Low dose rate prostate brachytherapy
Introduction
Low dose rate (LDR) brachytherapy is one of many proven radiation therapy strategies that are recommended for the curative treatment of men with prostate cancer. Derived from the Greek term “brakhus” or “brachy”, which translates to “short”, brachytherapy refers to radiation therapy techniques which are delivered by placing radioactive sources into or very near target tissues. Brachytherapy is commonly used to treat a variety of malignancies including cervical, uterine, breast, ocular, and skin cancers. In the context of prostate cancer, LDR brachytherapy is also frequently referred to as permanent prostate brachytherapy, given that the implanted radioactive sources are left within the prostate. This approach differs from high dose rate (HDR) brachytherapy, which is another radiation technique used to treat prostate cancer, where higher activity radioactive sources are temporarily placed into the prostate via catheter needles and then removed once the appropriate dose has been delivered.
The first documented use of use of brachytherapy for prostate cancer was in 1914, when Pasteau and Degrais reported the insertion of a radium source via a urethral catheter (1). Dr. Barringer from Memorial Hospital in New York subsequently described interstitial prostatic placement of radioactive needles, a breakthrough development that served as the genesis of the modern prostate brachytherapy technique (2). In the 1970’s, retropubic implantation of radiation sources via an open laparotomy incision was utilized with some frequency, but this technique was hindered by the inherent risks and logistics of a surgical procedure. Furthermore, radiation dosing was often suboptimal due to the free-hand, relatively blind source placement (3,4). Ultimately, with the development and refinement of trans-rectal ultrasound (TRUS) imaging, transperineal image-guided techniques emerged and have continued to evolve into the safe, effective, and modern LDR brachytherapy approaches used today (5).
Rationale
The use of LDR brachytherapy for the management of prostate cancer is driven both by the practical aspects of the technique and the biologic behavior of the disease. With the anatomical location of the prostate, accurate deposition of radioactive sources can be reliably performed via a minimally-invasive transperineal approach under TRUS image guidance. From a radiobiologic standpoint, the degree of dose escalation provided by LDR brachytherapy, compared to other radiation techniques, may be more effective in killing tumor cells (6). Most patients receiving definitive external beam radiation therapy (EBRT) in the modern era receive escalated doses of 74–86.4 Gray (Gy), which have been shown to improve biochemical control rates compared to doses less than 70 Gy (7). However, further dose escalation with EBRT can be limited by increasing risks of toxicity involving the bladder and rectum (8). While the physical prescription doses commonly used with LDR brachytherapy are approximately 1.5–2 times higher than those with EBRT, their biologic effective dose (BED) can be up to 3 times greater (9). Increasing BED, which is a parameter used to compare different dose delivery regimens, is calculated based on expected tissue responses to radiation and has been shown to be an important predictor of improved clinical outcomes in patients receiving radiation for prostate cancer (10). While the prescription dose for brachytherapy is typically intended to cover the whole prostate gland, doses greater than 150% of the prescription dose are deposited in regions in close proximity (<5 mm) to the implanted radionuclides within the prostate (Figure 1A). Given that many sources are often implanted in the peripheral zone, these focal “hot spots” are ideally distributed in the regions where most prostate cancers develop. This degree of focal dose heterogeneity is not possible with EBRT and may further improve cancer control (11).
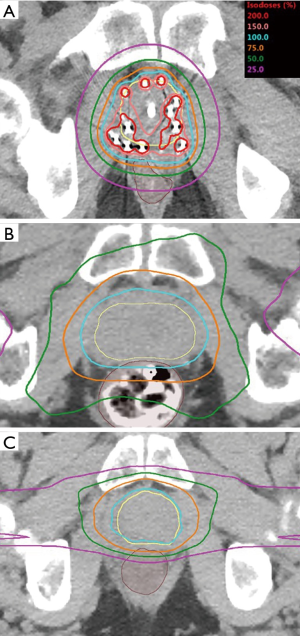
Another advantage of brachytherapy compared to EBRT, is the favorable dose distribution to surrounding nearby normal tissues. The photons produced by modern LDR brachytherapy sources have relatively low average energy (21–31 keV) and are rapidly attenuated by the surrounding tissues, which allow high quality implants to achieve unparalleled dose conformality. Figure 1 demonstrates the favorable relative dose distribution afforded by LDR brachytherapy (Figure 1A) compared to a modern EBRT photon treatment with intensity modulate radiation therapy (IMRT) (Figure 1B) or even pencil beam scanning intensity-modulated proton beam therapy (IMPT) (Figure 1C). The figure demonstrates the entrance dose delivered by either IMRT or IMPT as beams traverse up to 20 cm of normal tissue between the patient’s external surface and the target tissue of the prostate, leading to much higher volumes of tissue receiving low to moderate dose (1–50% of prescription) exposure. Entrance dose is obviated with LDR brachytherapy, given that the radiation source is inserted directly into the prostate, thus minimizing unnecessary radiation exposure to the normal tissues.
LDR brachytherapy technique and dosimetry
Delivery of high-quality permanent prostate brachytherapy is dependent on the accurate placement of the radioactive sources to maximize tumor control, while minimizing toxicity. This approach requires a skilled brachytherapy clinician working in concert with physicists, dosimetrists, and others to perform a technique that is safe, reproducible, and robust to inherent clinical uncertainties. Figure 2 shows a visual representation of real-time TRUS image guided source placement through transperineally implanted 14 to 18 gauge hollow catheters or needles. LDR brachytherapy is typically an outpatient surgical procedure with the patient receiving either general anesthesia or intravenous sedation in combination with spinal anesthesia. Visualization of the urethra, which represents a critical organ at risk for brachytherapy toxicity, is accomplished by placing a Foley catheter at the time of the procedure. Needles are typically placed under TRUS image guidance through holes in a physical template that is mounted against the patient’s perineum. The approximate locations of the templated holes are superimposed on the ultrasound monitor image in order to facilitate real-time guidance for needle positioning. LDR brachytherapy sources can be preloaded into the needles for subsequent deposition or inserted through hollow needles in the prostate with the use of a mechanical device, such as a Mick Applicator (12).

Although focal brachytherapy implants, which only treat the portion of the prostate that is known to contain tumor have been investigated, radiation coverage of the entire gland remains standard practice (13,14). In order to assure adequate dosimetric coverage of the prostate, while minimizing radiation dose to the nearby normal tissues, a plan must be generated to optimize radionuclide source placement. The number and distribution of needles to be placed for source deposition is dependent on a number of factors including measured prostate volume, patient pelvic anatomy, physicist/clinician preferences, and the physical properties of the radioisotope to be implanted. Furthermore, the source activity of the radionuclide must be considered, as this can significantly alter dose distributions (15).
Pre-planning is a technique that was pioneered by the Seattle Prostate Institute, in which the TRUS images of the prostate are obtained at some time prior to the brachytherapy procedure and used to generate an acceptable distribution of needles and sources (16). Pre-planned prostate brachytherapy depends on ensuring that the TRUS images obtained at the time of the implant closely match those in the plan to allow appropriate needle and source localization. Intraoperative planning is an alternative technique that can be used to determine source distributions for an individual patient at the time of the brachytherapy procedure. This technique has a number of variations in its execution, including creating a plan on the day of the implant using a TRUS image set obtained immediately prior to treatment, and dynamic planning where subsequent source positions are adjusted throughout the procedure based upon the placement of the preceding seeds (17). Changes in prostate volume or shape on the day of the procedure may be better accommodated by intraoperative planning than preplanning, with a resultant improvement in dosimetry (18). Compared to preplanning, intraoperative planning may prolong the time required in the operating room for the implant, but this may be mitigated by eliminating the need for a pre-procedural TRUS study (19). Excellent clinical outcomes have been reported with both techniques and the selection of either will be dictated by clinician preference, along with variables unique to their practice environment.
