Prostate cancer screening—when to start and how to screen?
Introduction
Currently, only two countries in the world—Lithuania and Kazakhstan—have an organized population-based screening program for prostate cancer (PCa) (1,2). Screening is still a controversial issue in most countries because there are both benefits and harms associated with such practice. Even experts and opinion leaders in the field disagree on who, when and how to best screen for PCa and interpret the evidence differently regarding the magnitude of prevention of PCa deaths and metastatic disease versus the risks of overdiagnosis and side-effects of treatment such as erectile dysfunction and urinary incontinence (3). For instance, an often cited estimate is that “there is a small but finite benefit from PCa screening in terms of PCa mortality—about 1 fewer PC death/1,000 men screened over 10 years” (4). This fails to address the time-to-event nature of the data (5). Computer simulation screening analysis have shown that the benefit increases with time, with 9 fewer deaths/1,000 men screened followed for their entire life span, i.e., closer to 1/100 rather than 1/1,000 (6). Although organized screening with the blood test prostate-specific antigen (PSA) has been shown to be more effective than opportunistic PSA-testing in terms of reducing PCa mortality (7), most guideline groups recommend against mass screening, as the benefits do not exceed the disadvantages with overdiagnosis and over-treatment. Therefore, shared, or informed, decision-making has been the trend in recent years, implying that the decision whether or not to be screened for PCa should be an individual one, after a discussion about the benefits and harms between the man and his health care provider (4,8). Simple decision support tools are available to facilitate such discussions (9). All experts agree that screening should take place only after shared decision making, and that increased use of active surveillance (monitoring) for low risk PCa is desirable (2,3). Risk-stratified screening algorithms have also become increasingly popular, with or without additional biomarkers added to the PSA-test to determine the need for biopsy (10). The objective of this article is to review the current literature regarding when to start PCa screening and how to screen.
Methods
Several known recommendations, as well as articles selected after searching PubMed, were included for this review. Several guidelines for PCa screening and “guideline to the guidelines” have been reported (11-15) (Table 1). All guidelines agree that any PCa screening ought to take place in the context of shared decision making. The United States Preventive Services Task Force (USPSTF) 2017 draft recommendation recommends shared decision making starting between the ages 55–69 (8). The American Cancer Society (ACS) and the American College of Physicians (ACP) recommend starting discussions about PSA testing at age 50 (16-18) and the American Society of Clinical Oncology (ASCO) recommends discussing the appropriateness of screening with men with a life expectancy >10 years (19).
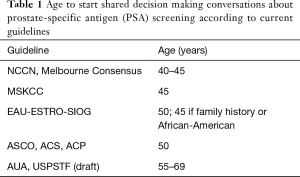
Full table
As for the suggested screening algorithms, we narrowed this review down to guidelines describing more detailed risk-stratified approaches to PSA-screening—the current trend in the academic urologic oncology community to improve the balance between benefits and harms. To this end, we included select guidelines currently available in Europe and the U.S.: the European Association of Urology (EAU)-European Society for Radiotherapy & Oncology (ESTRO)-International Society of Geriatric Oncology (SIOG) Guidelines on Prostate Cancer (20), the National Comprehensive Cancer Center (NCCN) Guidelines Prostate Cancer Early Detection Guideline (10), the Memorial Sloan Kettering Cancer Center (MSKCC) Recommendations for Prostate Cancer Screening and the American Urological Association (AUA) Guideline (21), as well as articles published in the last 5 years from the PubMed search. When searching PubMed, Medical Subject Heading (MeSH) terms such as “Prostatic Neoplasms”, “Mass Screening”, “Risk Factors”, “Age Factors”, etc., were used, supplemented by relevant keywords to include in-process citations and PubMed articles not indexed for Medline. Publication types such as Case Reports, Letter and Editorial were excluded from the search. A total of 43 full-text articles were selected for the final review. These were categorized into one of five categories: age to start, risk factors, PSA, magnetic resonance imaging (MRI) and other screening alternatives.
Results
Does screening reduce PC mortality?
The European Randomized Study of Screening for Prostate Cancer (ERSPC) is the world’s largest randomized controlled trial (RCT) on PSA-screening including 162,388 men aged 55–69 years in 8 European countries (22). The 13-year follow-up report showed that PSA-screening every 2–4 years reduces PC mortality by 21%. The reduction in PCa mortality was even larger—44% at 14 years—in the Göteborg trial where 20,000 men ages 50–64 were randomized to biennial PSA-screening or a control group (7,23). The U.S. Prostate Lung Colorectal and Ovarian (PLCO) cancer screening trial randomized 76,685 men aged 55–74 years but did not show any difference in PCa mortality between the screening and control arm (24). The reason for this was high pre-screening rates in both arms and a high contamination rate in the control arm; i.e., the two arms were subjected to almost the same amount of screening (25). However, with these discrepancies accounted for, both the ERSPC and PLCO trials provide compatible evidence that PSA screening reduces PC mortality (26). There is also compelling evidence from observational data. In the U.S., where the PSA test was introduced as a screening tool in the early 90’s, the age-adjusted death rate from PCa dropped 51% between 1993 to 2014 (27).
When to start PCa screening
Age
There is no consensus regarding the age at which to initiate PSA-testing (Table 1). Most guidelines recommend that discussions about PSA screening start around ages 45–55 (10,20,21) with well-informed men in good health and a life expectancy of at least 10–15 years. The core age group in the ERSPC trial started screening between ages 55–69. The AUA guideline supports starting screening at age 55 based on the ERSPC trial and because of the risk of overdiagnosis (and overtreatment) in younger men, but also acknowledge that men at higher risk for PCa can start before 55.
