Different stages in drug development for muscle-invasive bladder cancer
Introduction
Despite intensive research, investigation of novel agents in clinical trials and optimization of surgical strategies, the prognosis of patients with muscle-invasive bladder cancer (MIBC) have remained virtually the same during the last three decades. This is in stark contrast to other cancers, where novel agents could be discovered and implemented into daily clinical practice to improve patient outcomes. Therefore, in MIBC there is a high unmet need to identify novel treatments, strategies for co-targeting or improvement of patient selection for systemic treatments.
One of the most important recent discoveries for the treatment of MIBC is the introduction of checkpoint inhibition. Being aware of this major breakthrough, we aimed to discuss different stages of drug discovery in MIBC, beside checkpoint inhibition, on the examples of three selected targets.
Insufficient patient selection as a reason for unsuccessful clinical trials?
Several novel targeted therapies have been investigated in patients with MIBC without promising anti-tumor activities in clinical trials. On the example of Her2 we aim to elucidate reasons for these frustrating results.
Description of target
Human EGFR2 (Her-2/neu, c-erbB2 or ERBB2) is a transmembrane receptor important in angiogenesis, cell growth, and survival signaling in several malign entities, e.g., gastric, breast, bladder cancer (1). Her2 expression as well as prognostic implications may vary widely, importantly; MIBC has the third highest rate of Her2 expression and amplification after cancers of the breast and stomach (1-3). Not surprisingly, investigators caught this target for therapeutic access also in MIBC. However, Her2 up to date has not proven to be an efficient target for treatment of MIBC (4-7). Even though reasons for the unsuccessful treatment of MIBC have not been unveiled and remain to be elucidated. Recent studies have been able to demonstrate that patient selection for Her2-based therapies in urothelial carcinoma may be insufficient if done by fluorescence in situ hybridization (FISH) and/or immuno-histochemistry (IHC) alone: the presence neither of ERBB2 amplification nor of Her2 overexpression in urothelial carcinoma seems not to be a mere warrantor of treatment success.
State of current misapprehension
In advanced bladder cancer, there is a tendency (if not conviction) to newly subclassify urothelial carcinoma according to molecular subtypes. Several groups discovered molecular subtypes of MIBC that divide MIBC on a higher level into basal- and luminal-like subtypes (1,8-10). Importantly, all levels of Her2 are enriched in luminal-like tumors, such as amplification rate, mRNA and protein overexpression (11,12).
A most recent debate in the EAU’s journal European Urology concerning the “phase III, double-blind, randomized trial that compared maintenance Lapatinib versus placebo after first-line chemotherapy in patients with human Epidermal Growth Factor Receptor 1/2-positive metastatic bladder cancer” (7) gives an insight into the state of current studies in bladder cancer. The study included patients with metastatic urothelial carcinoma whose tumors overexpressed Her1 and Her2 in FISH and IHC and who did not have progressive disease during chemotherapy (four to eight cycles). They were randomly assigned to lapatinib or placebo after completion of first-line/initial chemotherapy for metastatic disease. The study did not find significant improvements in outcome by the addition of maintenance lapatinib to standard of care.
In a letter to the editor, Eriksson et al. (13) point out, that according to recent findings (11,12,14,15), in advanced bladder cancer Her2-positive tumors come in different flavors, suggesting that knowing Her2 status alone is not enough. Contrary, a more comprehensive molecular profiling may be required to obtain the complete picture. In particular, our group has contributed new understanding of Her2 status at the DNA, RNA, and protein levels (11). We showed discrepancies when investigating Her2 on DNA, RNA and protein level. Hence, patient selection for the above mentioned study has most likely confounded its outcome.
Future prospects
Responding to the letter to editor, the authors of the initially mentioned study agree that knowing Her2 status alone is not enough (16). They put in discussion that although the identification of novel treatments with anti-tumor activity is the ultimate goal, exploring its efficacy without taking the biological knowledge into account may be insufficient. They suggest approaching future treatments through a combination of existing and novel diagnostic tools. Molecular profiling of MIBC and identification of a new generation of biomarkers may likely be the best way to achieve this goal. While this, however, seems two steps ahead of current abilities and possibilities, nowadays knowledge calls for consistently designed clinical studies with a closer look not only at amplification rate and expression, but mainly at DNA alterations, gene and protein expression. The suggested blueprint by Kiss et al. may be a step towards the right direction (11), although its clinical significance needs to be proven in clinical trials.