Documentation and assessment of post-implant dosimetry is an important component in maintaining a high quality LDR brachytherapy practice. The American Brachytherapy Society (ABS) consensus guidelines recommend that CT-based postoperative dosimetry be performed within 60 days of the brachytherapy procedure (20). This process allows accurate identification of implanted sources, the clinical target volume (prostate), and nearby organs at risk. Three-dimensional dosimetry can then be calculated and relevant doses to structures should be reported. Many studies have identified dosimetric parameters that are associated with patient outcomes following LDR brachytherapy monotherapy. A minimum dose of ≥140 Gy for iodine-125 (125I) and ≥100 Gy for palladium-103 (103Pd) received by at least 90% of the prostate volume (D90%) has been shown in large retrospective series to be correlated to improved biochemical control rates (21,22). Additional parameters, including the minimum dose to 100% of the prostate volume (D100%) and the percentage of the prostate volume receiving 100% or greater of the prescription dose (V100%) have been investigated, but were less predictive of outcomes (23). Post-implant measures of dose to both the rectum and urethra have also been identified as risk factors for subsequent toxicity. A rectal V100% (the volume of rectum receiving 100% or greater of the prescription dose) greater than 1 cc is associated with an increased risk of developing Grade 2 or greater gastrointestinal adverse effects (24-26). Although predictors of urinary toxicity, including stricture development, are less well-defined, a maximum dose to the urethra of less than 150% of prescription dose is frequently utilized as a dosimetric goal (27). Future research, ideally with patients treated in a prospective and standardized manner, is needed to help identify which parameters are most closely associated with optimal patient outcomes.
LDR brachytherapy sources
According to the International Commission on Radiologic Units and Measurements, LDR brachytherapy is defined by the use of a radiation source with a dose rate of less than 2 Gy per hour (28). Currently, there are three radioactive sources commercially available with excellent technical and clinical data supporting their routine use; 125I, 103Pd, and cesium-131 (131Cs). The corresponding physical characteristics and common clinical dose applications for these radionuclides are listed in Table 1.

Full table
125I has been utilized for prostate brachytherapy since 1965, and based on the most recent survey of practicing physicians, continues to be the predominant source used in clinical practice (29). Whereas 131Cs was introduced in 2004 and accordingly has a relatively smaller volume of published experience (30). The mean energies of each of these three radionuclides are all comparable, meaning that their physical radiation dose distributions and emitted photon attenuation properties are also similar. The notable difference between the sources is the longer half-life (t1/2), or rate of radioactive decay for 125I (t1/2 =59.4 days) relative to 103Pd (t1/2 =17 days) or 131Cs (t1/2 =9.7 days). The lower physical doses that are commonly prescribed with 103Pd and 131Cs are primarily a consequence of their shorter half-lives and are based on consensus review of the clinical literature (20). A more rapid delivery of radiation dose is conferred by the shorter t1/2 of 103Pd and 131Cs, which has led some to theorize these isotopes may confer a superior anti-tumor effect, from a radiobiological standpoint (31,32). One small randomized clinical trial comparing tumor control outcomes between 125I and 103Pd has been conducted, with initial results demonstrating no difference in outcomes between the two radionuclides at 3 years following treatment (33). This equivalence in oncologic outcomes has been further confirmed in large retrospective studies (34,35). The choice of radionuclide used is largely dependent on clinician preference and experience, with each of the sources described above representing an acceptable and effective option for routine use in permanent prostate brachytherapy.
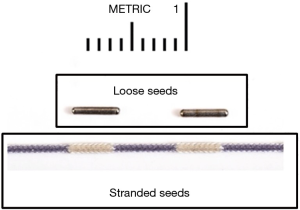
Brachytherapy sources are typically cylindrical in shape and housed within a non-ferromagnetic metal jacket in order to allow MRI compatibility. Most commercially available radionuclides used for prostate brachytherapy measure approximately 4.5 mm in length and are 800 µm (0.8 mm) in diameter. Sources are available in a “loose” format as individual seeds or they can be linked, aka “stranded”, together at regular intervals (typically 1 cm) within surgical suture material (Figure 3). Loose seeds can offer greater flexibility in seed spacing and may improve some post-implant dosimetric measures; however stranded seeds have a lower risk of migration outside of the peri-prostatic tissues and may result in better long-term dose coverage (36-38). Physicians often rely on their previous experience for selecting their source format, but both loose and stranded sources remain commonly used in modern prostate brachytherapy (29).
Patient selection criteria for LDR brachytherapy
Appropriate patient selection is a vital process for ensuring optimal outcomes with the use of permanent prostate brachytherapy. When considering LDR brachytherapy as a treatment strategy for prostate cancer, both oncologic and non-oncologic clinical factors must be evaluated on an individual patient basis. Consultation with an experienced practitioner in LDR brachytherapy can help men best determine whether this technique is appropriate for use in the management of their prostate cancer. Additionally, identification of patients who do not meet the appropriate criteria for the use of LDR prostate brachytherapy should be counseled about alternative treatment approaches that may be better suited to their clinical presentation.
Oncologic considerations
In patients with newly diagnosed prostate cancer, risk group stratification is commonly utilized as a means to predict prognosis and determine treatment strategies. Multiple consensus groups have proposed risk group stratifications based on pre-biopsy serum prostate specific antigen (PSA) level, clinical tumor classification (or T stage), and biopsy-determined International Society of Urological Pathology (ISUP) Gleason grade group (39-42). Published guidelines from the ABS, American College of Radiology (ACR), and the European Society for Radiotherapy and Oncology (ESTRO) and others have recommended that LDR brachytherapy is a reasonable option to consider for men with all risk group categories of non-metastatic prostate cancer (20,43,44). Although these guidelines were formulated based on expert review of the significant body of published literature on the topic, there is currently no level I evidence investigating appropriate patient selection. Therefore, these guidelines must be interpreted with some level of caution given the variability in institutional reporting of outcomes and techniques.
According the American Urological Association (AUA), American Society for Radiation Oncology (ASTRO), and Society of Urologic Oncology (SUO) guideline, active surveillance is now recommended as the preferred treatment for patients with low risk prostate cancer [National Comprehensive Cancer Network (NCCN): cT1c–T2a, ISUP Grade Group 1, and PSA <10 ng/mL] (45). Of note, however, in a recently published study which randomized low and intermediate risk patients to active surveillance, EBRT, or prostatectomy the 10-year rate of development of metastases was significantly greater in the active surveillance arm compared to the other two active treatment arms (46). Thus, recommendations and methods pertaining to active surveillance may continue to evolve. Amongst men with low-risk disease who are not appropriate candidates for active surveillance or prefer to pursue immediate treatment, permanent prostate brachytherapy alone is considered an appropriate oncologic treatment. The optimal use of LDR brachytherapy, whether as monotherapy or in combination with either supplemental EBRT and/or androgen deprivation therapy (ADT), remains uncertain in some patients with intermediate risk prostate cancer (NCCN: cT2b–c, ISUP Grade Group 2–3, or PSA 10–20 ng/mL). Much of this uncertainty stems from the heterogeneous clinical outcomes observed within this risk group, which have led to proposed subgroups of “favorable” and “unfavorable” intermediate risk (47-49). The recently presented RTOG 0232 study, which randomized patients with predominantly favorable intermediate risk prostate cancer to either LDR brachytherapy alone or LDR brachytherapy plus supplemental EBRT, found that the addition of EBRT did not improve long-term disease control, but did increase the rate of treatment-related toxicity (50). One factor driving concerns about the efficacy of LDR monotherapy is the greater likelihood of tumor extension beyond the prostate in those with intermediate risk disease prostate cancer (51). However, the radial extent of tumor is rarely greater than 5 mm and should be expected to be covered by a tumoricidal dose with appropriately implemented permanent prostate brachytherapy (52,53). Thus, LDR brachytherapy alone appears to be a reasonable treatment option for most patients with favorable intermediate prostate cancer. Further study is needed to determine if there are subsets of patients whose outcomes are improved by adding supplement EBRT or ADT to permanent prostate brachytherapy. Treatment intensification is necessary to improve to the oncologic outcomes in patients with high risk prostate cancer (cT3a–cT4, ISUP Grade Group 4–5, or PSA >20 ng/mL). Given the risk of extra-prostatic dissemination in these patients, brachytherapy alone is not recommended. However, brachytherapy provides an excellent tool to provide radiation dose escalation to the prostate. There is a growing body of evidence supporting the improved outcomes achieved with the use of brachytherapy in combination with EBRT and ADT when compared to surgical and non-surgical treatment approaches (54,55). Thus, in clinically eligible patients receiving definitive radiation for high risk disease, brachytherapy boost should be considered as means to escalate the dose and potentially maximize long-term disease control.