Men in the Göteborg trial started screening between ages 50–64. A recent analysis comparing screened men in Göteborg, Sweden, to unscreened men in Malmö, Sweden, showed that regular screening starting at 50–54 could reduce PCa mortality by 17% at 17 years (28). The EAU Guideline recommends to start at age 50 for most men, except in men with a family history of PCa or African American men for whom the recommendation is to start at age 45 (20). The NCCN and MSKCC Guidelines support testing beginning at age 45 after shared decision-making (10,29). Indirect support for starting PSA screening no later than age 55 also comes from an Australian study of 598 prostate biopsies and 723 prostatectomy matched subjects, in which the rates of high-risk PCa (and insignificant PC) were similar between men ≤55 years and men >55 years (30). A sub study from the Göteborg screening trial, in which men were screened every 2 years between ages 50–70 investigated the effect of age at start and the number of screening occasions on the risk of PCa diagnosis, by following the age cohorts over time (e.g., starting at age 52 resulted in 9 screens and starting at 60 resulted in 5 screens). The study showed that starting screening at an earlier age advanced the time of PCa diagnosis but did not increase the risk of being diagnosed, suggesting that starting early does not increase the risk of overdiagnosis, whereas the age for stopping screening does (31). A study from Johns Hopkins showed that older men (75+) who underwent radiotherapy for PCa and who had no history of PSA testing presented with worse disease (more high-risk and high-grade PC) than men who were previously screened (32). Weight et al. (33) suggest that there is no advantage in starting screening at age 40 instead of 50. They compared screening in a younger group of men, aged 40–49, with men in their 50s and found greater risk of undergoing a biopsy and receiving the diagnosis of low-risk PCa (HR 2.4, 95% CI: 1.7–3.3 and HR 2.2, 95% CI: 1.12–4.0 respectively) for the younger group. The authors did not find any difference in PCa deaths between the groups, however, follow-up longer than the 17 years in the study is likely necessary to detect any difference in PCa mortality between groups. Another limitation of the study was the rather small sample size.
A recent analysis of the U.S. PLCO trial specifically studied characteristics of 151 men who died from PCa within 13 years of follow-up and were randomized to the screening arm. The authors found that more than half of these men were never screened and they were also older at study start than the average participant (66 vs. 62 years) (34).
Critical for balancing the benefits and harms of screening, particularly the risk of overdiagnosis, is the age to stop screening—which is covered in another article in this issue of TAU (35). For instance, stopping screening at age 70 can reduce overdiagnosis by 42% (36).
Risk factors
Men with family history of PCa and Afro-American race have increased risk of PCa (15,37,38). According to SEER data, the U.S. incidence of PCa among black men is 60% higher than in white men; the PCa mortality rate is also 2–3 times higher (39). As pointed out by Grill et al., taking a detailed family history is inexpensive and also family history is an independent predictor of PCa among other commonly considered risk factors (40). The likelihood of PCa diagnosis is increased by 2.1- to 2.5-fold in men with a first degree relative who had PCa diagnosed before the age of 60 (40,41). However, men with family history of PCa are at risk of low-grade PCa diagnosis with screening, similar to all men screened (42). In a study of 461 PCa patients treated with radical prostatectomy, Raheem et al. found no increased risk of aggressive PCa or biochemical recurrence among patients with first-degree relatives who died of PCa compared to those without family history (43).
However, whether screening should start sooner for these men remains an issue for debate. While both the NCCN and MSKCC screening guidelines (10,29) acknowledge the above-mentioned groups of men as high-risk groups, however they consider data on screening in diverse and high-risk populations as lacking since PCa screening has mainly been studied in Caucasian men. Of the two major screening trials, one, ERSPC, reported no information on race or family history, and the other one, PLCO, had enrolled approximately 4.4% African-Americans and 6.9% men had a positive family history. The guidelines mentioned above (NCCN and MSKCC) instead stress that the PSA value at age 45 is a stronger risk factor for long-term PCa death than both family history and race (44) suggesting no differential screening guidelines but start age 45 for most (10,29). However, there may be synergistic effects between a positive family history and elevated PSA. Some call for separate PCa screening guidelines for African-American men as there are differences between African-Americans and Caucasians in terms of PCa incidence, clinical course and outcomes, PSA levels and social barriers (45). In a retrospective analysis, Verges et al investigated the relationship of baseline PSA and risk of future PCa and its variance by race. Black men were more likely to be diagnosed with PCa (OR 1.62, P<0.0001) despite that the median baseline PSA was similar between black and white men. The risk was particularly high among black men younger than 70 years (46).
The EAU guidelines recommend starting PSA testing earlier, from 45 years, for men with a family history of PCa or African American men (20). The AUA guidelines recommend against routine screening for men below 55 years unless they are at higher risk; positive family history or African-American race (21). They advocate individualized and well-informed discussion regarding the uncertainty of benefit and the associated harms of screening, pointing out that a strong family history (two or more first degree relatives and/or PCa in multiple generations and/or early onset of PCa <60 years in relatives) is associated with an increased risk of disease and should be taken into account when discussing the potential benefit and harm.
Albright and colleagues analyzed data from 600,000 men in the population-based SEER registry with information on family history and found that the relative risks of lethal PCa varied with the number of affected first-degree relatives [RR 2.5 (95% CI: 2.3–2.7) if 1 relative and 5.3 (95% CI: 2.1–10.9) if 3+ relatives] (47).
Several germline single nucleotide polymorphisms (SNPs) have been associated with PCa risk (48). It has been suggested that SNPs can play a role in targeted screening (49,50). The ongoing PROFILE study will investigate the probability of detecting PCa with biopsy in men with family history by combining SNP profiling with clinical variables (51). With data from 4,528 men in the Prostate Cancer Prevention Trial (PCPT), Chen et al. developed a genetic risk score based on 29 PCa risk-associated SNPs and found that more high-grade PCa cases could be identified if family history was supplemented by the genetic risk score (52). Turner and colleagues carried out a randomized trial of 700 men aged 40–49 years randomized to counseling men regarding screening based on family history vs. family history plus a SNP-based genetic risk score. At 3 years of follow-up, the authors found no increases in anxiety or PSA screening utilization (neither overuse, nor underuse), rather suggesting a more targeted use of PSA screening in high risk men, without negative effects on quality-of-life (53).