Promising in clinical trials
Fibroblast growth factor receptor (FGFR) signaling in bladder cancer has been investigated for two decades (17-19). However, more than 10 years later, antibodies and strategies to target the FGFR pathway were subsequently developed (20,21). Almost 10 years later, clinical trials on targeting this pathway in solid tumors are initiated and performed. In this section, we aim to discuss the current status of FGFR targeting in bladder cancer.
Description of target
Fibroblast growth factors is a family of 18 growth and 4 homologous factors, that play a role in several processes including angiogenesis, proliferation and wound-healing (22,23). Four transmembrane glycoprotein receptors are known (FGFR1-4). They mediate the signal from these growth factors that mediate an activation of several pathways e.g., Erk, Mek, MAPK, NF-kB, Ras, Raf, Stat3, PI3K (23). Urothelial carcinoma shows a high frequency of genetic alterations particularly in FGFR3. Activating mutations, amplification and fusions of FGFR3 often result in an FGFR3 overexpression. However, about 50% of bladder cancers show an FGFR3 overexpression without genomic DNA alterations (1). It is well accepted, that FGFR signaling may have a pivotal role in urothelial carcinogens and therefore, seems to be a promising point of action for targeted therapies.
State of current research
There are different ways of targeting FGFR mediated pathways such as selective and nonselective FGFR tyrosine kinase inhibitors, monoclonal antibodies and antibody-drug-conjugates. An overview of the current ongoing or planed trials, investigating anti-tumor activity of FGFR targeting in bladder cancer is given in Table 1. FGFR targeting is performed using different strategies. B-701 is a monoclonal antibody directed against FGFR3 and has potential antineoplastic activity. In these trials (Table 1, No. 1 and 2), B-701 is either used in combination with pembrolizumab (Table 1, No. 1) a monoclonal antibody against programmed cell death protein 1 (PD-1) or with docetaxel (Table 1, No. 2). LY3076226 is a FGFR3 antibody conjugated with microtubule inhibitor, DM4 (Table 1, No. 3). In this trial, the tumors are screened for FGFR alterations. For the three selective FGFR tyrosine kinase inhibitors, BAY1163877, JNJ-42756493 and BGJ398, patients are preselected for FGFR alterations. For the first two (Table 1, No. 4 and 5), patients are selected according to high FGFR expression levels and FGFR mutations in archival tumors. A phase 2/3 trial investigating BAY1163877 (Rogaratinib) in progressive cisplatin-resistant urothelial carcinoma is about to start recruitment. For the third (Table 1, No. 6), patients with tumors that harbor amplification in FGFR1 or FGFR2 or mutations in FGFR3 are included. FPA144 (Table 1, No. 7) is an anti-FGF receptor 2b (FGFR2b) humanized monoclonal antibody that has two different mechanisms. First, it binds specifically to FGFR2b and prevents the binding of certain fibroblast growth factors that promote tumor growth. Second, it is engineered to enhance the recruitment of natural killer cells and drive immune-based killing of tumor cells by antibody-dependent cell-mediated cytotoxicity. Finally, AZD4547a selective FGFR1-3 tyrosine kinase inhibitor is used as one of 18 different treatments (Table 1, No. 8). This trial is designed for different advanced solid tumors, such as bladder cancer, lymphoma, or myeloma. Tumors are screened for the presence of different genomic alterations and subsequently assigned to the most promising target. In terms of FGFR, tumors with FGFR1-3 mutations or translocation receive FGFR Inhibitor AZD4547. Taken together, different strategies are used to target FGFR signaling in bladder cancer.
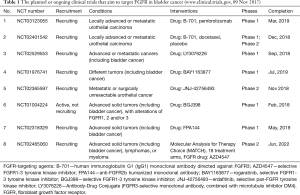
Full table
FGFR-targeting agents: B-701—human immunoglobulin G1 (IgG1) monoclonal antibody directed against FGFR3; AZD4547—selective FGFR1-3 tyrosine kinase inhibitor; FPA144—anti-FGFR2b humanized monoclonal antibody; BAY1163877—rogaratinib, selective FGFR1-3 tyrosine kinase inhibitor; BGJ398—selective FGFR1-3 tyrosine kinase inhibitor; JNJ-42756493—erdafitinib, selective pan-FGFR tyrosine kinase inhibitor; LY3076226—Antibody-Drug Conjugate (FGFR3-selective monoclonal antibody, combined with microtubule inhibitor DM4). FGFR, fibroblast growth factor receptor.
