Testosterone replacement therapy for physician assistants and nurse practitioners
Physician extenders are a growing force in healthcare today, often going beyond their initial role of complementing the growing shortage of primary care physicians (PCP). Currently, many physician assistants (PA) and nurse practitioners (NP) are moving toward specialty practices. However, mainstream curricula for PAs and NPs provide limited education in the specialty fields (1). Hypogonadism is a very common chief complaint in both endocrinology and urology (2). Of the males above age 40 who tested their testosterone levels, the incidence of low testosterone was 40% (3). Well trained PAs and NPs can manage most cases of hypogonadism requiring testosterone replacement therapy (TRT) independently. The goal of this review is to recognize the role and the limits to which physician extenders should be managing hypogonadism, and when physician collaboration or referral is necessary (4).
Introduction to testosterone and hypogonadism
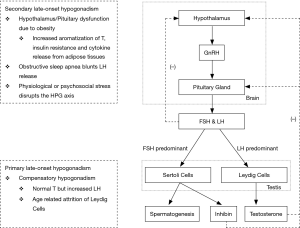
Testosterone is a steroid hormone that is predominantly produced by the Leydig cells in the testis and its production is controlled principally by luteinizing hormone (LH), a gonadotropin produced by the pituitary gland. Another gonadotropin, follicle stimulating hormone (FSH), acts on the Sertoli cells and aids in spermatogenesis. Both hormones are controlled by the production of gonadotropin-releasing hormone (GnRH) by the hypothalamus. These three organs create the hypothalamic-pituitary-gonadal (HPG) axis which ultimately controls the serum testosterone level. One of the functions of the HPG axis is the negative feedback system in which a normal or elevated serum testosterone level inhibits the production of GnRH, FSH and LH to prevent the overproduction of testosterone (5).
Hypogonadism is a deficiency of testosterone along with positive signs and symptoms. Testosterone production depends on two factors: the testes being able to produce the hormone, and the pituitary gland being able to stimulate the production of testosterone. Primary hypogonadism is when the pituitary gland is adequately producing FSH and LH, but the testis is incapable of producing enough testosterone due to underlying problems, such as a congenital defect or chemical exposure. These patients typically present with a low testosterone level and a high FSH and LH level (6). Secondary hypogonadism is when the pituitary gland is not producing enough FSH or LH to adequately stimulate the testis. These patients would present with low testosterone, FSH and LH levels (6). This is either due to underlying pathology within the pituitary gland or it can be due to problems with the hypothalamus (5,7). Prolactin, another hormone produced by the pituitary gland, directly inhibits GnRH production from the hypothalamus and hyperprolactinemia can also lead to secondary hypogonadism (5). Simply put, testicular pathology leads to primary hypogonadism while pituitary or hypothalamic pathology leads to secondary hypogonadism.
Testosterone decreases by 0.5–2% per year from age 30 and can eventually lead to late-onset hypogonadism (LOH) (8). Physiologically, the Leydig cells in the testis deteriorate with age, decreasing negative feedback and causing the pituitary gland to increase its secretion of LH as a compensatory response. At this stage, testosterone levels will be normal and LH levels will be elevated. This is known as compensatory hypogonadism. When this is coupled with the co-morbidities seen in the aging population, symptomatic hypogonadism can develop (6). Obesity and diabetes are co-morbidities that can inhibit the secretion of LH and disrupt this compensatory increase. Obesity increases the aromatization of testosterone, induces insulin resistance, and increases cytokine release from adipose tissues. The decreased LH means that the Leydig cells will be inadequately stimulated and secondary hypogonadism will develop (8).
A flowchart that summarizes the HPG axis and the physiology of hypogonadism can be seen in Figure 1.
Guidelines for TRT
Diagnosis
The Endocrine Society and the International Society of Sexual Medicine (ISSM) recommend TRT in men with symptomatic primary or secondary hypogonadism, as well as in adolescents with a delayed onset of puberty (9,10). The ISSM defines the normal testosterone level as above 346 ng/dL (12 nmol/L). The Endocrine Society as well as several other authors state that the normal range of serum testosterone depends on the laboratory performing the test and insurance companies. Regardless of what the normal values are, it is generally accepted that clinicians should correlate serum testosterone levels with the patient’s presenting symptoms when assessing the need for TRT (7).
