Predicting success after artificial urinary sphincter: which preoperative factors drive patient satisfaction postoperatively?
Introduction
The artificial urinary sphincter (AUS), considered the gold standard for treatment of post-prostatectomy incontinence, remains the ultimate treatment option after more conservative management has failed (1). Previous studies have demonstrated that AUS implantation has a positive impact on quality of life (QoL) (2-5). Most patients are satisfied with their increased continence postoperatively and use less absorbent pads per day while also feeling comfortable voiding in public restrooms (3-4,6).
Considering the 73–90% patient-reported success rates in prior studies, it appears that most, but not all, patients are satisfied with their levels of continence after AUS implantation (2-3,5). Therefore, determining which preoperative factors are the strongest determinants of postoperative satisfaction may help urologist better counsel patients regarding postoperative expectations.
This is a prospective, observational study to test the hypothesis that certain patient characteristics, such as more severe preoperative urinary incontinence, are associated with better patient-reported outcomes of AUS surgery. The aims of this study were to record and characterize preoperative factors known to impact objective AUS outcomes and use these to determine which factors are associated with patient-reported QoL measures. To the authors’ knowledge, this study is the first to examine what preoperative factors drive postoperative patient satisfaction.
Methods
Local institutional review board approval was obtained. Using a prospectively populated AUS database at a large academic referral center, men undergoing AUS implantation for post-prostatectomy incontinence between August 2012 and April 2016 were identified. Patient age, weight, and urologic history were collected. All patients presenting to this 3-surgeon urologic sub-specialty clinic completed an initial urologic visit questionnaire, which included questions on pad usage, urinary habits, and past urologic history pertaining to incontinence treatments. Prior surgical therapies were defined as bulking injections, sling placement, or prior AUS implantation.
Maximum pads per day (MxPPD) were used, as opposed to minimum, average, or range, to provide a more accurate measure of incontinence severity. Urodynamic testing (UDS) was done at surgeon discretion and was not routinely used. For those who did undergo UDS, strong desire, maximum capacity, Valsalva leak point pressure (VLPP), pressure at peak flow (pDetQmax) and average flow (Qavg) were recorded.
Patient-reported QoL and satisfaction were assessed postoperatively via telephone survey at least six months after AUS implantation. Patient contact was attempted with up to three calls on separate days. Patients were excluded from the study if they did not or were unable to answer the postoperative survey, the AUS device was non-functional, or the AUS had been explanted. Questionnaires used included the Expanded Prostate Cancer Index Composite Urinary Domain (EPIC-UD), validated in the post-prostatectomy population, and Urogenital Distress Inventory (UDI-6), validated in adults with incontinence (7,8). The EPIC-UD consists of 12 items separated into two sections: one on urinary function and the second on how problematic urinary function has been for the patient (9). The instrument generates a score from zero to one hundred, with higher values representing better QoL (10). The UDI-6 survey contains six questions pertaining to the patient’s “bother” with his urinary symptoms (11). The instrument generates a score from zero to one hundred, with higher values representing more urinary bother (11). Additional study-specific questions on postoperative daily pad usage were also administered. Percent change in pad usage was defined as the difference between maximum preoperative and postoperative pad usage, divided by maximum preoperative pad usage.
Statistical analyses were performed using JMP Pro Version 13.0 (SAS Institute Inc.; Cary, NC, USA). Descriptive statistics are reported as median [interquartile range (IQR)] based upon non-parametric data distribution. Postoperative pad usage and survey response scores were analyzed by preoperative patient characteristics and urodynamic measurements. Continuous variables were compared using the Wilcoxon-Rank sum test; binary variables were assessed using Chi-squared analysis. For all analyses, P≤0.05 was considered statistically significant.
