Chronic scrotal pain may lead to reduced sexual function and interest, while sexual activity may worsen chronic scrotal pain: “double jeopardy”
Introduction
There is very little data on the true incidence of chronic scrotal pain (CSP): estimates from studies from Switzerland based on a survey of urologists suggested an incidence of 350–400/100,000 cases of CSP yearly (1), while studies on men presenting to physician offices for unrelated conditions found the incidence to be much higher at 4.75% (2).
CSP refers to an ill understood complex of symptoms. Various terms have been used to describe the condition including orchialgia and orchiodynia, which really refer to the testicle itself, and chronic epididymitis. Both the European Urological Association (EAU) and the International Continence Society have adopted the generic term scrotal pain syndrome to include testicular pain syndrome, post-vasectomy pain syndrome, and epididymal pain syndrome (3-5).
It is well recognized that chronic pain in general may affect sexual function (6,7) due to the debilitating nature of chronic pain there may often be a lack of interest and diminished performance, but patients with scrotal/groin pain often have the additional problem of sex itself being painful, which may in turn be associated with a downhill spiral of reduced interest, diminished performance and actively avoiding sex (6-9). This then may be associated with relationship issues (9).
Unsurprisingly, sexual dysfunction heightens anger, frustration, and depression, all of which place a strain on a relationship (9). The partners of men with sexual dysfunction and depression often present with similar symptoms (10).
Although the available literatures recognizes sexuality as a very important factor in the quality of life (QoL) and well-being of patients and in spite of the fact that the sexual life is known to be highly disturbed in CSP patients, there are no previous studies that have evaluated the frequency and the importance of changes in the sexual desire or function in patients with CSP. In addition, the impact of sexual activity on CSP symptoms is recognized but poorly quantified.
The purpose of this study is to describe the impact of CSP on the sexual life of patients and conversely describe the impact of sexual activity on CSP.
Methods
Institution Research Ethics Board (REB) approval was obtained prior to commencement of the study. This is a retrospective review of a prospectively collected database of men presenting between Feb 2014 and Sep 2015 to a university program specializing in CSP. This clinic is a multi-disciplinary clinic in which the patient is evaluated simultaneously both by a specialist in pain medicine and urologists with special expertise in the investigation and management of CSP. The men completed a standardized questionnaire to elicit general information on the characteristics of the CSP with specific questions to identify activities and factors that aggravate or relieve the CSP.
A standardized questionnaire was used to obtain a detailed pain history including a validated numeric pain scale to quantify pain levels from 1–10 (11,12) (addendum attached). Validated questionnaires were used to elicit information on the impact on the men’s lives of the CSP (12). Impact on sexual life were measured with a standardized questionnaire validated for screening of sexual dysfunction (sexual health inventory for men: SHIM). The five questions are answered with a sum of ≤21 indicating sexual dysfunction (Table 1) (13).

Full table
A validated questionnaire to identify symptoms of testosterone deficiency (androgen deficiency in the aging male: ADAM score) was also completed by the men. This questionnaire has been validated as a screening test to identify men with symptoms of testosterone deficiency with a positive result if the patient answers, “Yes”, to questions 1 or 7 or any 3 (questions about libido and sexual capacity) of the other questions (14).
Mood was assessed by two validated questions: “during the past month, have you often been bothered by feeling down, depressed, or hopeless?” and “during the past month, have you often been bothered by little interest or pleasure in doing things?” with the standard answers being “not at all”, “several days”, “more than half the days”, and “nearly every day” for both of these questions (15).
If patients reported depressive symptoms, the patients were directly questioned about suicidal thoughts.
The information on the questionnaires was retrospectively reviewed.
The aim of this study is to describe the impact of CSP on the sexual life of patients and the impact of sexual activity on the symptoms of CSP.
Results
From Feb 2014 to Sep 2015, a total of 131 men presenting for assessment of CSP completed questionnaires. The mean age of the men was 43±12 (SD) years with a mean duration of CSP of 4.7±5.95 years.
The underlying reported etiology of CSP varied from surgery [vasectomy (20.6%), hernia repair (4.6%), trans-urethral prostatectomy, hydrocelectomy, nephrectomy, orchidectomy and knee surgery (total 8.5%)], trauma (12%), infections (11.5%) and idiopathic (43.5%).
The severity of the CSP was also variable, with the men reporting that the severe pain episodes occurred with an average pain score of 7.2±2 (on a 10-point numeric scale) with episodes occurring on average 40%±30% of the time.
A high number of men complained about the CSP keeping them from normal sexual activities, with close to 60% noting that the CSP kept them from normal activities either “a lot” or “some” (Table 2). Close to 40% of these patients (45/116) noted that the CSP in general affected their sexual activity “a lot”, while 42% (49/116) noticed some negative impact and only 23% (22/97) noted no effect on sexual activity. The more severe the pain level, the greater the impact on sexual activity, with 54% (45/83) of men having pain scores between 7–10 out of 10 (Pain scores at or above the average pain scores for the group) noting that the pain kept them from normal sexual activity “a lot” compared to 0% (0/33) of the men with pain levels between 1–6 (P<0.01: Fisher’s exact test). Conversely, 39% (13/33) of the men with pain scores between 1–6 reported no limitations on normal sexual activity, compared to only 10% (9/89) of the men with higher pain scores (P<0.01: chi squared test).