Patient considerations
While oncologic factors must be considered prior to brachytherapy in order optimize treatment efficacy, clinical patient selection is vital to minimize the risk of treatment-related toxicity. The ABS consensus guidelines offer a detailed review of important considerations during the course of patient evaluation, including a list of contra-indications to LDR prostate brachytherapy which are shown in Table 2 (20). Larger prostate volume can present challenges to achieving adequate dosimetry and may also predispose men to a greater risk of acute post-implant urinary toxicity (56). A prostate greater than 60 cm3 increases the risk that the posterior aspects of the pubic arch will interfere with appropriate needle placement and subsequent source positioning (57). An average prostate volume downsizing of approximately 30% can be achieved to facilitate brachytherapy with short-term ADT (~4 months) using a luteinizing hormone-releasing hormone (LHRH) agonist plus an oral anti-androgen (58,59). A thorough assessment of patient urinary function should be performed prior to performing LDR brachytherapy. Patients with existing decrements in urinary function, as measured by International Prostate Symptom Score (IPSS) and peak urinary flow rates, have repeatedly been shown to be at greater risk of developing urinary toxicity after LDR brachytherapy (60-62). It is important to note the upfront presence of urinary symptoms should not eliminate a patient for consideration of brachytherapy. Medical interventions such as oral alpha blockers, with or without phosphodiesterase 5 inhibitors, prostate downsizing with ADT, or a carefully coordinated, limited transurethral resection of the prostate (TURP) can improve symptomatology sufficiently to mitigate the risk of a subsequent brachytherapy implants (63,64). The impact of a pre-existing TURP on a patient’s candidacy for permanent prostate brachytherapy must be evaluated on a case-by-case basis. Although many studies have shown good oncologic and toxicity outcomes in patients with a prior TURP, others have suggested an increased risk of incontinence (65-67). In-office cystoscopy, careful urinary symptom assessment, and multi-parametric magnetic resonance imaging (MRI) can be helpful to determine whether the deficit will significantly deter a safe implant. These factors, along with any others unique to each patient’s presentation and history should be considered as a part of the shared decision-making process between physicians and patients.

Full table
Oncologic efficacy
Many large series have been published which consistently demonstrate the excellent clinical outcomes for men treated with LDR brachytherapy. Data from a selected group of these studies are shown in Table 3. While some heterogeneity is present with regard to the definitions of risk groups and outcomes reported, these reports consistently demonstrate the excellent oncologic outcomes achieved with permanent prostate brachytherapy. For patients with low risk prostate cancers, PSA control rates of greater than 90% are common, which persist upon long-term follow-up up extending more than a decade after treatment. Additionally, LDR brachytherapy often results in biochemical control of 75–95% in patients with intermediate risk disease. Although randomized studies comparing treatment modalities for prostate cancer are lacking, high quality retrospective analyses confirm very favorable PSA control rates for brachytherapy relative to EBRT or RP in low/intermediate risk patients (55). Patients with high risk disease are relatively underrepresented in most large series assessing LDR brachytherapy outcomes. Although Table 3 shows that reasonable biochemical control rates can be seen with brachytherapy alone, these outcomes seem to be improved with the addition of supplemental EBRT and/or ADT (55). The long-term cause-specific survival (CSS) estimates exceeding 95% in all series further supports the curative nature of prostate cancer treatment with LDR brachytherapy.

Full table
Supplemental EBRT, which is typically administered either prior to or following brachytherapy to a dose of 20–50.4 Gy in 1.8–2.2 Gy fractions, has been incorporated by some clinicians in an effort to improve treatment outcomes in patients with intermediate or high-risk disease. This additional radiation dose can help ensure adequate dose coverage to regions that are not adequately treated with permanent prostate brachytherapy or deliver dose to the seminal vesicles and pelvic lymph nodes that are inaccessible with brachytherapy and may harbor micrometastases. However, most retrospective studies suggest supplemental EBRT does not significantly improve clinical outcomes for intermediate risk prostate cancer compared to high quality LDR implants alone (76,77). Additionally, the rates of grade 2+ toxicity are significantly increased with the dose escalation of combination treatment (78). The lack of apparent benefit from supplemental EBRT has been confirmed by a recently presented phase III randomized trial (RTOG 0232) (50). Nevertheless, there may be specific subgroups of intermediate risk patients that benefit from the use of EBRT with LDR, and as such, treatment should be determined on an individual basis at the discretion of the treating radiation oncologist.
An LDR brachytherapy boost is an excellent, and potentially underutilized clinical tool to escalate dose and improve outcomes in patients with high risk prostate cancers (54,79). This hypothesis was tested in ASCENDE-RT, a Phase III randomized trial comparing EBRT alone (78 Gy in 39 fractions) to EBRT (46 Gy in 23 fractions) + LDR brachytherapy (115 Gy with 125I) in men with intermediate or high-risk prostate receiving 12 months of ADT (80). Although biochemical control rates were significantly improved with the addition of brachytherapy (83% vs. 62% at 9 years), no differences in distant metastases or survival have been reported. Combination therapy was associated with increased rates of GU toxicity and with modest decrements in patient-reported quality of life (QOL) (81,82). Long-term follow-up of ASCENDE-RT and further study will be needed to determine if the addition of brachytherapy to EBRT is superior to EBRT alone in other clinical endpoints including metastasis-free, cause-specific, and overall survival.
While ADT is a common and effective strategy to downsize the prostate in preparation for brachytherapy, its role in improving cancer control rates in this setting remains uncertain. Multiple randomized studies have shown that adding ADT to EBRT, in selected intermediate and high-risk patients improves biochemical control, and in some cases cause-specific and overall survival are improved compared to EBRT alone (83). A recent large database review of over 2,000 men with intermediate risk prostate cancer treated with LDR brachytherapy demonstrated that while the addition of ADT modestly improved biochemical control (89% vs. 86%) at 10 years follow-up, its use was actually associated with worse overall survival (84% vs. 86%) (84). The authors hypothesized that this survival decrement may have been the result of increased cardiac mortality related to ADT. An ABS Task Group has reviewed the available literature regarding the role of ADT in combination with brachytherapy. Based on the review of 52 studies with over 40,000 patients the authors concluded there was little evidence to demonstrate a benefit of adding ADT to brachytherapy in low or favorable intermediate risk patients (85). The use of 3–12 months of ADT was found to improve biochemical control, but not necessarily cause-specific or overall survival, for patients with suboptimal post-implant dosimetry, unfavorable intermediate risk or high-risk disease. Prospective, randomized trials are needed to further clarify the optimal use and duration of ADT in the setting of prostate brachytherapy.