Results
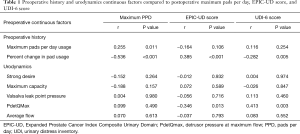
Two hundred thirty eight patients underwent AUS implantation between August 2012 and April 2016. One hundred and one out of 238 (42%) patients completed the telephone survey and were included in analyses. Of these, 58 (57%) underwent preoperative urodynamics. Median patient age at surgery was 69 [63–75] years and BMI was 29 [26–32] kg/m2. MxPPD was 5 [3–9] preoperatively and 2 [1–3] postoperatively (r=0.255, P=0.011) (Table 1). Median postoperative EPIC-UD score was 82 [67–89] and UDI-6 score was 22 [11–36].
Full table
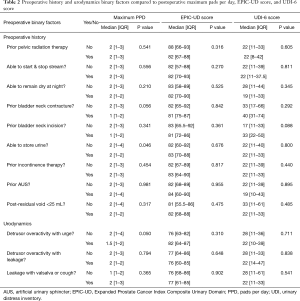
MxPPD after AUS was significantly lower in patients with the preoperative ability to store urine {2 [1–2] with vs. 2 [1–4] without, respectively, P=0.046} (Table 2). Percent change in pad usage was negatively associated with postoperative MxPPD (r=−0.536, P<0.001) and positively associated with increased postoperative QoL according to both EPIC (r=0.385, P<0.001) and UDI-6 (r=−0.282, P=0.005) results (Table 1). There was no difference in pad usage or survey score with regards to the ability to start and stop the urinary stream, dryness at night, previous surgical incontinence therapy, or a post void residual of greater or less than 25 mL (Table 2).
Full table
Postoperative MxPPD was lower in men with urodynamically-proven detrusor overactivity {1.5 [1–2] with vs. 2 [1–4] without, respectively, P=0.050}, although not associated with postoperative EPIC-UD or UDI-6 scores (Table 2). Detrusor pressure at maximum flow (PDetQMax) was negatively associated with EPIC-UD score (r=−0.346, P=0.013) and positively associated with UDI-6 score (r=0.413, P=0.003), meaning that higher pressure at peak flow was associated with worse QoL on both measures (Table 1). There was no difference in pad usage or QoL scores with regard to capacity at strong desire or maximum bladder capacity, VLPP, average urine flow, urgency incontinence, or stress incontinence (Tables 1,2).
Forty-one (41%) patients had a history of prior pelvic radiation exposure. There was no significant difference between patients who had or had not received prior pelvic radiation with regards to postoperative PPD {2 [1–3] with vs. 2 [1–3] without, P=0.541}, EPIC-UD score {82 [67–88] with vs. 83 [66–93] without, P=0.316}, or UDI-6 score {22 [8–42] with vs. 22 [11–33] without, P=0.605} (Table 2).
Ten (10%) patients had previously undergone AUS implantation prior to the surgical episode analyzed in this study. There was no significant difference between patients who had or had not undergone prior AUS implantation surgery with regard to postoperative PPD {2 [1–3] with vs. 2 [1–4] without, P=0.981}, EPIC-UD score {82 [68–89] with vs. 84 [60–90] without, P=0.955}, or UDI-6 score {22 [11–39] with vs. 19 [10–43] without, P=0.895} (Table 2).
Discussion
Implantation of the AUS has been shown to improve patient quality of life in the setting of post-prostatectomy incontinence (2-5). As such, determining which preoperative factors predict satisfaction with AUS implantation can help optimally counsel surgical candidates pursuing this procedure. This will enable preoperative expectations to be more accurately established, which in turn has been shown to be associated with better patient satisfaction and QoL (12).
Patients with greater MxPPD preoperatively were found to have greater MxPPD postoperatively. This follows logically, as MxPPD is indicative of incontinence severity. Further support of this observation was found in the subset of patients able to store urine preoperatively, who subsequently had a lower median postoperative MxPPD compared to those who could not store urine prior to AUS. However, this difference was statistically but not clinically significant. Prior studies have demonstrated a decrease in median pad usage after AUS implantation, but to the author’s knowledge this is the first study to demonstrate a correlation between the quantity of preoperative and postoperative pad usage (4-6). Percent change in pad usage from before to after surgery was the only pad-related measure predictive of quality of life survey scores. However, this measure cannot be calculated preoperatively.