Additionally, physician extenders should be aware of the increased prevalence of low testosterone in males with metabolic syndrome (11). Grosman et al. found that hypertension, increased triglycerides and elevated waist circumference have the strongest association with decreased testosterone (12). Dhindsa et al. found that 50% of obese diabetic men above 45 years old have low free testosterone levels (13). This association has been acknowledged by the Endocrine Society who have recommended routine testosterone measurement in all patients with type 2 diabetes mellitus (14).
The symptoms of hypogonadism include decreased libido, erectile dysfunction, testicular atrophy, decreased muscle mass and body hair, depression, low energy, and gynecomastia. Some males can even present with infertility (6,15). Although these may be non-specific findings, recognizing them in the context of low testosterone suggests clinically significant hypogonadism (7). Questionnaires, such as the Androgen Deficiency in the Aging Male (ADAM) questionnaire or the aging male symptoms (AMS) rating, can help evaluate the symptoms of hypogonadism. However, it is important to note that while these tests are very sensitive, they are not very specific (16-19).
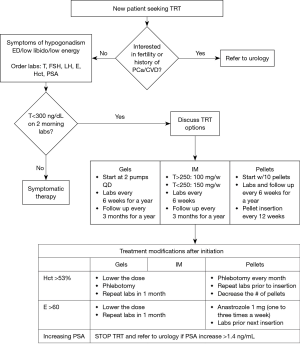
Patients who qualify for TRT should have their serum levels of free and total testosterone, estradiol, hematocrit, and PSA measured before starting TRT. Ideally, the diagnosis is made with two low morning serum total testosterone levels (7,15,20,21).
Treatment
Once hypogonadism is diagnosed and TRT is decided as the treatment option, the different options for TRT must be discussed. There are several ways to administer TRT and methods include topical preparations, intranasal gels, intramuscular (IM) injections and subdermal implants (7,9,22). The advantages and disadvantages of each are shown in Table 1.
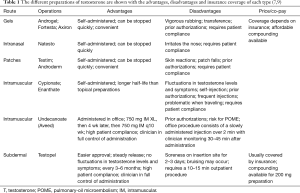
Full table
Gels and patches are the two types of topical preparations. Patches often cause skin irritation, while gels run the risk of transference to others; however, this can be avoided with a clothing barrier (23,24). Intranasal gels need to be reapplied during the day and can cause irritation to the nasal passage (25). One of the benefits of the topical and intranasal preparations is that the dose can be quickly altered when needed (7).
IM options include testosterone cypionate and enanthate, which are self-administered once every 1–4 weeks. Most clinicians start 100 mg weekly or 200 mg every 2 weeks and titrate as needed depending on follow up labs. IM testosterone has been shown to have a greater risk of side effects than other preparations (7,26). As with the other self-administered medications, non-compliance and patients self-adjusting the dose are important factors that affect treatment and require patient education. With proper compliance and education, they can be beneficial as patients will be able to avoid trips to the clinic once the dose is properly titrated.
Testosterone undecanoate (750 mg/3 mL) is a long acting preparation of IM testosterone that needs to be administered with an initial dose of 750 mg, followed 4 weeks later by another 750 mg dose. After that, the 750 mg IM injection should be administered once every 10 weeks. This preparation should be administered in the office as a slow injection over 2 min and patients should be monitored for 30 to 45 min after administration because of an uncommon but real risk of pulmonary-oil micro embolism (POME) (27).
Subdermal implants are the option for extended release TRT. They are administered in a 10 to 15 min procedure in the office and this is repeated every 3 to 6 months depending on follow up laboratory results. Patients tend to like this option because they do not have to self-inject or apply gels repeatedly (7). It is particularly helpful in patients who travel regularly, and the extended release decreases the “up and down” feeling often experienced with the IM injections. This “up and down” phenomenon is due to the variable release of the hormone into the bloodstream leading to peaks and troughs beyond the normal range of serum testosterone levels (5,7,28,29). The disadvantages of subdermal implants include the need for regular office visits, pain and bruising at the site of insertion, as well as the minimal risk of infection and pellet extrusion (7).
Contraindications and adverse effects
A thorough review of the patient’s past medical history when evaluating the patient for TRT is vital as patients with certain co-morbidities may need to be referred to a physician or they may be contraindicated for TRT (4).
TRT is contraindicated in men with benign prostatic hyperplasia (BPH) and severe lower urinary tract symptoms (LUTS). If the International Prostate Symptom Score (IPSS) is above 19, which indicates severe symptoms (usually due to an enlarged prostate), the patient should be referred to a urologist (7,30). The effect of testosterone on prostate cancer (PCa) development is controversial although most studies show no association between TRT and PCa (31-36). The Endocrine Society recommends against TRT in patients with a history of PCa, while ISSM recommends that it can only be prescribed and followed up by urologists (9,10). Therefore, if PCa is suspected or PSA elevation is noticed on TRT, referral to urology is advisable.