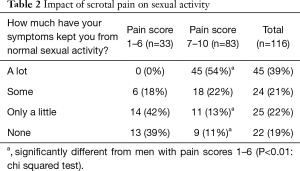
Full table
While many complained that the CSP kept them from normal sexual activities, many also were found to have sexual dysfunction.
Overall, 63% (27/43) of the men who answered the SHIM questionnaire, had scores of ≤21 indicating some erectile dysfunction (Table 3). Not surprisingly, men with more severe pain were significantly more likely to have evidence of sexual dysfunction, with 70% of the men with pain scores of 7 or higher having SHIM scores in the range indicating sexual dysfunction, compared to 40% in men with pain scores of 6 or lower (P<0.01: Fisher’s exact test).

Full table
Unfortunately, very few men answered the question about sexual frequency, but of those who did, interestingly, 25% (7/28) reported having no sex at all while 21.42% (6/28) reported having sex once monthly.
Overall, 43% (55/128) of men with CSP reported that they had diminished libido based on the ADAM score (Table 4). Unlike the other questions on sexual function, diminished libido was not more common in men with more severe pain. Among our patients, 76% (97/128) overall had a positive ADAM score. Men with more severe pain were significantly more likely to have a positive ADAM score with 79% (70/89) of the men with pain scores 7 or higher being positive compared to 69% (27/39) of the men with pain scores 6 or lower (P<0.01: chi squared test).
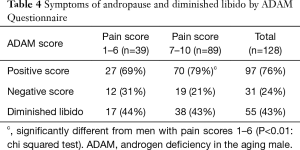
Full table
Sex and ejaculations may worsen the pain associated with CSP (Table 5). Among our patients, 38% (48/128) reported that pain increased with or following sex. Patients who had more severe pain were significantly more likely to have a worsening of the pain following sex, with 44% (39/89) of the men with pain scores from 7–10 out of 10 finding that sex worsened the pain, compared to 23% (9/39) of men with pain scores less than 7 (P<0.01: chi squared test). Patients also frequently reported that ejaculation worsened the pain, with increasing pain following ejaculation in 37% (47/128) of the men. Again, men with more severe pain were more likely to experience worsening of pain following ejaculation, with 40% (36/89) with pain scores 7 or higher finding that ejaculation increases pain levels, compared to 28% (11/39) for those with pain scores 6 or lower. Interestingly, increase in pain was not universal following ejaculation, since 1.5% (2/128) noticed a reduction in pain following ejaculation.
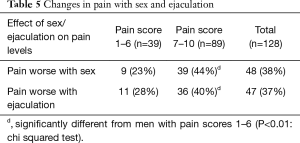
Full table
Discussion
Both chronic pain and sexual dysfunction are common problems managed by physicians. Pain is considered chronic when it exceeds 3 months in duration (16). When pain becomes chronic, it increases in complexity and leads to various psycho-social problems, including marital dissatisfaction and distress (17). A study by Flor et al. (18) regarding the impact of chronic pain of different origins on the spouse revealed that in 67% of the patients, the marital relationship was negatively affected by the chronic pain with a significant reduction in marital satisfaction.
An association between chronic pain in general and sexual dysfunction has been recognized, Osborne and Maruta (19) examined marital and sexual adjustment in chronic pain patients. In their study, 50 married chronic pain patients and their spouses were interviewed independently. Seventy-eight percent of their patients reported either elimination (16%) or reduction (62%) in sexual activity. However, 65% of their patients reported coital frequency of at least once a week (in 42% the frequency was 2–3 times a week) post onset of pain.
In another study, Maruta and Osborne (8) studied sexual activity in 66 married patients with a history of chronic pain of non-malignant origin for 6 months or more. Almost two thirds reported deterioration in sexual activity, with decreased frequency and quality of sexual functioning. Sixty-eight percent of patients were experiencing problems with orgasmic activities. There was a profound effect on libido with 58% of the patients noting a lack of sexual interest.
Unfortunately, chronic pain and its treatment can impair our ability to express sexuality by decreasing patients’ sexual interest and function. Few studies have evaluated the frequency of changes in sexual desire or function in people with chronic pain. One study of patients enrolled in chronic pain treatment programs in England found that 73% had some degree of sexual problems (9). Other studies support these findings (20-22). Approximately one half to two thirds of patients report reduced frequency in their sexual relationships as a result of chronic pain.