Although Lynch syndrome is associated with a 2- to 5-fold increase in risk for PCa (54), there are currently no specific screening recommendations for men with Lynch syndrome in any of the major guidelines. BRCA2 mutations have similarly been associated with a 2- to 6-fold increase risk for PCa (55,56). Men with BRCA2 mutations have a high PCa mortality despite PSA testing (57). BRCA2 mutations are associated with early onset and poor prognosis of PCa (58,59). The NCCN guidelines for Genetic/Familial High-Risk Assessment: Breast and Ovarian (60) recommend that men with BRCA1/2 mutations start PCa screening at age 40. However, due to insufficient data, this practice is currently not supported by the panel for the NCCN guidelines on PCa early detection v2.2017 (10). There are ongoing screening studies in genetically higher-risk cohorts, such as carriers of germ-line mutations in BRCA1/2 and Lynch syndrome, that will investigate the clinical utility of these genetic variants as there are suggestions that these patients benefit from screening and earlier diagnosis and treatment (61,62).
Mutations in the tumor suppressor gene HOXB13 is associated with increased risk of PCa (63). In a study in a Swedish population, the relative risk of PCa was 3.4-fold higher in carriers of this mutation compared to matched cases (64). In their review on screening for familial and hereditary PC, Lynch et al propose that PSA testing could be supplemented by testing alleles, such as BRCA2 and HOXB13, in families with unfavorable family cancer history, and encourages that genomic sequencing protocols be included in future population studies (65).
Gulati et al. addressed the lack of guidelines for PSA screening in subgroups with higher risks (BRCA1/2 carriers) and analyzed these subgroups further in computer simulation models, finding that more lives could indeed be saved by screening these subgroups compared to average risk groups, but also found that more intensive screening did not necessarily improve the balance between benefits and harm of screening (66). Muhlberger et al. found contradictory findings using a decision-analytic model, and suggested that PCa screening should take into account age, individual quality-of-life preferences and familiar predisposition as that optimized the benefit-harm balance, whereas screening men with average PCa risk yielded life expectancy gains but potential losses in quality-adjusted life expectancy (67).
How to screen
Total PSA
Total PSA measured in serum or plasma has been the mainstay as a screening tool for PCa since its introduction in the late 1980’s to the early-mid 1990’s (68,69) The evidence is growing regarding the value of the “baseline” PSA measured in midlife for risk-stratification of future screening intensity, as shown by several large population-based observational studies (e.g., Malmö Preventive Project, Malmö Diet and Cancer, Västerbotten Intervention Project, the Baltimore Longitudinal Study of Aging, the Physician’s Health Study) (28,70-72). Recently, one of the first baseline PSA cohort studies, The Olmsted Study, reported 17-year follow-up data from the population of 1,052 men who were screened biennially with PSA, DRE and TRUS starting between the ages 40–49 and were compared to men who began screening in their 50’s. The younger cohort was more likely to undergo prostate biopsy (HR 2.4, 95% CI: 1.8–3.3) and be diagnosed with PCa (HR 2.2, 95% CI: 1.1–4.0), however, longer follow-up is needed to determine the effect of PCa mortality (33). The prospective German study PROBASE, starting screening at 45 is currently under way (73).
Taken together, these studies support starting screening in midlife and stratifying risk based on PSA in midlife to tailor further re-screening intervals to risk of future metastasis and/or PCa death with more frequent screening if higher risk and less frequent screening if lower risk. These studies, together with data from the randomized trials, have helped inform the screening algorithms outlined by the NCCN, EAU and MSKCC guidelines (10,20,29). For example, the MSKCC guideline recommends starting at age 45 and considering biopsy if the PSA ≥3 ng/mL. If the PSA is ≥1 but <3 ng/mL, the guideline suggest returning for PSA testing every 2–4 years. If the PSA <1 ng/mL, returning for PSA testing at 6–10 years (29).
For over two decades, a PSA cut-off of ≥4 ng/mL was used to recommend biopsy. However, the Prostate Cancer Prevention Trial (PCPT) in 2004, in which all men had an end-of-study biopsy regardless of PSA-level, changed this paradigm. PCa was found in 15% of men with PSA <4.0 ng/mL, and of which 15% were Gleason score 7 or higher. Many cancers were found in the PSA-range 3–4 ng/mL. After this trial, PSA is no longer seen as a dichotomous marker (“normal” vs. “elevated”) but as a continuum of risk.
Rather than the one-size-fits-all, more personalized and risk-stratified strategies have been proposed. Recently, some groups have suggested to perform further risk assessment already at PSA values >1.0 ng/mL, as suggested by Brawley et al. (74) or having discussions and performing clinical workup including considerations of additional biomarkers and/or urology referral for men above >1.5 ng/mL, as suggested by Crawford et al. (75), as men below these cut-points have low risk for significant PC. Simple cut-offs for urology referral would facilitate the work of primary care physicians, however, whether these approaches would be clinically feasible is not known, in terms of number of referrals and resources needed. Moreover, the number of men needed to screen and biopsy to find one high-grade cancer and prevent PCa mortality with these approaches are not known.
Several studies and guidelines also emphasize repeating the PSA before biopsy, due to commonly observed fluctuations in this measurement (29,76-78).
MRI
Due to its ability to help detect, localize and characterize PC, multiparametric (mp)MRI plays an important role in a wide array of areas regarding PCa diagnosis, risk stratification, staging and treatment planning (79). In recent years, mpMRI has become increasingly utilized also in the pre-diagnostic setting, i.e., before prostate biopsy. EAU guidelines cite a recent systematic review, where prostate MRI in the pre-diagnostic setting had a negative predictive value (NPV) ranging from 63% to 98% and a positive predictive value (PPV) ranging from 34% to 68%, respectively (80). Because we have yet to see studies with consistently high NPV in excluding PCa on biopsy (81)—especially in the community setting—it is still too early to make recommendations on the routine use of pre-biopsy mpMRI in biopsy-naïve patients. A recent systematic review showed that the accuracy of mpMRI is highly variable depending on the setting and that sharpened risk stratification before mpMRI can help improve the accuracy of prebiopsy mpMRI (81). Several recent studies (82-86) have shown higher detection rate of clinically significant PCa when using targeted biopsies compared to systematic biopsies, mainly in the repeat biopsy setting (87), however, there are several contradictory studies (88-90). The EAU guideline recommends MRI before repeat biopsy, which should consist of both targeted and systematic biopsies, if the clinical suspicion of PCa persists despite negative biopsies (20). Moreover, the MRI rating system PI-RADS v.2 has been shown to have low specificity (91,92) and a moderate inter-reader reproducibility (92-95).