Toxicity and QOL outcomes
The clinical utility and applicability of LDR brachytherapy is not only dependent on its tumor treatment effects, but also on the demonstration of a favorable toxicity profile. For patients who often have a variety of similarly efficacious options for treating their prostate cancer, including radical prostatectomy (RP) or other radiation modalities, the expected acute and long-term side effects are often important factors to be considered in treatment decisions (86).
Acute adverse effects in the immediate post-implant setting are infrequent. The risk of peri-procedural infection is less than 5%, given that the needles are inserted into the prostate transperineally and not transrectally (87). Transient urinary retention requiring catheter placement occurs as a consequence of prostate edema in approximately 2–10% of patients undergoing permanent prostate brachytherapy, and typically resolves shortly following the procedure (60). Larger prostate gland volume, pre-existing urinary symptoms, and greater number of needles at the time of implant are identified risk factors for acute retention. The use neoadjuvant ADT or post-implant corticosteroids have been shown to decrease the risk of retention in the immediate post-brachytherapy setting (88-90).
Acute and late genitourinary (GU) toxicities are the most commonly reported adverse effects after LDR prostate brachytherapy. The symptomatology of patients is often both irritative and obstructive in nature and peaks between 1–4 months after the procedure depending on the half-life of the isotope used, with the former being slower to resolve in the first 6 months following implantation (91,92). Evaluation of the temporal trends in patient-reported urinary symptoms, as measured by IPSS, after permanent prostate brachytherapy reveals that more than 80% and 95% of men return to their baseline function within 6 and 12 months of the procedure, respectively (93). Furthermore, the use of prophylactic alpha-blockers prior to and immediately following LDR brachytherapy has been demonstrated to lessen the severity and duration of post-implant urinary symptoms (94). The rate of acute physician-reported Grade 2 and Grade 3 GU toxicities in large retrospective series ranges between 15–30% and 5–10%, respectively (56,95). Significant late GU toxicity after LDR brachytherapy is relatively uncommon, with reported rates of less than 10% for Grade 2 and substantially less than 3% for Grade 3–4 (96). Risk factors associated with the development of any grade 2 or greater urinary toxicity include larger prostate volume, elevated baseline IPSS score, and higher radiation prescription dose (56,96). These observations have helped guide consensus recommendations for the optimization of patient selection and treatment delivery in order to ensure the risk of treatment-related urinary toxicity is minimized.
Given the proximity of the anterior rectal wall to the prostate, radiation-related gastrointestinal (GI) toxicity can occur after permanent prostate brachytherapy. However, the great majority (>85%) of patients report no significant change in self-assessed bowel symptoms at any time point following LDR brachytherapy (97). Grade 2 or 3 GI toxicity rates of 5–10% and 1–3%, respectively, have been reported in published case series (98-100). The volume of the rectum receiving 100% of the prescription dose (V100%) and physician learning curve have been identified as factors associated with an increased risk of rectal toxicity (24). While rectal ulceration is an uncommon late complication of permanent prostate brachytherapy, occurring in less than 1% of patients, multi-disciplinary evaluation when this occurs is vital to optimize management and long-term outcomes (101).
A number of publications have compared patient reported QOL assessments prior to and after definitive treatment for prostate cancer with brachytherapy, EBRT, or RP (102-106). Although there are heterogeneity amongst these studies with regard to the QOL instruments used for evaluation, patient populations, and treatment protocols their findings are remarkably similar. Most confirmed that treatment modality did not significantly affect overall QOL measures, but did impact specific symptom domains. Generally, patients treated with brachytherapy experience lower rates of urinary incontinence and maintain erectile function/sexual QOL better than those undergoing RP. Conversely, symptoms of urinary bother (obstruction/irritation) and bowel function are more significantly affected by brachytherapy than RP. While most studies show little difference in QOL between EBRT and brachytherapy, some do suggest that that brachytherapy may have less effect on the sexual and bowel domains (102,103). While these data are useful to reference when counseling patients, they are limited by their retrospective study design. One randomized trial comparing LDR brachytherapy to RP has been conducted and further supports the above findings. While enrollment (n=168) in this study did not meet its accrual goal needed to assess differences in oncologic efficacy, QOL evaluation demonstrated less decrement in urinary domain, sexual domain, and patient satisfaction score for patients treated with brachytherapy compared to RP (107).
Risk of radiation induced malignancies
While therapeutic radiation exposure to normal tissue has been shown to increase the rate of a second malignancy (SM), especially in pediatric patients, assertions that a similar risk is present following prostate brachytherapy have largely been proven untrue. A radiobiologic modelling study estimated that based on permanent prostate brachytherapy dosing in three representative patients, the excess absolute risk of developing a SM involving the rectum or bladder was less than 1 and 2 per 10,000 person-years, respectively (108). Large database analyses have further demonstrated no difference in the rate of SM when comparing patients treated with LDR brachytherapy to those undergoing RP or even those in the general population (109-111). These findings are augmented by a meta-analysis which reported a small increase in SM involving colon, bladder following treatment with EBRT, but not brachytherapy for prostate cancer (112). Taken together, these data show that concerns related to SM following LDR brachytherapy are not supported by the current data and should not be prohibitive to its use in appropriately selected patients.
Radiation safety
Many amongst the general population, including cancer patients, have pre-existing fears about the perceived risks associated with radiation therapy (113). Multiple studies have been conducted to assuage concerns related to potential radiation exposure in the setting of LDR prostate brachytherapy. Post-implant radiation exposures to spouses and other family members, as assessed by worn dosimeter monitoring studies, have been calculated at 0.07–0.1 millisieverts (mSv) following either 125I or 103Pd prostate brachytherapy (114). For a sense of prospective, this amount of exposure is less than the amount of cosmic radiation experienced annually by an individual living at sea level (115). Further calculations have shown that in a worst case scenario, a patient could hold a child on his lap for at least 30 minutes every day immediately following a 125I implant and not exceed a 0.5 mSv exposure threshold (116). While these objective measures of exposure are comforting, patients are still counseled to avoid prolonged close contact with the general public, especially pregnant women and children, to ensure any unnecessary risk is avoided. Radiation exposure to medical personnel during LDR brachytherapy is minimal and much lower than many other medical settings involving radiation, particularly cardiac procedures involving fluoroscopy. In a detailed study of sources of radiation during LDR brachytherapy, the net duration of fluoroscopy “on-time’ was the most significant contributing factor to radiation exposure (117).
New developments in LDR prostate brachytherapy
Many radiation oncologists are routinely incorporating multiparametric magnetic resonance imaging (mpMRI) into their brachytherapy practices as a means to improve patient selection, treatment planning, and dosimetric analysis (118). The use of mpMRI has been shown to improve the detection of otherwise clinically occult extraprostatic tumor, which can be useful for determining the need for supplemental EBRT (119). There is also some early interest in mpMRI imaging to guide focal brachytherapy, with implants delivering dose to only the visible tumor lesion or involved hemi-gland, as a means to minimize treatment-related toxicity (14). While early reports of these treatments suggest they are technically feasible and can reduce doses to nearby organs at risk, their long-term oncologic efficacy remains unproven (120-122).
Another development for patients undergoing permanent prostate brachytherapy is an FDA-approved temporary retroprostatic polymer spacer (SpaceOAR, Augmenix, Waltham MA). Transperineal injection of this material, which is resorbed naturally within 4–6 months, anterior to Denonvilliers’ fascia has been shown to displace the rectum posteriorly by an average distance of 1 cm (123). A randomized clinical trial in prostate cancer patients treated with EBRT has shown the use of the spacer provides a modest, but statistically significant, improvement in GI/GU toxicity and QOL (124). Early results with its use in the setting of LDR brachytherapy have shown promising dosimetric and toxicity outcomes (125).