Future prospects
As shown in Table 1, several promising clinical trials investigating FGFR targeting in bladder cancer will be completed in the nearest future. Trials that use other combinations or mechanisms of action will definitely be initiated. Therefore, after more than 20 years of research in FGFR signaling in bladder cancer, targeting this pathway finally is investigated in clinical trials.
From petri dish to clinical trials?
Intensive research is ongoing to discover novel therapies for patients with MIBC. In this section, we aim to introduce a recently discovered treatment paradigm with promising results in vitro and in vivo. The human placenta and many cancers share several features such as high proliferation rate, invasion into adjacent tissue, immune escape as well as the expression of specifically modified chondroitin sulfate (CS) chains (24). Here we discuss a strategy targeting these CS chains in cancer.
Description of target
CS are carbohydrate modifications attached to cellular and extra-cellular proteins and play a key role in malaria pathogenesis (25). The malaria parasite Plasmodium falciparum has evolved a protein, called VAR2CSA that mediates attachment of infected erythrocytes to a distinct type of CS chains in the placental syncytium giving rise to pregnancy-associated malaria in endemic regions of the world (26,27). This evolution happened over millions of years; therefore, VAR2CSA is not recognized by the immune system of humans. For unknown reasons, many tumors re-express placental-type CS as a secondary oncofetal CS (ofCS) modification. Recently, a recombinant malarial VAR2CSA (rVAR2) protein was discovered that could be conveniently utilized to detect ofCS in human cancer (28,29). rVAR2 has a broad potential for its use in cancer diagnostics and treatment. For example, V5-tagged rVAR2 can be used for detection of ofCS by immunohistochemistry or flow cytometry. On the other hand, it can be used for drug delivery and targeted cancer therapy. Interestingly, rVAR2 in conjugation with different toxins showed anti-tumor activity in several cancers (28).
rVAR2 in MIBC
The expression of ofCS in MIBC is independent from the genomic landscape and molecular subtypes (30). We showed that ofCS are overexpressed in 25% of MIBC across all molecular subtypes. Importantly, the expression of ofCS is even higher in cisplatin-resistant MIBC, suggesting its use as a target for second-line treatment option for MIBC not responding to cisplatin. In vitro several bladder cancer cell lines showed high ofCS expression compared to cell lines of other cancers (28). This expression data indicates that rVAR2 might be a promising target for MIBC. rVAR2 in conjugation with the hemiasterlin toxin analog KT886 (VDC886), a tubulus toxin, eliminated all MIBC cell lines in the low-nanomolar IC50 concentration range (30). These IC50 were among the lowest when compared to cell lines from other cancer types. In vivo, VDC886 strongly retarded tumor growth of orthotopic cisplatin-resistant bladder cancer xenografts and showed dramatic anti-tumor activity. Importantly, no side effects of VDC886 were observed during treatment. Taken together, rVAR2 may offer therapeutic access and new treatment paradigms for MIBC not responding to cisplatin.
Future prospects
Several issues on rVAR2 need further investigation. First, it should be evaluated, whether treatment benefit is dependent on the expression of ofCS, to determine the predictive role of ofCS expression. Second, VDC886 has only been investigated in cisplatin-resistant MIBC in vivo. However, the overexpression of ofCS in 25% of treatment naïve MIBC suggests its use also at this stage of the disease. This may be either in a comparative manner or in combination with cisplatin-based regimens. Finally, manufacturing of VDC886 for its use in patients should be pursued and this treatment paradigm will need to be tested in clinical trials.
Discussion
MIBC is a highly aggressive disease to which half of the patient will succumb despite optimal staging and treatment (31). This prognosis has remained virtually the same during the last three decades (32). MIBC lags behind in discovery and establishment of novel treatment, what is in contrast to many other cancers. The emerging evidence for treatment success with checkpoint inhibition (33-35) is a major breakthrough for MIBC therapy. However, without ignoring this recent evidence, we aimed to elucidate different stages of drug development for MIBC other than checkpoint inhibition.
MIBC is a highly heterogeneous disease which is reflected not only by the large variety in patient outcomes but also in the tremendous biological and genomic variations (1). Therefore, it is unlikely that one treatment will fit for all patients. On the other hand, patient selection for targeted therapies using a single biomarker may also be insufficient. Despite biological similarities between cancers of breast and bladder cancer, e.g., in terms of molecular subtyping, amplification status or protein expression of Her2 are predictive for successful treatment in one but not in the other disease. Consequently, in MIBC a more comprehensive analysis of the biological landscape will be needed to select likely responders for Her2 targeted therapy.