The NCCN guideline panel shares a similar opinion as the EAU; that current data has not convincingly—and consistently—shown that MRI can improve detection for clinically significant PCa in the initial biopsy setting (10). The AUA guideline also agrees that the current data supports the use of MRI in patients with previous negative biopsy and with persistent suspicion of PC, but not in other settings such as screening (96).
Up to recently, there were no prospective studies on MRI in the biopsy-naïve setting until Ahmed and colleagues reported the PROMIS study (97), which suggests that mpMRI can be used as a triage test before first prostate biopsy to avoid unnecessary biopsies, reduce over-diagnosis of clinically insignificant PCa and improve detection of clinically significant cancer (96). MRI was more accurate than transrectal ultrasound (TRUS)-biopsy in terms of both sensitivity; 93% vs. 48%, and NPV; 89% vs. 74%. There are several ongoing trials that will elucidate the role of MRI as a screening tool, such as the Göteborg-2 trial which is a large population-based randomized clinical screening trial with PSA and MRI. This trial was preceded by a pilot study within the 10th round of the Göteborg-1 trial which assessed the role of MRI in screening by investigating three different screening strategies; the two strategies that included MRI and PSA (with different thresholds) had significantly higher sensitivity than the strategy with only PSA (98). This pilot included 384 both biopsy-naïve men and men with previous biopsy. A separate pilot study from Toronto included 47 biopsy-naïve men who underwent MRI and systematic biopsies, as well as targeted biopsies if the MRI showed a suspicious lesion (99). MRI performed better than PSA in predicting PCa (OR 2.7 vs. 1.1). PRECISION is another ongoing trial; a multicenter RCT that investigates whether MRI-targeted biopsy is non-inferior to standard TRUS-guided biopsy for the diagnosis of clinically significant PCa in men without prior biopsy (100).
Other alternatives
There are currently no alternative first-line screening tests, such as total-PSA, but several markers exist that are intended to be used as reflex markers [e.g., free-to-total (F/T) PSA, Prostate Health Index (PHI), 4Kscore, PCA3] in men with indications for biopsy (e.g., elevated PSA, positive DRE etc.). The NCCN guideline recommends consideration of F/T PSA, PHI or 4Kscore before initial biopsy (10). The same tests, or the urine test PCA3, are recommended also before repeat biopsy. The PCA3 test has been studies in multiple studies and has been shown to be useful mainly for repeat biopsy. This is because of the rather high risk (13%) of high-grade disease among men with low PCA3 values at initial biopsy (101). These reflex markers have been shown to improve the specificity of PSA and help reduce the number of unnecessary biopsies (10,15,76,102,103). The PHI and 4Kscore are widely used tests and both have been validated in multiple studies in thousands of patients including prospective multicenter validation studies (104-106). For instance, and a study done in over 600 men with abnormal PSA and/or DRE in routine U.S. care reduced 65% of biopsies with the use of the 4Kscore (107). In a prospective multicenter study of nearly 900 men with PSA 2–10 ng/mL, use of the PHI test with a cut-off of 25 for biopsy reduced about 40% of unnecessary biopsies (105). However, a recent study showed that while the clinical use of PHI indeed reduced biopsies among men with PSA 4–10 ng/mL, the risk of high grade cancer in men not biopsied in the PHI group was estimated to be about 1 in 3, far too many missed cancers for acceptable clinical use (101).
The most common cause of a PSA-elevation is a large prostate gland (benign prostatic hyperplasia, BPH). Because of this, PSA density is an adjunct that can help discriminate PCa from BPH (108). It is calculated as the PSA value (ng/mL) divided by prostate volume (cc) measured by TRUS. A lower PSA density implies a higher probability of BPH. Using a PSA density cut-off of >0.15 ng/mL/cc to recommend biopsy can reduce unnecessary biopsies, however, with the caveat that TRUS volume measurement can be user dependent and the sensitivity of this cut-off is insufficient, missing 31% of cancers among men with PSA 4–10 ng/mL in one study (10,109).
PSA velocity has no place in the decision-making regarding biopsy after screening as argued by Vickers et al. (110).
The STHLM3-test (a combination of several biomarkers, clinical information and genetic polymorphisms) has been proposed as a first-line test (74,111). The study invited nearly 150,000 men aged 50–69 to STHLM3 testing compared to PSA alone. The predictive accuracy (AUC) was higher for the STHLM3 test for high-grade PCa (Gleason Score 7 or higher) compared to PSA alone and reduced the number of unnecessary biopsies by 32% (95% CI: 23–39) (74,111). However, the cost-effectiveness of using the test for screening is not known and the marginal added value of each of the individual components of the STHLM3 test remain to be understood (112,113).
A new urine exosome assay (ExoDx Prostate IntelliScore) was recently validated by McKiernan et al., showing a reduction of 27% of biopsies at the cost of missing 8% PCa Gleason Score 7 or higher (114). However, this test has yet only been evaluated in a single study.