There was an indication from this present study that CSP was associated with both a loss of sexual interest and decreased sexual functioning. With specific questioning, 43% of the men with CSP reported a significant decline in libido, 64% had evidence of sexual dysfunction and 60% felt that the CSP had kept them from normal sexual activity either a “lot” or at least “some” of the time. It was likely a multi-factorial cause for the diminished libido and sexual function, possibly including the debilitation from the chronic pain itself, depression (reported in 33% of the men in our study), reduced QoL due to both the pain and the limitations caused by the chronic pain and the negative feedback experience by many of the men with CSP from worsening pain with sexual activity (38%) and ejaculations (37%).
The effect on sexual functioning and interest seems to be most profound in men with more severe pain with men having pain scores of 7/10 or higher being significantly more likely to report that the pain has kept them from their normal sexual activity, have evidence of sexual dysfunction (reduced ability to have adequate erections for satisfactory sexual activity), have symptoms of testosterone deficiency and have worsening of pain with sex and/or ejaculation than men with pain scores 6/10 or lower.
This study is limited by the cross sectional nature of the study and given that the patients were referred to a specialized scrotal pain clinic, the results may not be generalizable.
In summary, there is double jeopardy for men with CSP: they have diminished libido and sexual function likely due to the chronic pain and because the pain is in the scrotum, sex and ejaculations may exacerbate the pain, making it difficult for the men to continue with sex leading to a further loss of interest. This is in turn, is associated with a worsened QoL for the men.
Conclusions
Clinicians treating men with CSP should be aware of the twin prongs of the chronic pain condition associated with a reduced QoL and reduced interest in sex as well as sexual activity itself being associated with increasing pain which in turn is also associated with an even further reduction in sexual interest, sexual function and lower QoL.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institution Research Ethics Board (REB) approval was obtained prior to commencement of the study.
References
- Strebel RT, Leippold T, Luginbuehl T, et al. Chronic scrotal pain syndrome: management among urologists in Switzerland. Eur Urol 2005;47:812-6. [Crossref] [PubMed]
- Ciftci H, Savas M, Yeni E, et al. Chronic orchialgia and associated diseases. Current Urology 2010;4:67-70. [Crossref]
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187:116-26. [Crossref] [PubMed]
- Fall M, Baranowski AP, Elneil S, et al. EAU guidelines on chronic pelvic pain. Eur Urol 2010;57:35-48. [Crossref] [PubMed]
- Fall M, Baranowski AP, Fowler CJ, et al. EAU guidelines on chronic pelvic pain. Eur Urol 2004;46:681-9. [Crossref] [PubMed]
- Anderson RU, Wise D, Sawyer T, et al. Sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome: improvement after trigger point release and paradoxical relaxation training. J Urol 2006;176:1534-8; discussion 1538-9. [Crossref] [PubMed]
- Ciftci H, Savas M, Gulum M, et al. Evaluation of sexual function in men with orchialgia. Arch Sex Behav 2011;40:631-4. [Crossref] [PubMed]
- Maruta T, Osborne D. Sexual activity in chronic pain patients. Psychosomatics 1978;19:531-7. [Crossref] [PubMed]
- Ambler N, Williams AC, Hill P, et al. Sexual difficulties of chronic pain patients. Clin J Pain 2001;17:138-45. [Crossref] [PubMed]
- Shabsigh R, Anastasiades A, Cooper KL, et al. Female sexual dysfunction, voiding symptoms and depression: common findings in partners of men with erectile dysfunction. World J Urol 2006;24:653-6. [Crossref] [PubMed]
- Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S240-52. [Crossref] [PubMed]
- Nickel JC, Siemens DR, Nickel KR, et al. The patient with chronic epididymitis: characterization of an enigmatic syndrome. J Urol 2002;167:1701-4. [Crossref] [PubMed]
- Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res 2005;17:307-19. [Crossref] [PubMed]
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000;49:1239-42. [Crossref] [PubMed]
- Whooley MA, Avins AL, Miranda J, et al. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med 1997;12:439-45. [Crossref] [PubMed]
- Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986;3:S1-226. [PubMed]
- Block AR. Investigation of the response of the spouse to chronic pain behavior. Psychosom Med 1981;43:415-22. [Crossref] [PubMed]
- Flor H, Turk DC, Scholz OB. Impact of chronic pain on the spouse: marital, emotional and physical consequences. J Psychosom Res 1987;31:63-71. [Crossref] [PubMed]
- Osborne D, Maruta T. Sexual adjustment and chronic back pain. Med Aspects Hum Sex 1980;14:94-113.
- Lutz MC, Roberts RO, Jacobson DJ, et al. Cross-sectional associations of urogenital pain and sexual function in a community based cohort of older men: olmsted county, Minnesota. J Urol 2005;174:624-8; discussion 628. [Crossref] [PubMed]
- Muller A, Mulhall JP. Sexual dysfunction in the patient with prostatitis. Curr Opin Urol 2005;15:404-9. [Crossref] [PubMed]
- Trinchieri A, Magri V, Cariani L, et al. Prevalence of sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome. Arch Ital Urol Androl 2007;79:67-70. [PubMed]