Solutions are being sought to improve real-time dosimetry calculations during LDR brachytherapy (126,127). Such advances would allow rapid assessment of implants quality and correction of any detected deficiencies at the time of implant (128). Another recent development of note, is a novel 103Pd line source, which provides a unique dose distribution compared to standardly used point sources (129). Ongoing research and development are necessary to continue to advance the technology and techniques for prostate brachytherapy, with the goal of improving oncologic and safety outcomes for patients.
Conclusions
LDR brachytherapy has been proven with decades of reported outcomes to be a safe, convenient, and highly efficacious approach in the management and cure of localized prostate cancer that should remain an option for all appropriate patients. Additionally, the cost-effectiveness of LDR brachytherapy, for both patients and providers, has been shown to compare very favorably to other treatment approaches, including RP and EBRT (130,131). However, despite this evidence, the use of brachytherapy for all risk groups of prostate cancer has declined by as much as 50% over the past decade (132,133). This waning utilization has been hypothesized to be driven by the emergence of competing treatment modalities or a lack of radiation oncologists with the skills needed to perform high quality brachytherapy implants. It remains imperative that radiation oncology and urology training programs provide trainees with appropriate exposure to brachytherapy in order ensure patient access to this treatment in the future. As current and future research further refines the optimal use and delivery of LDR brachytherapy, its use should be discussed with all clinically appropriate patients with localized prostate cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Holm HH. The history of interstitial brachytherapy of prostatic cancer. Semin Surg Oncol 1997;13:431-7. [Crossref] [PubMed]
- Barringer BS. Radium in the treatment of carcinoma of the bladder and prostate. JAMA 1916;67:1442-5. [Crossref]
- Whitmore WF Jr, Hilaris B, Grabstald H. Retropubic implantation to iodine 125 in the treatment of prostatic cancer. J Urol 1972;108:918-20. [Crossref] [PubMed]
- Blasko JC, Ragde H, Luse RW, et al. Should brachytherapy be considered a therapeutic option in localized prostate cancer? Urol Clin North Am 1996;23:633-50. [Crossref] [PubMed]
- Iversen P, Bak M, Juul N, et al. Ultrasonically guided 125iodine seed implantation with external radiation in management of localized prostatic carcinoma. Urology 1989;34:181-6. [Crossref] [PubMed]
- King CR, DiPetrillo TA, Wazer DE. Optimal radiotherapy for prostate cancer: predictions for conventional external beam, IMRT, and brachytherapy from radiobiologic models. Int J Radiat Oncol Biol Phys 2000;46:165-72. [Crossref] [PubMed]
- Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys 2009;74:1405-18. [Crossref] [PubMed]
- Zaorsky NG, Keith SW, Shaikh T, et al. Impact of Radiation Therapy Dose Escalation on Prostate Cancer Outcomes and Toxicities. Am J Clin Oncol 2018;41:409-15. [PubMed]
- Jani AB, Hand CM, Lujan AE, et al. Biological effective dose for comparison and combination of external beam and low-dose rate interstitial brachytherapy prostate cancer treatment plans. Med Dosim 2004;29:42-8. [Crossref] [PubMed]
- Marshall RA, Buckstein M, Stone NN, et al. Treatment outcomes and morbidity following definitive brachytherapy with or without external beam radiation for the treatment of localized prostate cancer: 20-year experience at Mount Sinai Medical Center. Urol Oncol 2014;32:38.e1-7. [Crossref] [PubMed]
- Herstein A, Wallner K, Merrick G, et al. There is a wide range of predictive dosimetric factors for I-125 and Pd-103 prostate brachytherapy. Am J Clin Oncol 2008;31:6-10. [Crossref] [PubMed]
- Yu Y, Anderson LL, Li Z, et al. Permanent prostate seed implant brachytherapy: report of the American Association of Physicists in Medicine Task Group No. 64. Med Phys 1999;26:2054-76. [Crossref] [PubMed]
- Polders DL, Steggerda M, van Herk M, et al. Establishing implantation uncertainties for focal brachytherapy with I-125 seeds for the treatment of localized prostate cancer. Acta Oncol 2015;54:839-46. [Crossref] [PubMed]
- Al-Qaisieh B, Mason J, Bownes P, et al. Dosimetry Modeling for Focal Low-Dose-Rate Prostate Brachytherapy. Int J Radiat Oncol Biol Phys 2015;92:787-93. [Crossref] [PubMed]
- Elliott SL, Beaufort CL, Millar JL. Practical considerations in the selection of seed strength for prostate implants. J Appl Clin Med Phys 2015;16:53-61. [Crossref] [PubMed]
- Blasko J, Ragde H, Schumacher D. Transperineal percutaneous iodine-125 implantation for prostatic carcinoma using transrectal ultrasound and template guidance. Endocurie Hypertherm Oncol 1987;3:131-9.
- Nag S, Ciezki JP, Cormack R, et al. Intraoperative planning and evaluation of permanent prostate brachytherapy: report of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys 2001;51:1422-30. [Crossref] [PubMed]
- Zelefsky MJ, Yamada Y, Marion C, et al. Improved conformality and decreased toxicity with intraoperative computer-optimized transperineal ultrasound-guided prostate brachytherapy. Int J Radiat Oncol Biol Phys 2003;55:956-63. [Crossref] [PubMed]
- Gewanter RM, Wuu C, Laguna JL, et al. Intraoperative preplanning for transperineal ultrasound-guided permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 2000;48:377-80. [Crossref] [PubMed]
- Davis BJ, Horwitz EM, Lee WR, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy 2012;11:6-19. [Crossref] [PubMed]
- Stone NN, Potters L, Davis BJ, et al. Customized dose prescription for permanent prostate brachytherapy: insights from a multicenter analysis of dosimetry outcomes. Int J Radiat Oncol Biol Phys 2007;69:1472-7. [Crossref] [PubMed]
- Kollmeier MA, Stock RG, Stone N. Biochemical outcomes after prostate brachytherapy with 5-year minimal follow-up: importance of patient selection and implant quality. Int J Radiat Oncol Biol Phys 2003;57:645-53. [Crossref] [PubMed]
- Potters L, Cao Y, Calugaru E, et al. A comprehensive review of CT-based dosimetry parameters and biochemical control in patients treated with permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 2001;50:605-14. [Crossref] [PubMed]
- Keyes M, Spadinger I, Liu M, et al. Rectal toxicity and rectal dosimetry in low-dose-rate (125)I permanent prostate implants: a long-term study in 1006 patients. Brachytherapy 2012;11:199-208. [Crossref] [PubMed]
- Han BH, Wallner KE. Dosimetric and radiographic correlates to prostate brachytherapy-related rectal complications. Int J Cancer 2001;96:372-8. [Crossref] [PubMed]
- Price JG, Stone NN, Stock RG. Predictive factors and management of rectal bleeding side effects following prostate cancer brachytherapy. Int J Radiat Oncol Biol Phys 2013;86:842-7. [Crossref] [PubMed]
- Crook JM, Potters L, Stock RG, et al. Critical organ dosimetry in permanent seed prostate brachytherapy: defining the organs at risk. Brachytherapy 2005;4:186-94. [Crossref] [PubMed]
- International Commission on Radiological Units and Measurements. Dose and volume specification for reporting intracavitary therapy in gynecology. ICRU report, vol 38. Bethesda, Md., U.S.A.: ICRU; 1985.