FGFR signaling in MIBC has caught the attention of researchers for years (36-38). However, only recently drugs have been investigated in clinical trials with promising anti-tumor activity (39). Although DNA alterations such as activating mutations and amplifications are most commonly found in MIBC (1,38), patient selection for these trials are based on gene expression analysis. This is in line with one of the conclusions from the most recent update of The Cancer Genome Atlas consortium of MIBC (1). Based on gene expression, they characterized a molecular subtype, the “Luminal-papillary” and concluded that targeted therapies, e.g., by targeting the FGFR pathway, may be most beneficial in these tumors. Moreover, in several datasets this subset of tumors showed the most favorable prognosis when compared to other subtypes (10,40). Therefore, it remains to be proven whether targeting FGFR pathways improves patient outcomes or these ongoing trials select a subset of MIBC with a lower aggressiveness and therefore, more favorable prognosis.
We are aware that many other promising targets are currently discovered in vitro and in vivo. However, we believe that the novelty of using a protein from the malaria parasite, Plasmodium falciparum, to target MIBC is particularly noteworthy (30). This treatment paradigm is independent on the genomic landscape, molecular subtype and different pathway activities. rVAR2 is primarily used for drug delivery, while the toxin conjugated to rVAR2 could be exchanged. We used the toxin KT886 in our study, which is too toxic to be used in doses to reach therapeutic levels. In conjugation with rVAR2 the doses could be reduced, while the anti-tumor activity was maintained. This treatment paradigm seems to be promising; however, it warrants further pre-clinical testing before implanting and investigating in clinical trials.
In conclusion, clinical trials investigating ineffective drugs may not only be frustrating because of the lack of anti-tumor activity but also due to insufficient patient selection. Novel promising treatments, based on the genomic landscape of MIBC are currently investigated in clinical trials. Ironically, we may be able to use paradigms from the malaria parasite, Plasmodium falciparum, a severe human hostile, for successful cancer therapy in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017;171:540-56.e25. [PubMed]
- Ohta JI, Miyoshi Y, Uemura H, et al. Fluorescence in situ hybridization evaluation of c-erbB-2 gene amplification and chromosomal anomalies in bladder cancer. Clin Cancer Res 2001;7:2463-7. [PubMed]
- Kruger S, Weitsch G, Buttner H, et al. HER2 overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic implications. Int J Cancer 2002;102:514-8. [Crossref] [PubMed]
- Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 2007;25:2218-24. [Crossref] [PubMed]
- Wulfing C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 2009;115:2881-90. [Crossref] [PubMed]
- Oudard S, Culine S, Vano Y, et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur J Cancer 2015;51:45-54. [Crossref] [PubMed]
- Powles T, Huddart RA, Elliott T, et al. Phase III, Double-Blind, Randomized Trial That Compared Maintenance Lapatinib Versus Placebo After First-Line Chemotherapy in Patients With Human Epidermal Growth Factor Receptor 1/2-Positive Metastatic Bladder Cancer. J Clin Oncol 2017;35:48-55. [Crossref] [PubMed]
- Sjodahl G, Lauss M, Lovgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res 2012;18:3377-86. [Crossref] [PubMed]
- Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 2014;111:3110-5. [Crossref] [PubMed]
- Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25:152-65. [Crossref] [PubMed]
- Kiss B, Wyatt AW, Douglas J, et al. Her2 alterations in muscle-invasive bladder cancer: Patient selection beyond protein expression for targeted therapy. Sci Rep 2017;7:42713. [Crossref] [PubMed]
- Eriksson P, Sjodahl G, Chebil G, et al. HER2 and EGFR amplification and expression in urothelial carcinoma occurs in distinct biological and molecular contexts. Oncotarget 2017;8:48905-14. [PubMed]