Multivariable approaches to reduce the number of biopsies have long been proposed through the use of risk calculators, e.g., the ERSPC or PCPT risk calculators, which combine clinical information with PSA into predicted risk of high-grade PCa on biopsy (17). MRI has been proposed to be used in combination with PSA and/or other biomarkers or risk calculators to further stratify risk and is currently being studied. In a retrospective study of 1,159 men who underwent MRI before targeted and systematic transperineal biopsies, Radtke and colleagues proposed a risk model for risk of PCa based on age, clinical parameters (PSA, prostate volume, DRE) and PI-RADS v.1. score. The risk model’s performance characteristic (AUC 0.81) was superior to commonly used risk calculators and PIRADS v.1. alone to discriminate between the presence and absence of clinically significant PCa (115).
Conclusions
Urologists agree on the fact that PSA screening reduces PCa mortality. Total PSA is still currently the preferred screening tool and a powerful marker of future risk of metastasis (AUC 0.86) and PCa death (116,117). Yet, there is no consensus on the age to start PSA screening, due to insufficient data. The different guidelines recommend starting at ages 45, 50 and 55. Discussions between a patient and a physician on the pros and cons of screening and shared decision-making are crucial.
There is strong consensus regarding which men are at increased risk for PC; men with family history of PCa and African-American race/men, but there is no consensus regarding the screening practices for these men; EAU, MSKCC and NCCN recommend PSA testing at the age of 45 for these men and AUA strongly recommends shared decision-making after discussion concerning the impact of the individual man’s risk.
International guidelines do not currently recommend mpMRI before initial biopsy decision, but considerations in patients with persistently rising PSA and previous negative biopsies. While there are promising pilot studies, current data is still insufficient to support a role for MRI in biopsy decision making in the screening context because studies have not shown consistently high NPVs. Future, larger-scale prospective studies are around the corner (87,98).
Acknowledgements
We sincerely thank Dr. Ola Bratt for his guidance regarding hereditary risk.
Funding: This work was supported in part by funds from the Sidney Kimmel Center for Prostate and Urologic Cancers, a Specialized Programs of Research Excellence grant (P50-CA92629) from the National Cancer Institute to Dr. Howard Scher, and a National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30-CA008748) to Memorial Sloan Kettering Cancer Center, a grant from the National Cancer Institute as part of the Cancer Intervention and Surveillance Modelling Network (U01CA199338-02), the David H. Koch prostate cancer research fund and the Prevent Cancer Foundation.
Footnote
Conflicts of Interest: Dr. SV Carlsson has received travel support from Astellas. The other authors have no conflicts of interest to declare.
References
- Gondos A, Krilaviciute A, Smailyte G, et al. Cancer surveillance using registry data: Results and recommendations for the Lithuanian national prostate cancer early detection programme. Eur J Cancer 2015;51:1630-7. [Crossref] [PubMed]
- Ishkinin Y, Zhylkaidarova A, Nurgaliyev N, et al. Population-based Prostate Cancer Screening in Kazakhstan. Iran J Public Health 2017;46:917-22. [PubMed]
- Carlsson S, Leapman M, Carroll P, et al. Who and when should we screen for prostate cancer? Interviews with key opinion leaders. BMC Med 2015;13:288. [Crossref] [PubMed]
- Barry MJ, Simmons LH. Prevention of Prostate Cancer Morbidity and Mortality: Primary Prevention and Early Detection. Med Clin North Am 2017;101:787-806. [Crossref] [PubMed]
- Carlsson S, Vickers AJ, Roobol M, et al. Prostate cancer screening: facts, statistics, and interpretation in response to the US Preventive Services Task Force Review. J Clin Oncol 2012;30:2581-4. [Crossref] [PubMed]
- Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med 2012;367:595-605. [Crossref] [PubMed]
- Arnsrud Godtman R, Holmberg E, Lilja H, et al. Opportunistic testing versus organized prostate-specific antigen screening: outcome after 18 years in the Goteborg randomized population-based prostate cancer screening trial. Eur Urol 2015;68:354-60. [Crossref] [PubMed]
- U.S. Preventive Services Task Force. Prostate cancer screening draft recommendations. Available online: https://screeningforprostatecancer.org/
- Vickers AJ, Edwards K, Cooperberg MR, et al. A simple schema for informed decision making about prostate cancer screening. Ann Intern Med 2014;161:441-2. [Crossref] [PubMed]
- Carroll PR, Parsons JK, Andriole G, et al. NCCN Guidelines Insights: Prostate Cancer Early Detection, Version 2.2016. J Natl Compr Canc Netw 2016;14:509-19. [Crossref] [PubMed]
- Loeb S. Guideline of guidelines: prostate cancer screening. BJU Int 2014;114:323-5. [PubMed]
- Cabarkapa S, Perera M, McGrath S, et al. Prostate cancer screening with prostate-specific antigen: A guide to the guidelines. Prostate Int 2016;4:125-9. [Crossref] [PubMed]
- Greene KL, Punnen S, Carroll PR. Evolution and immediate future of US screening guidelines. Urol Clin North Am 2014;41:229-35. [Crossref] [PubMed]
- Lewis R, Hornberger B. The current state of prostate-specific antigen testing. Jaapa 2016;29:51-3. [Crossref] [PubMed]
- Stewart RW, Lizama S, Peairs K, et al. Screening for prostate cancer. Semin Oncol 2017;44:47-56. [Crossref] [PubMed]
- Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2017;67:100-21. [Crossref] [PubMed]
- Carlsson SV, Roobol MJ. What's new in screening in 2015? Curr Opin Urol 2016;26:447-58. [Crossref] [PubMed]
- Wilt TJ, Harris RP, Qaseem A, et al. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015;162:718-25. [Crossref] [PubMed]
- Nam RK, Oliver TK, Vickers AJ, et al. Prostate-specific antigen test for prostate cancer screening: American Society of Clinical Oncology provisional clinical opinion. J Oncol Pract 2012;8:315-7. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol 2013;190:419-26. [Crossref] [PubMed]
- Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384:2027-35. [Crossref] [PubMed]
- Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010;11:725-32. [Crossref] [PubMed]
- Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 2012;104:125-32. [Crossref] [PubMed]
- Pinsky PF, Blacka A, Kramer BS, et al. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Clin Trials 2010;7:303-11. [Crossref] [PubMed]
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the Effects of Screening on Prostate Cancer Mortality in the ERSPC and PLCO Trials. Ann Intern Med 2017;167:449-55. [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Carlsson S, Assel M, Ulmert D, et al. Screening for Prostate Cancer Starting at Age 50-54 Years. A Population-based Cohort Study. Eur Urol 2017;71:46-52. [Crossref] [PubMed]
- Vickers AJ, Eastham JA, Scardino PT, et al. The Memorial Sloan Kettering Cancer Center Recommendations for Prostate Cancer Screening. Urology 2016;91:12-8. [Crossref] [PubMed]
- Dantanarayana ND, Hossack T, Cozzi P, et al. Men under the age of 55 years with screen detected prostate cancer do not have less significant disease compared to older men in a population of patients in Australia. BMC Urol 2015;15:124. [Crossref] [PubMed]
- Godtman RA, Carlsson S, Holmberg E, et al. The Effect of Start and Stop Age at Screening on the Risk of Being Diagnosed with Prostate Cancer. J Urol 2016;195:1390-6. [Crossref] [PubMed]
- Tosoian JJ, Alam R, Gergis C, et al. Unscreened older men diagnosed with prostate cancer are at increased risk of aggressive disease. Prostate Cancer Prostatic Dis 2017;20:193-6. [Crossref] [PubMed]
- Weight CJ, Narayan VM, Smith D, et al. The Effects of Beginning Population Based PSA Screening at Age 40. Urology 2017;110:127-33. [Crossref] [PubMed]
- Shoag J, Mittal S, Halpern JA, et al. Lethal Prostate Cancer in the PLCO Cancer Screening Trial. Eur Urol 2016;70:2-5. [Crossref] [PubMed]
- Hugosson J. Stopping screening, when and how? Transl Androl Urol 2018;7:46-53.