- Buyyounouski MK, Davis BJ, Prestidge BR, et al. A survey of current clinical practice in permanent and temporary prostate brachytherapy: 2010 update. Brachytherapy 2012;11:299-305. [Crossref] [PubMed]
- Bice WS, Prestidge BR, Kurtzman SM, et al. Recommendations for permanent prostate brachytherapy with (131)Cs: a consensus report from the Cesium Advisory Group. Brachytherapy 2008;7:290-6. [Crossref] [PubMed]
- King CR. LDR vs. HDR brachytherapy for localized prostate cancer: the view from radiobiological models. Brachytherapy 2002;1:219-26. [Crossref] [PubMed]
- Ling CC. Permanent implants using Au-198, Pd-103 and I-125: radiobiological considerations based on the linear quadratic model. Int J Radiat Oncol Biol Phys 1992;23:81-7. [Crossref] [PubMed]
- Wallner K, Merrick G, True L, et al. 125I versus 103Pd for low-risk prostate cancer: preliminary PSA outcomes from a prospective randomized multicenter trial. Int J Radiat Oncol Biol Phys 2003;57:1297-303. [Crossref] [PubMed]
- Kollmeier MA, Pei X, Algur E, et al. A comparison of the impact of isotope ((125)I vs. (103)Pd) on toxicity and biochemical outcome after interstitial brachytherapy and external beam radiation therapy for clinically localized prostate cancer. Brachytherapy 2012;11:271-6. [Crossref] [PubMed]
- Peschel RE, Colberg JW, Chen Z, et al. Iodine 125 versus palladium 103 implants for prostate cancer: clinical outcomes and complications. Cancer J 2004;10:170-4. [Crossref] [PubMed]
- Major T, Agoston P, Frohlich G, et al. Loose versus stranded seeds in permanent prostate brachytherapy: dosimetric comparison of intraoperative plans. Phys Med 2014;30:909-13. [Crossref] [PubMed]
- Reed DR, Wallner KE, Merrick GS, et al. A prospective randomized comparison of stranded vs. loose 125I seeds for prostate brachytherapy. Brachytherapy 2007;6:129-34. [Crossref] [PubMed]
- Birckhead BJ, Fossum CC, Deufel CL, et al. Stranded seed displacement, migration, and loss after permanent prostate brachytherapy as estimated by Day 0 fluoroscopy and 4-month postimplant pelvic x-ray. Brachytherapy 2016;15:714-21. [Crossref] [PubMed]
- Network NCC. NCCN Clinical Practice Guidelines-Prostate Cancer (Version 2.2017). 2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed September 23, 2017.
- Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol 2008;53:68-80. [Crossref] [PubMed]
- Horwich A, Parker C, Bangma C, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v129-33. [Crossref] [PubMed]
- Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007;177:2106-31. [Crossref] [PubMed]
- Ash D, Flynn A, Battermann J, et al. ESTRO/EAU/EORTC recommendations on permanent seed implantation for localized prostate cancer. Radiother Oncol 2000;57:315-21. [Crossref] [PubMed]
- Davis BJ, Taira AV, Nguyen PL, et al. ACR appropriateness criteria: Permanent source brachytherapy for prostate cancer. Brachytherapy 2017;16:266-76. [Crossref] [PubMed]
- Sanda M, Chen R, Crispino T, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. American Urological Association. 2017. Available online: (aua/astro/suo-guideline-2017). Accessed September 23,2017.http://www.auanet.org/guidelines/clinically-localized-prostate-cancer-new-
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415-24. [Crossref] [PubMed]
- Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol 2013;64:895-902. [Crossref] [PubMed]
- Merrick GS, Butler WM, Galbreath RW, et al. Stratification of brachytherapy-treated intermediate-risk prostate cancer patients into favorable and unfavorable cohorts. J Contemp Brachytherapy 2015;7:430-6. [Crossref] [PubMed]
- Sengupta S, Davis BJ, Mynderse LA, et al. Permanent prostate brachytherapy: pathologic implications as assessed on radical prostatectomy specimens of broadening selection criteria for monotherapy. Urology 2006;68:810-4. [Crossref] [PubMed]
- Prestidge BR, Winter K, Sanda MG, et al. Initial Report of NRG Oncology/RTOG 0232: A phase 3 study comparing combined external beam radiation and transperineal interstitial permanent brachytherapy with brachytherapy alone for selected patients with intermediate-risk prostatic carcinoma. Int J Radiat Oncol Biol Phys 2016;96:S4. [Crossref]
- Eifler JB, Feng Z, Lin BM, et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int 2013;111:22-9. [Crossref] [PubMed]
- Davis BJ, Haddock MG, Wilson TM, et al. Treatment of extraprostatic cancer in clinically organ-confined prostate cancer by permanent interstitial brachytherapy: is extraprostatic seed placement necessary? Tech Urol 2000;6:70-7. [PubMed]
- Davis BJ, Pisansky TM, Wilson TM, et al. The radial distance of extraprostatic extension of prostate carcinoma: implications for prostate brachytherapy. Cancer 1999;85:2630-7. [Crossref] [PubMed]
- Kishan AU, Shaikh T, Wang PC, et al. Clinical outcomes for patients with gleason score 9-10 prostate adenocarcinoma treated with radiotherapy or radical prostatectomy: a multi-institutional comparative analysis. Eur Urol 2017;71:766-73. [Crossref] [PubMed]
- Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int 2012;109 Suppl 1:22-9. [Crossref] [PubMed]
- Keyes M, Miller S, Moravan V, et al. Predictive factors for acute and late urinary toxicity after permanent prostate brachytherapy: long-term outcome in 712 consecutive patients. Int J Radiat Oncol Biol Phys 2009;73:1023-32. [Crossref] [PubMed]
- Fukada J, Shigematsu N, Nakashima J, et al. Predicting pubic arch interference in prostate brachytherapy on transrectal ultrasonography-computed tomography fusion images. J Radiat Res 2012;53:753-9. [Crossref] [PubMed]
- Solhjem MC, Davis BJ, Pisansky TM, et al. Prostate volume before and after permanent prostate brachytherapy in patients receiving neoadjuvant androgen suppression. Cancer J 2004;10:343-8. [Crossref] [PubMed]
- Petit JH, Gluck C, Kiger WS 3rd, et al. Bicalutamide alone prior to brachytherapy achieves cytoreduction that is similar to luteinizing hormone-releasing hormone analogues with less patient-reported morbidity. Urol Oncol 2008;26:372-7. [Crossref] [PubMed]
- Crook J, McLean M, Catton C, et al. Factors influencing risk of acute urinary retention after TRUS-guided permanent prostate seed implantation. Int J Radiat Oncol Biol Phys 2002;52:453-60. [Crossref] [PubMed]
- Martens C, Pond G, Webster D, et al. Relationship of the International Prostate Symptom score with urinary flow studies, and catheterization rates following 125I prostate brachytherapy. Brachytherapy 2006;5:9-13. [Crossref] [PubMed]
- Ikeda T, Shinohara K. Peak flow rate is the best predictor of acute urinary retention following prostate brachytherapy: our experience and literature review. Int J Urol 2009;16:558-60. [Crossref] [PubMed]
- Singh DV, Mete UK, Mandal AK, et al. A comparative randomized prospective study to evaluate efficacy and safety of combination of tamsulosin and tadalafil vs. tamsulosin or tadalafil alone in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. J Sex Med 2014;11:187-96. [Crossref] [PubMed]