The assessment of the cardiovascular risk associated with TRT yields conflicting results. The ISSM evaluated multiple studies and concluded that the studies showing an association between TRT and cardiovascular risk have systematic errors (37-39). Higher quality studies often showed no association, and some even showed a decrease in mortality with TRT (36,40-44). However, it is important to note the FDA has concluded that there is a possible increased risk of cardiovascular events (such as a myocardial infarction and stroke) with TRT and all testosterone prescribers should discuss these risks with their patients. A possible mechanism supporting the risk for cardiovascular events would be polycythemia, considering that testosterone stimulates erythropoietin (EPO) production. With that said, erythrocytosis with a hematocrit of over 50% is a contraindication for testosterone therapy and if the hematocrit exceeds 53% while the patient is on TRT, a phlebotomy along with a decrease in TRT dose is recommended (5,7,36). A complete list of indications and contraindications of TRT is shown in Table 2.

Full table
Apart from the increased hematocrit that was previously discussed, another side effect with TRT is decreased spermatogenesis because testosterone is a contraceptive, albeit a poor one (30). Exogenous testosterone inhibits gonadotropin production and decreases intra-testicular production of testosterone, leading to decreased fertility and testicular atrophy (30). TRT can also manipulate lipid levels. An increase in total cholesterol, a decrease in HDL, and a minimal change in LDL have been demonstrated in some studies; but these changes are more commonly associated with diet and is normally managed by the PCP (22,35,36). TRT can also increase serum estradiol levels, which can lead to gynecomastia. Aromatase inhibitors, such as anastrozole, are used to inhibit the peripheral conversion of testosterone into estradiol (45). TRT is also linked with an increase in serum prostate specific antigen (PSA) levels and up to a 1.4 ng/mL increase with TRT is allowed by the Endocrine Society guidelines. Urology referral is required if there is any increase beyond that (7,9). Additionally, TRT can worsen symptoms of obstructive sleep apnea (9).
The discussion about the association between TRT and venous thromboembolism (VTE), such as deep vein thromboses (DVT) and pulmonary embolism (PE) is controversial. The FDA requires prescribers to discuss the risks of VTE with patients (46). Some studies describe observing this adverse effect among patients using TRT, like Martinez et al. in 2016 (47). However, various other studies have shown no association between the two. Sharma et al. concluded that there was no increase in the risk of DVT after starting TRT (48). Another study among 217 men over the age of 65 showed no difference in the prevalence of thrombotic events in patients with and without TRT (49). In summary, the evidence linking VTEs and TRT is mixed and should be discussed with the patient.
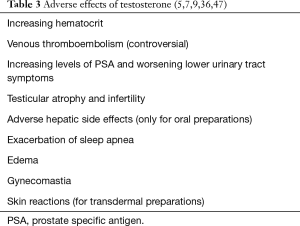
A complete list of adverse effects due to TRT is shown in Table 3.
An algorithm that summarizes TRT prescription and follow-up is shown in Figure 2.
Financial benefits
One of the important considerations of allowing physician extenders to manage TRT and other non-complicated specialty cases is the financial benefit. Many practices use current procedural terminology (CPT) codes which assigns a specific relative value unit (RVU) for each service or procedure provided by the healthcare provider. This is a standardized way to determine reimbursements for physicians and physician extenders. Physician extenders typically receive 85% of the physician’s rate for the same service and the same number of RVUs, as allowed by the scope of practice in the state of practice. However, if the physician extender provides a service while the physician is in the office the practice is reimbursed 100% even though the physician did not provide the service (50).
Physician extenders in the outpatient setting allows physicians to attend to new patients and more complicated cases, which are typically billed as Level 4 and receive better RVUs than non-complicated cases and follow ups. Simultaneously, the physician extender can attend to the TRT patients, which are typically billed as Level 3 and have lower RVUs. All encounters are billed under the physician’s name which significantly increases RVUs and overall practice revenue. On top of the financial gain, this also allows more appointments to be available to the patients (51). These benefits have been documented in emergency medicine and oncology (52,53).
A pilot study was conducted at our practice where one physician referred patients to one nurse practitioner one clinic day a week for 6 months. The total charges billed amounted to $413,000 and the net collections generated by the nurse practitioner was over $103,900 after taking the cost of the equipment used into account. The revenue during the pilot covered over 100% of the annual salary of this nurse practitioner.