- Vickers AJ, Sjoberg DD, Ulmert D, et al. Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC Med 2014;12:26. [Crossref] [PubMed]
- Kicinski M, Vangronsveld J, Nawrot TS. An epidemiological reappraisal of the familial aggregation of prostate cancer: a meta-analysis. PLoS One 2011;6:e27130. [Crossref] [PubMed]
- Bratt O, Drevin L, Akre O, et al. Family History and Probability of Prostate Cancer, Differentiated by Risk Category: A Nationwide Population-Based Study. J Natl Cancer Inst 2016;108:djw110. [Crossref] [PubMed]
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Bethesda, MD, , based on November 2008 SEER data submission, posted to the SEER web site, 2009.http://seer.cancer.gov/csr/1975_2006/
- Grill S, Fallah M, Leach RJ, et al. Incorporation of detailed family history from the Swedish Family Cancer Database into the PCPT risk calculator. J Urol 2015;193:460-5. [Crossref] [PubMed]
- Chen YC, Page JH, Chen R, et al. Family history of prostate and breast cancer and the risk of prostate cancer in the PSA era. Prostate 2008;68:1582-91. [Crossref] [PubMed]
- Randazzo M, Muller A, Carlsson S, et al. A positive family history as a risk factor for prostate cancer in a population-based study with organised prostate-specific antigen screening: results of the Swiss European Randomised Study of Screening for Prostate Cancer (ERSPC, Aarau). BJU Int 2016;117:576-83. [Crossref] [PubMed]
- Raheem OA, Cohen SA, Parsons JK, et al. A Family History of Lethal Prostate Cancer and Risk of Aggressive Prostate Cancer in Patients Undergoing Radical Prostatectomy. Sci Rep 2015;5:10544. [Crossref] [PubMed]
- Vertosick EA, Poon BY, Vickers AJ. Relative value of race, family history and prostate specific antigen as indications for early initiation of prostate cancer screening. J Urol 2014;192:724-8. [Crossref] [PubMed]
- Shenoy D, Packianathan S, Chen AM, et al. Do African-American men need separate prostate cancer screening guidelines? BMC Urol 2016;16:19. [Crossref] [PubMed]
- Verges DP, Dani H, Sterling WA, et al. The Relationship of Baseline Prostate Specific Antigen and Risk of Future Prostate Cancer and Its Variance by Race. J Natl Med Assoc 2017;109:49-54. [Crossref] [PubMed]
- Albright FS, Stephenson RA, Agarwal N, et al. Relative Risks for Lethal Prostate Cancer Based on Complete Family History of Prostate Cancer Death. Prostate 2017;77:41-8. [Crossref] [PubMed]
- Eeles R, Goh C, Castro E, et al. The genetic epidemiology of prostate cancer and its clinical implications. Nat Rev Urol 2014;11:18-31. [Crossref] [PubMed]
- Kader AK, Sun J, Reck BH, et al. Potential impact of adding genetic markers to clinical parameters in predicting prostate biopsy outcomes in men following an initial negative biopsy: findings from the REDUCE trial. Eur Urol 2012;62:953-61. [Crossref] [PubMed]
- Xu J, Sun J, Kader AK, et al. Estimation of absolute risk for prostate cancer using genetic markers and family history. Prostate 2009;69:1565-72. [Crossref] [PubMed]
- Castro E, Mikropoulos C, Bancroft EK, et al. The PROFILE Feasibility Study: Targeted Screening of Men With a Family History of Prostate Cancer. Oncologist 2016;21:716-22. [Crossref] [PubMed]
- Chen H, Liu X, Brendler CB, et al. Adding genetic risk score to family history identifies twice as many high-risk men for prostate cancer: Results from the prostate cancer prevention trial. Prostate 2016;76:1120-9. [Crossref] [PubMed]
- Turner AR, Lane BR, Rogers D, et al. Randomized trial finds that prostate cancer genetic risk score feedback targets prostate-specific antigen screening among at-risk men. Cancer 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:437-49. [Crossref] [PubMed]
- Moran A, O'Hara C, Khan S, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer 2012;11:235-42. [Crossref] [PubMed]
- Mersch J, Jackson MA, Park M, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015;121:269-75. [Crossref] [PubMed]
- Akbari MR, Wallis CJ, Toi A, et al. The impact of a BRCA2 mutation on mortality from screen-detected prostate cancer. Br J Cancer 2014;111:1238-40. [Crossref] [PubMed]
- Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 2013;31:1748-57. [Crossref] [PubMed]
- Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol 2015;68:186-93. [Crossref] [PubMed]
- Daly MB, Pilarski R, Berry M, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J Natl Compr Canc Netw 2017;15:9-20. [Crossref] [PubMed]
- Bancroft EK, Page EC, Castro E, et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol 2014;66:489-99. [Crossref] [PubMed]
- Castro E, Goh CL, Eeles RA. Prostate cancer screening in BRCA and Lynch syndrome mutation carriers. Am Soc Clin Oncol Educ Book 2013. [Crossref] [PubMed]
- Zhang J, Xiao L, Qin Z, et al. Association between germline homeobox B13 (HOXB13) G84E allele and prostate cancer susceptibility: a meta-analysis and trial sequential analysis. Oncotarget 2016;7:67101-10. [PubMed]
