostate cancer: finding the wolf in sheep's clothing or the sheep in wolf's clothing? Curr Opin Urol 2017;27:238-45. [Crossref] [PubMed]
- Robertson NL, Hu Y, Ahmed HU, et al. Prostate cancer risk inflation as a consequence of image-targeted biopsy of the prostate: a computer simulation study. Eur Urol 2014;65:628-34. [Crossref] [PubMed]
- Alberts AR, Roobol MJ, Drost FH, et al. Risk-stratification based on magnetic resonance imaging and prostate-specific antigen density may reduce unnecessary follow-up biopsy procedures in men on active surveillance for low-risk prostate cancer. BJU Int 2017;120:511-9. [Crossref] [PubMed]
- van den Bergh RC, Roemeling S, Roobol MJ, et al. Prospective validation of active surveillance in prostate cancer: the PRIAS study. Eur Urol 2007;52:1560-3. [Crossref] [PubMed]
- Alberts AR, Schoots IG, Bokhorst LP, et al. Risk-based Patient Selection for Magnetic Resonance Imaging-targeted Prostate Biopsy after Negative Transrectal Ultrasound-guided Random Biopsy Avoids Unnecessary Magnetic Resonance Imaging Scans. Eur Urol 2016;69:1129-34. [Crossref] [PubMed]
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40. [Crossref] [PubMed]
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 2016;40:244-52. [PubMed]
- Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol 2013;64:544-52. [Crossref] [PubMed]
- Kotb AF, Tanguay S, Luz MA, et al. Relationship between initial PSA density with future PSA kinetics and repeat biopsies in men with prostate cancer on active surveillance. Prostate Cancer Prostatic Dis 2011;14:53-7. [Crossref] [PubMed]
- Abdi H, Zargar H, Goldenberg SL, et al. Multiparametric magnetic resonance imaging-targeted biopsy for the detection of prostate cancer in patients with prior negative biopsy results. Urol Oncol 2015;33:165 e1-7.
- Washino S, Okochi T, Saito K, et al. Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int 2017;119:225-33. [Crossref] [PubMed]
- Pessoa RR, Viana PC, Mattedi RL, et al. Value of 3-Tesla multiparametric magnetic resonance imaging and targeted biopsy for improved risk stratification in patients considered for active surveillance. BJU Int 2017;119:535-42. [Crossref] [PubMed]
- Wickham H. ggplot2. Elegant Graphics for Data Analysis. New york: Springer-Verlag; 2009.
- Da Rosa MR, Milot L, Sugar L, et al. A prospective comparison of MRI-US fused targeted biopsy versus systematic ultrasound-guided biopsy for detecting clinically significant prostate cancer in patients on active surveillance. J Magn Reson Imaging 2015;41:220-5. [Crossref] [PubMed]
- Walton Diaz A, Shakir NA, George AK, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol 2015;33:202.e1-202.e7. [Crossref] [PubMed]
- Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer 2016;122:884-92. [Crossref] [PubMed]
- Recabal P, Assel M, Sjoberg DD, et al. The Efficacy of Multiparametric Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Risk Classification for Patients with Prostate Cancer on Active Surveillance. J Urol 2016;196:374-81. [Crossref] [PubMed]
- Tran GN, Leapman MS, Nguyen HG, et al. Magnetic Resonance Imaging-Ultrasound Fusion Biopsy During Prostate Cancer Active Surveillance. Eur Urol 2017;72:275-81. [Crossref] [PubMed]
- Kamrava M, Kishan AU, Margolis DJ, et al. Multiparametric magnetic resonance imaging for prostate cancer improves Gleason score assessment in favorable risk prostate cancer. Pract Radiat Oncol 2015;5:411-6. [Crossref] [PubMed]
- Nougaret S, Robertson N, Golia Pernicka J, et al. The performance of PI-RADSv2 and quantitative apparent diffusion coefficient for predicting confirmatory prostate biopsy findings in patients considered for active surveillance of prostate cancer. Abdom Radiol (NY) 2017;42:1968-74. [Crossref] [PubMed]
- Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol 2002;167:1664-9. [Crossref] [PubMed]
- Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst 2013;105:1050-8. [Crossref] [PubMed]
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415-24. [Crossref] [PubMed]
- Yamamoto T, Musunuru B, Vesprini D, et al. Metastatic Prostate Cancer in Men Initially Treated with Active Surveillance. J Urol 2016;195:1409-14. [Crossref] [PubMed]
- Nyame YA, Almassi N, Haywood SC, et al. Intermediate-Term Outcomes for Men with Very Low/Low and Intermediate/High Risk Prostate Cancer Managed by Active Surveillance. J Urol 2017;198:591-9. [Crossref] [PubMed]
- Klotz L. Active Surveillance for Intermediate Risk Prostate Cancer. Curr Urol Rep 2017;18:80. [Crossref] [PubMed]
- Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68:438-50. [Crossref] [PubMed]
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol 2015;33:3379-85. [Crossref] [PubMed]
- Meng X, Rosenkrantz AB, Taneja SS. Role of prostate magnetic resonance imaging in active surveillance. Transl Androl Urol 2017;6:444-52. [Crossref] [PubMed]
- Pessoa RR, Viana PC, Mattedi RL, et al. Value of 3-Tesla multiparametric magnetic resonance imaging and targeted biopsy for improved risk stratification in patients considered for active surveillance. BJU Int 2017;119:535-42. [Crossref] [PubMed]
- Hansen NL, Barrett T, Koo B, et al. The influence of prostate-specific antigen density on positive and negative predictive values of multiparametric magnetic resonance imaging to detect Gleason score 7-10 prostate cancer in a repeat biopsy setting. BJU Int 2017;119:724-30. [Crossref] [PubMed]
- Lai WS, Gordetsky JB, Thomas JV, et al. Factors predicting prostate cancer upgrading on magnetic resonance imaging-targeted biopsy in an active surveillance population. Cancer 2017;123:1941-8. [Crossref] [PubMed]
- Radtke JP, Kuru TH, Bonekamp D, et al. Further reduction of disqualification rates by additional MRI-targeted biopsy with transperineal saturation biopsy compared with standard 12-core systematic biopsies for the selection of prostate cancer patients for active surveillance. Prostate Cancer Prostatic Dis 2016;19:283-91. [Crossref] [PubMed]
- Nassiri N, Margolis DJ, Natarajan S, et al. Targeted Biopsy to Detect Gleason Score Upgrading during Active Surveillance for Men with Low versus Intermediate Risk Prostate Cancer. J Urol 2017;197:632-9. [Crossref] [PubMed]
- Radtke JP, Wiesenfarth M, Kesch C, et al. Combined Clinical Parameters and Multiparametric Magnetic Resonance Imaging for Advanced Risk Modeling of Prostate Cancer-Patient-tailored Risk Stratification Can Reduce Unnecessary Biopsies. Eur Urol 2017;72:888-96. [Crossref] [PubMed]
- Duffield AS, Lee TK, Miyamoto H, et al. Radical prostatectomy findings in patients in whom active surveillance of prostate cancer fails. J Urol 2009;182:2274-8. [Crossref] [PubMed]