Conclusions
Symptomatic hypogonadism is a common complaint in urology offices and TRT is the gold standard of treatment. Physician extenders, like NPs and PAs, are capable of independently diagnosing and evaluating men with low testosterone levels and starting them on TRT when appropriate. It is vital that physician extenders understand the indications, risks, and adverse effects to ensure that patients are counselled and treated appropriately. Physician extenders must be familiar with the guidelines and recommendations about monitoring patients on TRT and must clearly understand when to consult and refer the patient to a specialist. Physician extenders do play an important role in the safe management of patients on TRT, as long as a proper treatment algorithm is established by the physician.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- (AACN) AAoCoN. Criteria for Evaluation of Nurse Practitioner Programs 5th Edition. 2016. Available online: http://www.nonpf.org/?page=15
- Samplaski MK, Loai Y, Wong K, et al. Testosterone use in the male infertility population: prescribing patterns and effects on semen and hormonal parameters. Fertil Steril 2014;101:64-9. [Crossref] [PubMed]
- Smyth C, Sulkes D, Bhan J, et al. Incidence of Low Testosterone Is 40% in Tested Men > 40 Years; Highest Prevalence Found in Men Living in Southern States: Results from a Nationwide Database. Available online: http://www.prognos.ai/wp-content/uploads/AACE2014_lowtestosterone_Abstract-1353.final_.pdf
- Lee Y. Androgen deficiency syndrome in older people. J Am Assoc Nurse Pract 2014;26:179-86. [Crossref] [PubMed]
- Basaria S. Male hypogonadism. Lancet 2014;383:1250-63. [Crossref] [PubMed]
- Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag 2009;5:427-48. [PubMed]
- Srinivas-Shankar U, Sharma D. Testosterone treatment in elderly men. Adv Ther 2009;26:25-39. [Crossref] [PubMed]
- Rastrelli G, Carter EL, Ahern T, et al. Development of and Recovery from Secondary Hypogonadism in Aging Men: Prospective Results from the EMAS. J Clin Endocrinol Metab 2015;100:3172-82. [Crossref] [PubMed]
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536-59. [Crossref] [PubMed]
- Dean JD, McMahon CG, Guay AT, et al. The International Society for Sexual Medicine's Process of Care for the Assessment and Management of Testosterone Deficiency in Adult Men. J Sex Med 2015;12:1660-86. [Crossref] [PubMed]
- Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care 2011;34:1669-75. [Crossref] [PubMed]
- Grosman H, Rosales M, Fabre B, et al. Association between testosterone levels and the metabolic syndrome in adult men. Aging Male 2014;17:161-5. [Crossref] [PubMed]
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010;33:1186-92. [Crossref] [PubMed]
- Dandona P, Dhindsa S. Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab 2011;96:2643-51. [Crossref] [PubMed]
- Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol 2008;159:507-14. [Crossref] [PubMed]
- Morley JE, Perry HM 3rd, Kevorkian RT, et al. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas 2006;53:424-9. [Crossref] [PubMed]
- Chueh KS, Huang SP, Lee YC, et al. The comparison of the aging male symptoms (AMS) scale and androgen deficiency in the aging male (ADAM) questionnaire to detect androgen deficiency in middle-aged men. J Androl 2012;33:817-23. [Crossref] [PubMed]
- Emmelot-Vonk MH, Verhaar HJ, Nakhai-Pour HR, et al. Low testosterone concentrations and the symptoms of testosterone deficiency according to the Androgen Deficiency in Ageing Males (ADAM) and Ageing Males' Symptoms rating scale (AMS) questionnaires. Clin Endocrinol (Oxf) 2011;74:488-94. [Crossref] [PubMed]
- Bernie AM, Scovell JM, Ramasamy R. Comparison of questionnaires used for screening and symptom identification in hypogonadal men. Aging Male 2014;17:195-8. [Crossref] [PubMed]
- Grech A, Breck J, Heidelbaugh J. Adverse effects of testosterone replacement therapy: an update on the evidence and controversy. Ther Adv Drug Saf 2014;5:190-200. [Crossref] [PubMed]
- Lawrence KL, Stewart F, Larson BM. Approaches to male hypogonadism in primary care. Nurse Pract 2017;42:32-7. [Crossref] [PubMed]
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:5-15. [Crossref] [PubMed]
- Holyoak JD, Crawford ED, Meacham RB. Testosterone and the prostate: implications for the treatment of hypogonadal men. Curr Urol Rep 2008;9:500-5. [Crossref] [PubMed]