- Karlsson R, Aly M, Clements M, et al. A population-based assessment of germline HOXB13 G84E mutation and prostate cancer risk. Eur Urol 2014;65:169-76. [Crossref] [PubMed]
- Lynch HT, Kosoko-Lasaki O, Leslie SW, et al. Screening for familial and hereditary prostate cancer. Int J Cancer 2016;138:2579-91. [Crossref] [PubMed]
- Gulati R, Cheng HH, Lange PH, et al. Screening Men at Increased Risk for Prostate Cancer Diagnosis: Model Estimates of Benefits and Harms. Cancer Epidemiol Biomarkers Prev 2017;26:222-7. [Crossref] [PubMed]
- Muhlberger N, Boskovic K, Krahn MD, et al. Benefits and harms of prostate cancer screening - predictions of the ONCOTYROL prostate cancer outcome and policy model. BMC Public Health 2017;17:596. [Crossref] [PubMed]
- Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 1987;317:909-16. [Crossref] [PubMed]
- Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991;324:1156-61. [Crossref] [PubMed]
- Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40-55 and long term risk of metastasis: case-control study. BMJ 2013;346:f2023. [Crossref] [PubMed]
- Lilja H, Cronin AM, Dahlin A, et al. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer 2011;117:1210-9. [Crossref] [PubMed]
- Preston MA, Batista JL, Wilson KM, et al. Baseline Prostate-Specific Antigen Levels in Midlife Predict Lethal Prostate Cancer. J Clin Oncol 2016;34:2705-11. [Crossref] [PubMed]
- Arsov C, Becker N, Hadaschik BA, et al. Prospective randomized evaluation of risk-adapted prostate-specific antigen screening in young men: the PROBASE trial. Eur Urol 2013;64:873-5. [Crossref] [PubMed]
- Brawley OW, Thompson IM Jr, Gronberg H. Evolving Recommendations on Prostate Cancer Screening. Am Soc Clin Oncol Educ Book 2016;35:e80-7. [Crossref] [PubMed]
- Crawford ED, Rosenberg MT, Partin AW, et al. An Approach Using PSA Levels of 1.5 ng/mL as the Cutoff for Prostate Cancer Screening in Primary Care. Urology 2016;96:116-20. [Crossref] [PubMed]
- Ankerst DP, Gelfond J, Goros M, et al. Serial Percent Free Prostate Specific Antigen in Combination with Prostate Specific Antigen for Population Based Early Detection of Prostate Cancer. J Urol 2016;196:355-60. [Crossref] [PubMed]
- Lavallee LT, Binette A, Witiuk K, et al. Reducing the Harm of Prostate Cancer Screening: Repeated Prostate-Specific Antigen Testing. Mayo Clin Proc 2016;91:17-22. [Crossref] [PubMed]
- Eastham JA, Riedel E, Scardino PT, et al. Variation of serum prostate-specific antigen levels: an evaluation of year-to-year fluctuations. JAMA 2003;289:2695-700. [Crossref] [PubMed]
- Ueno Y, Tamada T, Bist V, et al. Multiparametric magnetic resonance imaging: Current role in prostate cancer management. Int J Urol 2016;23:550-7. [Crossref] [PubMed]
- Futterer JJ, Briganti A, De Visschere P, et al. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur Urol 2015;68:1045-53. [Crossref] [PubMed]
- Moldovan PC, Van den Broeck T, Sylvester R, et al. What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol 2017;72:250-66. [Crossref] [PubMed]
- Valerio M, Donaldson I, Emberton M, et al. Detection of Clinically Significant Prostate Cancer Using Magnetic Resonance Imaging-Ultrasound Fusion Targeted Biopsy: A Systematic Review. Eur Urol 2015;68:8-19. [Crossref] [PubMed]
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313:390-7. [Crossref] [PubMed]
- Wegelin O, van Melick HHE, Hooft L, et al. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? Eur Urol 2017;71:517-31. [Crossref] [PubMed]
- van Hove A, Savoie PH, Maurin C, et al. Comparison of image-guided targeted biopsies versus systematic randomized biopsies in the detection of prostate cancer: a systematic literature review of well-designed studies. World J Urol 2014;32:847-58. [Crossref] [PubMed]
- Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68:438-50. [Crossref] [PubMed]
- Wallis CJD, Haider MA, Nam RK. Role of mpMRI of the prostate in screening for prostate cancer. Transl Androl Urol 2017;6:464-71. [Crossref] [PubMed]
- Baco E, Rud E, Eri LM, et al. A Randomized Controlled Trial To Assess and Compare the Outcomes of Two-core Prostate Biopsy Guided by Fused Magnetic Resonance and Transrectal Ultrasound Images and Traditional 12-core Systematic Biopsy. Eur Urol 2016;69:149-56. [Crossref] [PubMed]
- Tonttila PP, Lantto J, Paakko E, et al. Prebiopsy Multiparametric Magnetic Resonance Imaging for Prostate Cancer Diagnosis in Biopsy-naive Men with Suspected Prostate Cancer Based on Elevated Prostate-specific Antigen Values: Results from a Randomized Prospective Blinded Controlled Trial. Eur Urol 2016;69:419-25. [Crossref] [PubMed]
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40. [Crossref] [PubMed]