Patients with detrusor overactivity associated with urinary urgency on preoperative UDS used fewer pads postoperatively compared to patients without urodynamic-proven urinary urgency, though the difference was statistically, not clinically, significant. We speculate that detrusor overactivity associated with urinary urgency prompts patients to void more frequently, therefore causing them to store lower volumes than men without detrusor overactivity, resulting in fewer opportunities to “leak through” with stress. An alternative theory is that AUS implantation may convert men with “wet” OAB to “dry” OAB by preventing leakage with low amplitude bladder contractions, resulting in fewer incontinence episodes due to detrusor overactivity alone.
Our findings may result from a reduction in detrusor hyperactivity, much in the same way mid-urethral slings have been noted to eliminate overactive bladder in up to half of women receiving sling implantation (13,14). It has been theorized that mid-urethral sling placement in females prevents urine from entering the urethra and providing the stimulus that triggers an urge to void (14). Similarly, this can be hypothesized for AUS placement, with the additional possibility that sensory denervation of the urethra resulting from its circumferential dissection may further reduce urethral afferent signaling that triggers urinary urgency. While a 2011 study by Lai, et al. stated that there is no contraindication to AUS implantation in men with mixed incontinence, prior investigations have not demonstrated a correlation between detrusor overactivity and decreased postoperative pad usage as demonstrated in our study (4,15-17).
Higher PDetQMax was associated with worse QoL as measured by EPIC-UD score and UDI-6 score. We use preoperative urodynamics primarily to evaluate bladder storage parameters in AUS candidates, but noted this association in our analysis. An explanation for this finding is not readily apparent; although it is intriguing that it was consistently seen across two measures of postoperative satisfaction. Higher preoperative PDetQMax may be a marker of overall bladder quality or contractility, or alternatively, a marker of outlet obstruction (as with some element of bladder neck contracture). The clinical significance of this finding and its impact on patient selection is unclear. Further investigation into this association and whether it persists postoperatively may shed some light onto this finding.
In the current study, patients with prior pelvic radiation reported no difference in QoL or MxPPD postoperatively compared to those without prior radiation exposure. This supports previous studies noting that radiation plays no significant role in AUS implantation outcomes as measured by pad usage, patient-reported satisfaction, and need for surgical revision (18-20). Some studies have also demonstrated low rates of cuff erosion and infection in both irradiated and non-irradiated patients (18-20). Based on these publications and the findings of this study, prior radiation exposure may not play as major a role in patient post-operative QoL as one might expect and should not be the sole factor discouraging implantation of an AUS device.
This study was limited in that time from surgery to survey was not standardized and was variable (range, 6–42 months), which may serve as a confounder in light of previous studies showing patient QoL with AUS results decreases over time (5). Another limitation is that only 42% of patients receiving AUS implantation participated in the postoperative survey, and the loss of the remaining data may have altered our findings. Additionally, urodynamic measures can demonstrate significant variability due to patient and operator characteristics, and was not obtained for all patients. Strengths of this study include our large sample size of 101 patients, our inclusion of both patient history and urodynamic characteristics, and the implementation of widely validated surveys.
Preoperative pad usage is predictive of postoperative pad usage, though not predictive of patient-reported QoL. However, patients who demonstrated detrusor overactivity associated with urge on preoperative urodynamics utilized fewer pads postoperatively. AUS implantation, while targeting stress incontinence, may benefit overactive bladder as well. Overall, a history of radiation therapy did not seem to worsen QoL. It appears that few preoperative factors are related to post-AUS implantation QoL as measured by pad use and on patient-reported QoL instruments.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Local institutional review board approval was obtained (IRB 15-301).