- Brousil P, Hussain M, Lynch M, et al. Modified transurethral resection of the prostate (TURP) for men with moderate lower urinary tract symptoms (LUTS) before brachytherapy is safe and feasible. BJU Int 2015;115:580-6. [Crossref] [PubMed]
- Moran BJ, Stutz MA, Gurel MH. Prostate brachytherapy can be performed in selected patients after transurethral resection of the prostate. Int J Radiat Oncol Biol Phys 2004;59:392-6. [Crossref] [PubMed]
- Wallner K, Lee H, Wasserman S, et al. Low risk of urinary incontinence following prostate brachytherapy in patients with a prior transurethral prostate resection. Int J Radiat Oncol Biol Phys 1997;37:565-9. [Crossref] [PubMed]
- Kittel JA, Reddy CA, Smith KL, et al. Long-Term Efficacy and Toxicity of Low-Dose-Rate (1)(2)(5)I Prostate Brachytherapy as Monotherapy in Low-, Intermediate-, and High-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys 2015;92:884-93. [Crossref] [PubMed]
- Blasko JC, Grimm PD, Sylvester JE, et al. Palladium-103 brachytherapy for prostate carcinoma. Int J Radiat Oncol Biol Phys 2000;46:839-50. [Crossref] [PubMed]
- Zelefsky MJ, Kuban DA, Levy LB, et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys 2007;67:327-33. [Crossref] [PubMed]
- Henry AM, Al-Qaisieh B, Gould K, et al. Outcomes following iodine-125 monotherapy for localized prostate cancer: the results of leeds 10-year single-center brachytherapy experience. Int J Radiat Oncol Biol Phys 2010;76:50-6. [Crossref] [PubMed]
- Taira AV, Merrick GS, Butler WM, et al. Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys 2011;79:1336-42. [Crossref] [PubMed]
- Crook J, Borg J, Evans A, et al. 10-year experience with I-125 prostate brachytherapy at the Princess Margaret Hospital: results for 1,100 patients. Int J Radiat Oncol Biol Phys 2011;80:1323-9. [Crossref] [PubMed]
- Morris WJ, Keyes M, Spadinger I, et al. Population-based 10-year oncologic outcomes after low-dose-rate brachytherapy for low-risk and intermediate-risk prostate cancer. Cancer 2013;119:1537-46. [Crossref] [PubMed]
- Funk RK, Davis BJ, Mynderse LA, et al. Permanent prostate brachytherapy monotherapy with I-125 for low- and intermediate-risk prostate cancer: outcome in 966 patients. Int J Radiat Oncol Biol Phys 2015;93:E213-4. [Crossref]
- Fellin G, Mirri MA, Santoro L, et al. Low dose rate brachytherapy (LDR-BT) as monotherapy for early stage prostate cancer in Italy: practice and outcome analysis in a series of 2237 patients from 11 institutions. Br J Radiol 2016;89. [Crossref] [PubMed]
- Merrick GS, Wallner KE, Galbreath RW, et al. Is supplemental external beam radiation therapy necessary for patients with higher risk prostate cancer treated with 103Pd? Results of two prospective randomized trials. Brachytherapy 2015;14:677-85. [Crossref] [PubMed]
- Merrick GS, Butler WM, Wallner KE, et al. Impact of supplemental external beam radiotherapy and/or androgen deprivation therapy on biochemical outcome after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 2005;61:32-43. [Crossref] [PubMed]
- Yorozu A, Kuroiwa N, Takahashi A, et al. Permanent prostate brachytherapy with or without supplemental external beam radiotherapy as practiced in Japan: outcomes of 1300 patients. Brachytherapy 2015;14:111-7. [Crossref] [PubMed]
- Spratt DE, Zumsteg ZS, Ghadjar P, et al. Comparison of high-dose (86.4 Gy) IMRT vs combined brachytherapy plus IMRT for intermediate-risk prostate cancer. BJU Int 2014;114:360-7. [PubMed]
- Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys 2017;98:275-85. [Crossref] [PubMed]
- Rodda S, Tyldesley S, Morris WJ, et al. ASCENDE-RT: An analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:286-95. [Crossref] [PubMed]
- Rodda S, Morris WJ, Hamm J, et al. ASCENDE-RT: An analysis of health-related quality of life for a randomized trial comparing low-dose-rate brachytherapy boost with dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:581-9. [Crossref] [PubMed]
- Wolff RF, Ryder S, Bossi A, et al. A systematic review of randomised controlled trials of radiotherapy for localised prostate cancer. Eur J Cancer 2015;51:2345-67. [Crossref] [PubMed]
- Pickles T, Tyldesley S, Hamm J, et al. Brachytherapy for intermediate risk prostate cancer, androgen deprivation and the risk of death. Int J Radiat Oncol Biol Phys 2018;100:45-52. [Crossref] [PubMed]
- Keyes M, Merrick G, Frank SJ, et al. American Brachytherapy Society Task Group Report: Use of androgen deprivation therapy with prostate brachytherapy-A systematic literature review. Brachytherapy 2017;16:245-65. [Crossref] [PubMed]
- Xu J, Dailey RK, Eggly S, et al. Men's perspectives on selecting their prostate cancer treatment. J Natl Med Assoc 2011;103:468-78. [Crossref] [PubMed]
- Wallner K, Roy J, Harrison L. Low risk of perioperative infection without prophylactic antibiotics for transperineal prostate brachytherapy. Int J Radiat Oncol Biol Phys 1996;36:681-3. [Crossref] [PubMed]
- Mabjeesh NJ, Chen J, Stenger A, et al. Preimplant predictive factors of urinary retention after iodine 125 prostate brachytherapy. Urology 2007;70:548-53. [Crossref] [PubMed]
- Lee N, Wuu CS, Brody R, et al. Factors predicting for postimplantation urinary retention after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 2000;48:1457-60. [Crossref] [PubMed]
- Sacco DE, Daller M, Grocela JA, et al. Corticosteroid use after prostate brachytherapy reduces the risk of acute urinary retention. BJU Int 2003;91:345-9. [Crossref] [PubMed]
- Jacobs BL, Smith RP, Beriwal S, et al. Changes in lower urinary tract symptoms after prostate brachytherapy. J Contemp Brachytherapy 2011;3:115-20. [Crossref] [PubMed]
- Wallner K, Merrick G, True L, et al. I-125 versus Pd-103 for low-risk prostate cancer: morbidity outcomes from a prospective randomized multicenter trial. Cancer J 2002;8:67-73. [Crossref] [PubMed]
- Merrick GS, Butler WM, Lief JH, et al. Temporal resolution of urinary morbidity following prostate brachytherapy. Int J Radiat Oncol Biol Phys 2000;47:121-8. [Crossref] [PubMed]
- Merrick GS, Butler WM, Wallner KE, et al. Prophylactic versus therapeutic alpha-blockers after permanent prostate brachytherapy. Urology 2002;60:650-5. [Crossref] [PubMed]
- Mohammed N, Kestin L, Ghilezan M, et al. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:204-12. [Crossref] [PubMed]
- Keyes M, Miller S, Pickles T, et al. Late urinary side effects 10 years after low-dose-rate prostate brachytherapy: population-based results from a multiphysician practice treating with a standardized protocol and uniform dosimetric goals. Int J Radiat Oncol Biol Phys 2014;90:570-8. [Crossref] [PubMed]
- Merrick GS, Butler WM, Wallner KE, et al. Late rectal function after prostate brachytherapy. Int J Radiat Oncol Biol Phys 2003;57:42-8. [Crossref] [PubMed]
- Ohashi T, Yorozu A, Saito S, et al. Urinary and rectal toxicity profiles after permanent iodine-125 implant brachytherapy in Japanese Men: Nationwide J-POPS Multi-institutional Prospective Cohort Study. Int J Radiat Oncol Biol Phys 2015;93:141-9. [Crossref] [PubMed]