- Mertan FV, Greer MD, Shih JH, et al. Prospective Evaluation of the Prostate Imaging Reporting and Data System Version 2 for Prostate Cancer Detection. J Urol 2016;196:690-6. [Crossref] [PubMed]
- Seo JW, Shin SJ, Taik Oh Y, et al. PI-RADS Version 2: Detection of Clinically Significant Cancer in Patients With Biopsy Gleason Score 6 Prostate Cancer. AJR Am J Roentgenol 2017;209:W1-W9. [Crossref] [PubMed]
- Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. Interobserver Reproducibility of the PI-RADS Version 2 Lexicon: A Multicenter Study of Six Experienced Prostate Radiologists. Radiology 2016;280:793-804. [Crossref] [PubMed]
- Kasel-Seibert M, Lehmann T, Aschenbach R, et al. Assessment of PI-RADS v2 for the Detection of Prostate Cancer. Eur J Radiol 2016;85:726-31. [Crossref] [PubMed]
- Muller BG, Shih JH, Sankineni S, et al. Prostate Cancer: Interobserver Agreement and Accuracy with the Revised Prostate Imaging Reporting and Data System at Multiparametric MR Imaging. Radiology 2015;277:741-50. [Crossref] [PubMed]
- Fulgham PF, Rukstalis DB, Turkbey IB, et al. AUA Policy Statement on the Use of Multiparametric Magnetic Resonance Imaging in the Diagnosis, Staging and Management of Prostate Cancer. J Urol 2017;198:832-8. [Crossref] [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Grenabo Bergdahl A, Wilderang U, Aus G, et al. Role of Magnetic Resonance Imaging in Prostate Cancer Screening: A Pilot Study Within the Goteborg Randomised Screening Trial. Eur Urol 2016;70:566-73. [Crossref] [PubMed]
- Nam RK, Wallis CJ, Stojcic-Bendavid J, et al. A Pilot Study to Evaluate the Role of Magnetic Resonance Imaging for Prostate Cancer Screening in the General Population. J Urol 2016;196:361-6. [Crossref] [PubMed]
- Kasivisvanathan V, Jichi F, Klotz L, et al. A multicentre randomised controlled trial assessing whether MRI-targeted biopsy is non-inferior to standard transrectal ultrasound guided biopsy for the diagnosis of clinically significant prostate cancer in men without prior biopsy: a study protocol. BMJ Open 2017;7:e017863. [Crossref] [PubMed]
- Wei JT, Feng Z, Partin AW, et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer? J Clin Oncol 2014;32:4066-72. [Crossref] [PubMed]
- Reiter RE. Risk stratification of prostate cancer 2016. Scand J Clin Lab Invest Suppl 2016;245:S54-9. [Crossref] [PubMed]
- Patel HD, Chalfin HJ, Carter HB. Improving Prostate Cancer Screening and Diagnosis: Health Policy and Biomarkers Beyond PSA. JAMA Oncol 2016;2:867-8. [Crossref] [PubMed]
- Loeb S, Lilja H, Vickers A. Beyond prostate-specific antigen: utilizing novel strategies to screen men for prostate cancer. Curr Opin Urol 2016;26:459-65. [Crossref] [PubMed]
- Catalona WJ, Partin AW, Sanda MG, et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol 2011;185:1650-5. [Crossref] [PubMed]
- Parekh DJ, Punnen S, Sjoberg DD, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol 2015;68:464-70. [Crossref] [PubMed]
- Konety B, Zappala SM, Parekh DJ, et al. The 4Kscore(R) Test Reduces Prostate Biopsy Rates in Community and Academic Urology Practices. Rev Urol 2015;17:231-40. [PubMed]
- Benson MC, Whang IS, Pantuck A, et al. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol 1992;147:815-6. [Crossref] [PubMed]
- Lujan M, Paez A, Llanes L, et al. Prostate specific antigen density. Is there a role for this parameter when screening for prostate cancer? Prostate Cancer Prostatic Dis 2001;4:146-9. [Crossref] [PubMed]
- Vickers AJ, Thompson IM, Klein E, et al. A commentary on PSA velocity and doubling time for clinical decisions in prostate cancer. Urology 2014;83:592-6. [Crossref] [PubMed]
- Gronberg H, Adolfsson J, Aly M, et al. Prostate cancer screening in men aged 50-69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol 2015;16:1667-76. [Crossref] [PubMed]
- Carlsson SV, Kattan MW. The STHLM3 prostate cancer diagnostic study: calibration, clarification, and comments. Nat Rev Clin Oncol 2016.13. [PubMed]
- Carlsson SV, Kattan MW. Prostate cancer: Personalized risk - stratified screening or abandoning it altogether? Nat Rev Clin Oncol 2016;13:140-2. [Crossref] [PubMed]
- McKiernan J, Donovan MJ, O'Neill V, et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol 2016;2:882-9. [Crossref] [PubMed]
- Radtke JP, Wiesenfarth M, Kesch C, et al. Combined Clinical Parameters and Multiparametric Magnetic Resonance Imaging for Advanced Risk Modeling of Prostate Cancer-Patient-tailored Risk Stratification Can Reduce Unnecessary Biopsies. Eur Urol 2017;72:888-96. [Crossref] [PubMed]
- Vickers AJ, Cronin AM, Bjork T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ 2010;341:c4521. [Crossref] [PubMed]
- Stattin P, Vickers AJ, Sjoberg DD, et al. Improving the Specificity of Screening for Lethal Prostate Cancer Using Prostate-specific Antigen and a Panel of Kallikrein Markers: A Nested Case-Control Study. Eur Urol 2015;68:207-13. [Crossref] [PubMed]