References
- Montague DK. Artificial urinary sphincter: long-term results and patient satisfaction. Adv Urol 2012;2012:835290. [PubMed]
- Gousse AE, Madjar S, Lambert MM, et al. Artificial urinary sphincter for post-radical prostatectomy urinary incontinence: long-term subjective results. J Urol 2001;166:1755-8. [Crossref] [PubMed]
- Litwiller SE, Kim KB, Fone PD, et al. Post-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. J Urol 1996;156:1975-80. [Crossref] [PubMed]
- Trigo Rocha F, Gomes CM, Mitre AI, et al. A prospective study evaluating the efficacy of the artificial sphincter AMS 800 for the treatment of postradical prostatectomy urinary incontinence and the correlation between preoperative urodynamic and surgical outcomes. Urology 2008;71:85-9. [Crossref] [PubMed]
- Viers BR, Linder BJ, Rivera ME, et al. Long-Term Quality of Life and Functional Outcomes among Primary and Secondary Artificial Urinary Sphincter Implantations in Men with Stress Urinary Incontinence. J Urol 2016;196:838-43. [Crossref] [PubMed]
- Bukavina L, Chaparala H, Kartha G, et al. Public Restroom Habits in Patients After Artificial Urinary Sphincter Implantation. Urology 2015;86:171-5. [Crossref] [PubMed]
- Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56:899-905. [Crossref] [PubMed]
- Utomo E, Korfage IJ, Wildhagen MF, et al. Validation of the Urogenital Distress Inventory (UDI-6) and Incontinence Impact Questionnaire (IIQ-7) in a Dutch population. Neurourol Urodyn 2015;34:24-31. [Crossref] [PubMed]
- Available online: https://medicine.umich.edu/sites/default/files/content/downloads/EPIC-Urinary-2.2002.pdf
- Available online: https://medicine.umich.edu/sites/default/files/content/downloads/Scoring%20Instructions-EPIC.pdf
- Available online: http://www.bestresultspt.com/userfiles/files/Urogenital%20Distress%20Inventory%20UDI%206.pdf
- Firoozi F, Gill B, Ingber MS, et al. Increasing patient preparedness for sacral neuromodulation improves patient reported outcomes despite leaving objective measures of success unchanged. J Urol 2013;190:594-7. [Crossref] [PubMed]
- Choe JH, Choo MS, Lee KS. The impact of tension-free vaginal tape on overactive bladder symptoms in women with stress urinary incontinence: significance of detrusor overactivity. J Urol 2008;179:214-9. [Crossref] [PubMed]
- Segal JL, Vassallo B, Kleeman S, et al. Prevalence of persistent and de novo overactive bladder symptoms after the tension-free vaginal tape. Obstet Gynecol 2004;104:1263-9. [Crossref] [PubMed]
- Thiel DD, Young PR, Broderick GA, et al. Do clinical or urodynamic parameters predict artificial urinary sphincter outcome in post-radical prostatectomy incontinence? Urology 2007;69:315-9. [Crossref] [PubMed]
- Lai HH, Hsu EI, Boone TB. Urodynamic testing in evaluation of postradical prostatectomy incontinence before artificial urinary sphincter implantation. Urology 2009;73:1264-9. [Crossref] [PubMed]
- Lai HH, Boone TB. Implantation of artificial urinary sphincter in patients with post-prostatectomy incontinence, and preoperative overactive bladder and mixed symptoms. J Urol 2011;185:2254-9. [Crossref] [PubMed]
- Gomha MA, Boone TB. Artificial urinary sphincter for post-prostatectomy incontinence in men who had prior radiotherapy: a risk and outcome analysis. J Urol 2002;167:591-6. [Crossref] [PubMed]
- Martins FE, Boyd SD. Artificial urinary sphincter in patients following major pelvic surgery and/or radiotherapy: are they less favorable candidates? J Urol 1995;153:1188-93. [Crossref] [PubMed]
- Sathianathen NJ, McGuigan SM, Moon DA. Outcomes of artificial urinary sphincter implantation in the irradiated patient. BJU Int 2014;113:636-41. [Crossref] [PubMed]