- Stahlman J, Britto M, Fitzpatrick S, et al. Serum testosterone levels in non-dosed females after secondary exposure to 1.62% testosterone gel: effects of clothing barrier on testosterone absorption. Curr Med Res Opin 2012;28:291-301. [Crossref] [PubMed]
- Testosterone nasal gel (Natesto) for hypogonadism. Med Lett Drugs Ther 2015;57:73-4. [PubMed]
- Layton JB, Meier CR, Sharpless JL, et al. Comparative Safety of Testosterone Dosage Forms. JAMA Intern Med 2015;175:1187-96. [Crossref] [PubMed]
- Middleton T, Turner L, Fennell C, et al. Complications of injectable testosterone undecanoate in routine clinical practice. Eur J Endocrinol 2015;172:511-7. [Crossref] [PubMed]
- Di Luigi L, Sgrò P, Aversa A, et al. Concerns about serum androgens monitoring during testosterone replacement treatments in hypogonadal male athletes: a pilot study. J Sex Med 2012;9:873-86. [Crossref] [PubMed]
- Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab 1980;51:1335-9. [Crossref] [PubMed]
- Crosnoe LE, Grober E, Ohl D, et al. Exogenous testosterone: a preventable cause of male infertility. Transl Androl Urol 2013;2:106-13. [PubMed]
- Morgentaler A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol 2006;50:935-9. [Crossref] [PubMed]
- Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol 2013;189:S26-33. [Crossref] [PubMed]
- Endogenous Hormones and Prostate Cancer Collaborative Group, Roddam AW, Allen NE, et al. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 2008;100:170-83. [Crossref] [PubMed]
- Muller RL, Gerber L, Moreira DM, et al. Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the Reduction by Dutasteride of Prostate Cancer Events trial. Eur Urol 2012;62:757-64. [Crossref] [PubMed]
- Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 2005;60:1451-7. [Crossref] [PubMed]
- Fernandez-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010;95:2560-75. [Crossref] [PubMed]
- Xu L, Freeman G, Cowling BJ, et al. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013;11:108. [Crossref] [PubMed]
- Vigen R, O'Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 2013;310:1829-36. [Crossref] [PubMed]
- Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One 2014;9:e85805. [Crossref] [PubMed]
- Baillargeon J, Urban RJ, Kuo YF, et al. Risk of Myocardial Infarction in Older Men Receiving Testosterone Therapy. Ann Pharmacother 2014;48:1138-44. [Crossref] [PubMed]
- Shores MM, Smith NL, Forsberg CW, et al. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab 2012;97:2050-8. [Crossref] [PubMed]
- Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol 2013;169:725-33. [Crossref] [PubMed]
- Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf 2014;13:1327-51. [Crossref] [PubMed]
- Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of Testosterone Treatment in Older Men. N Engl J Med 2016;374:611-24. [Crossref] [PubMed]
- Tan RS, Cook KR, Reilly WG. High estrogen in men after injectable testosterone therapy: the low T experience. Am J Mens Health 2015;9:229-34. [Crossref] [PubMed]
- FDA adding general warning to testosterone products about potential for venous blood clots. US Food and Drug Administration, Food and Drug Administration. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm401746.htm
- Martinez C, Suissa S, Rietbrock S, et al. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ 2016;355:i5968. [Crossref] [PubMed]
- Sharma R, Oni OA, Chen G, et al. Association Between Testosterone Replacement Therapy and the Incidence of DVT and Pulmonary Embolism: A Retrospective Cohort Study of the Veterans Administration Database. Chest 2016;150:563-71. [Crossref] [PubMed]
- Ramasamy R, Scovell J, Mederos M, et al. Association Between Testosterone Supplementation Therapy and Thrombotic Events in Elderly Men. Urology 2015;86:283-5. [Crossref] [PubMed]
- Buppert C. Billing For Nurse Practitioner Services: Guidelines for NPs, Physicians, Employers, and Insurers. Available online: http://www.medscape.com/viewarticle/422935_5
- Pickard T. Calculating your worth: understanding productivity and value. J Adv Pract Oncol 2014;5:128-33. [PubMed]
- Moote M, Nelson R, Veltkamp R, et al. Productivity assessment of physician assistants and nurse practitioners in oncology in an academic medical center. J Oncol Pract 2012;8:167-72. [Crossref] [PubMed]
- Brook C, Chomut A, Jeanmonod RK. Physician assistants contribution to emergency department productivity. West J Emerg Med 2012;13:181-5. [Crossref] [PubMed]