- Serrano N, Moghanaki D, Asher D, et al. Comparative study of late rectal toxicity in prostate cancer patients treated with low-dose-rate brachytherapy: With or without supplemental external beam radiotherapy. Brachytherapy 2016;15:435-41. [Crossref] [PubMed]
- Zelefsky MJ, Yamada Y, Cohen GN, et al. Five-year outcome of intraoperative conformal permanent I-125 interstitial implantation for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2007;67:65-70. [Crossref] [PubMed]
- Leong N, Pai HH, Morris WJ, et al. Rectal ulcers and rectoprostatic fistulas after (125)I low dose rate prostate brachytherapy. J Urol 2016;195:1811-6. [Crossref] [PubMed]
- Pardo Y, Guedea F, Aguilo F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol 2010;28:4687-96. [Crossref] [PubMed]
- Zelefsky MJ, Poon BY, Eastham J, et al. Longitudinal assessment of quality of life after surgery, conformal brachytherapy, and intensity-modulated radiation therapy for prostate cancer. Radiother Oncol 2016;118:85-91. [Crossref] [PubMed]
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250-61. [Crossref] [PubMed]
- Chen RC, Basak R, Meyer AM, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA 2017;317:1141-50. [Crossref] [PubMed]
- Blanchard P, Davis JW, Frank SJ, et al. Quality of life after brachytherapy or bilateral nerve-sparing robot-assisted radical prostatectomy for prostate cancer: a prospective cohort. BJU Int 2018;121:540-8. [Crossref] [PubMed]
- Crook JM, Gomez-Iturriaga A, Wallace K, et al. Comparison of health-related quality of life 5 years after SPIRIT: Surgical Prostatectomy Versus Interstitial Radiation Intervention Trial. J Clin Oncol 2011;29:362-8. [Crossref] [PubMed]
- Murray L, Mason J, Henry AM, et al. Modelling second malignancy risks from low dose rate and high dose rate brachytherapy as monotherapy for localised prostate cancer. Radiother Oncol 2016;120:293-9. [Crossref] [PubMed]
- Hamilton SN, Tyldesley S, Hamm J, et al. Incidence of second malignancies in prostate cancer patients treated with low-dose-rate brachytherapy and radical prostatectomy. Int J Radiat Oncol Biol Phys 2014;90:934-41. [Crossref] [PubMed]
- Hinnen KA, Schaapveld M, van Vulpen M, et al. Prostate brachytherapy and second primary cancer risk: a competitive risk analysis. J Clin Oncol 2011;29:4510-5. [Crossref] [PubMed]
- Cosset JM, Belin L, Wakil G, et al. Second malignancies after permanent implant prostate cancer brachytherapy: A single-institution study of 675 patients treated between 1999 and 2003. Cancer Radiother 2017;21:210-5. [Crossref] [PubMed]
- Wallis CJ, Mahar AL, Choo R, et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ 2016;352:i851. [Crossref] [PubMed]
- Gillan C, Abrams D, Harnett N, et al. Fears and misperceptions of radiation therapy: sources and impact on decision-making and anxiety. J Cancer Educ 2014;29:289-95. [Crossref] [PubMed]
- Michalski J, Mutic S, Eichling J, et al. Radiation exposure to family and household members after prostate brachytherapy. Int J Radiat Oncol Biol Phys 2003;56:764-8. [Crossref] [PubMed]
- Agency USEP. Radiation Sources and Doses. United States Environmental Protection Agency. Available online: https://www.epa.gov/radiation/radiation-sources-and-doses. Accessed October 1 2017.
- Hanada T, Yorozu A, Shinya Y, et al. Prospective study of direct radiation exposure measurements for family members living with patients with prostate (125)I seed implantation: Evidence of radiation safety. Brachytherapy 2016;15:412-9. [Crossref] [PubMed]
- Schwartz DJ, Davis BJ, Vetter RJ, et al. Radiation exposure to operating room personnel during transperineal interstitial permanent prostate brachytherapy. Brachytherapy 2003;2:98-102. [Crossref] [PubMed]
- Pugh TJ, Pokharel SS. Magnetic resonance imaging in prostate brachytherapy: Evidence, clinical end points to data, and direction forward. Brachytherapy 2017;16:659-64. [Crossref] [PubMed]
- Pugh TJ, Frank SJ, Achim M, et al. Endorectal magnetic resonance imaging for predicting pathologic T3 disease in Gleason score 7 prostate cancer: implications for prostate brachytherapy. Brachytherapy 2013;12:204-9. [Crossref] [PubMed]
- Laing R, Franklin A, Uribe J, et al. Hemi-gland focal low dose rate prostate brachytherapy: An analysis of dosimetric outcomes. Radiother Oncol 2016;121:310-5. [Crossref] [PubMed]
- Nguyen PL, Chen MH, Zhang Y, et al. Updated results of magnetic resonance imaging guided partial prostate brachytherapy for favorable risk prostate cancer: implications for focal therapy. J Urol 2012;188:1151-6. [Crossref] [PubMed]
- Mahdavi SS, Spadinger IT, Salcudean SE, et al. Focal application of low-dose-rate brachytherapy for prostate cancer: a pilot study. J Contemp Brachytherapy 2017;9:197-208. [Crossref] [PubMed]
- Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2015;92:971-7. [Crossref] [PubMed]
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III Trial. Int J Radiat Oncol Biol Phys 2017;97:976-85. [Crossref] [PubMed]
- Beydoun N, Bucci JA, Chin YS, et al. First report of transperineal polyethylene glycol hydrogel spacer use to curtail rectal radiation dose after permanent iodine-125 prostate brachytherapy. Brachytherapy 2013;12:368-74. [Crossref] [PubMed]
- Lee J, Mian OY, Le Y, et al. Intraoperative Registered Ultrasound and Fluoroscopy (iRUF) for dose calculation during prostate brachytherapy: Improved accuracy compared to standard ultrasound-based dosimetry. Radiother Oncol 2017;124:61-7. [Crossref] [PubMed]
- Carrara M, Cutajar D, Alnaghy S, et al. Semiconductor real-time quality assurance dosimetry in brachytherapy. Brachytherapy 2018;17:133-45. [Crossref] [PubMed]
- Zelefsky MJ, Cohen GN, Taggar AS, et al. Real-time intraoperative evaluation of implant quality and dose correction during prostate brachytherapy consistently improves target coverage using a novel image fusion and optimization program. Pract Radiat Oncol 2017;7:319-24. [Crossref] [PubMed]
- Stock R, Beyer D, Kaminetsky J, et al. Performance of a palladium-103 line source for prostate brachytherapy implants: A Phase I trial. Brachytherapy 2017;16:1007-12. [Crossref] [PubMed]
- Laviana AA, Ilg AM, Veruttipong D, et al. Utilizing time-driven activity-based costing to understand the short- and long-term costs of treating localized, low-risk prostate cancer. Cancer 2016;122:447-55. [Crossref] [PubMed]
- Shah C, Lanni TB Jr, Ghilezan MI, et al. Brachytherapy provides comparable outcomes and improved cost-effectiveness in the treatment of low/intermediate prostate cancer. Brachytherapy 2012;11:441-5. [Crossref] [PubMed]
- Martin JM, Handorf EA, Kutikov A, et al. The rise and fall of prostate brachytherapy: use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer 2014;120:2114-21. [Crossref] [PubMed]
- Orio PF 3rd, Nguyen PL, Buzurovic I, et al. The decreased use of brachytherapy boost for intermediate and high-risk prostate cancer despite evidence supporting its effectiveness. Brachytherapy 2016;15:701-6. [Crossref] [PubMed]