- Eriksson P, Sjödahl G, Liedberg F. Re: Thomas Powles, Robert A. Huddart, Tony Elliott, et al. Phase III, Double-blind, Randomized Trial that Compared Maintenance Lapatinib versus Placebo after First-line Chemotherapy in Patients with Human Epidermal Growth Factor Receptor 1/2-positive Metastatic Bladder Cancer. J Clin Oncol 2017;35:48-55: Knowing HER2 Status is Not Enough: A Molecular Subtype Approach to Bladder Cancer is Also Needed. Eur Urol 2017;72:e135-6. [Crossref] [PubMed]
- Yan M, Schwaederle M, Arguello D, et al. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev 2015;34:157-64. [Crossref] [PubMed]
- Carey LA, Berry DA, Cirrincione CT, et al. Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib. J Clin Oncol 2016;34:542-9. [Crossref] [PubMed]
- Powles T, Gomez de Liano A, Ackerman C. Reply to Pontus Eriksson, Gottfrid Sjodahl, and Fredrik Liedberg's Letter to the Editor re: Thomas Powles, Robert A. Huddart, Tony Elliott, et al. Phase III, Double-blind, Randomized Trial that Compared Maintenance Lapatinib versus Placebo after First-line Chemotherapy in Patients with Human Epidermal Growth Factor Receptor 1/2-positive Metastatic Bladder Cancer. J Clin Oncol 2017;35:48-55. Knowing HER2 Status is Not Enough: A Molecular Subtype Approach to Bladder Cancer is Also Needed: Protein Expression to Predict Outcome to Targeted Therapy in Bladder Cancer: Too Little, Too Late? Eur Urol 2017;72:e137-8. [Crossref] [PubMed]
- Scotet E, Houssaint E. The choice between alternative IIIb and IIIc exons of the FGFR-3 gene is not strictly tissue-specific. Biochim Biophys Acta 1995;1264:238-42. [Crossref] [PubMed]
- Tetzke TA, Caton MC, Maher PA, et al. Effect of fibroblast growth factor saporin mitotoxins on human bladder cell lines. Clin Exp Metastasis 1997;15:620-9. [Crossref] [PubMed]
- Allen LE, Maher PA. Expression of basic fibroblast growth factor and its receptor in an invasive bladder carcinoma cell line. J Cell Physiol 1993;155:368-75. [Crossref] [PubMed]
- Gorbenko O, Ovcharenko G, Klymenko T, et al. Generation of monoclonal antibody targeting fibroblast growth factor receptor 3. Hybridoma (Larchmt) 2009;28:295-300. [Crossref] [PubMed]
- Zhao G, Li WY, Chen D, et al. A novel, selective inhibitor of fibroblast growth factor receptors that shows a potent broad spectrum of antitumor activity in several tumor xenograft models. Mol Cancer Ther 2011;10:2200-10. [Crossref] [PubMed]
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 2009;8:235-53. [Crossref] [PubMed]
- Raju R, Palapetta SM, Sandhya VK, et al. A Network Map of FGF-1/FGFR Signaling System. J Signal Transduct 2014;2014:962962. [PubMed]
- Holtan SG, Creedon DJ, Haluska P, et al. Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin Proc 2009;84:985-1000. [Crossref] [PubMed]
- Rogerson SJ, Chaiyaroj SC, Ng K, et al. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med 1995;182:15-20. [Crossref] [PubMed]
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 1996;272:1502-4. [Crossref] [PubMed]
- Salanti A, Dahlback M, Turner L, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med 2004;200:1197-203. [Crossref] [PubMed]
- Salanti A, Clausen TM, Agerbaek MO, et al. Targeting Human Cancer by a Glycosaminoglycan Binding Malaria Protein. Cancer Cell 2015;28:500-14. [Crossref] [PubMed]
- Clausen TM, Pereira MA, Al Nakouzi N, et al. Oncofetal Chondroitin Sulfate Glycosaminoglycans Are Key Players in Integrin Signaling and Tumor Cell Motility. Mol Cancer Res 2016;14:1288-99. [Crossref] [PubMed]
- Seiler R, Oo HZ, Tortora D, et al. An Oncofetal Glycosaminoglycan Modification Provides Therapeutic Access to Cisplatin-resistant Bladder Cancer. Eur Urol 2017;72:142-50. [Crossref] [PubMed]
- Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol 2003;21:690-6. [Crossref] [PubMed]
- Zehnder P, Studer UE, Skinner EC, et al. Unaltered oncological outcomes of radical cystectomy with extended lymphadenectomy over three decades. BJU Int 2013;112:e51-8. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312-22. [Crossref] [PubMed]
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]
- Cheng T, Roth B, Choi W, et al. Fibroblast growth factor receptors-1 and -3 play distinct roles in the regulation of bladder cancer growth and metastasis: implications for therapeutic targeting. PLoS One 2013;8:e57284. [Crossref] [PubMed]
- Turo R, Harnden P, Thygesen H, et al. FGFR3 expression in primary invasive bladder cancers and matched lymph node metastases. J Urol 2015;193:325-30. [Crossref] [PubMed]
- Helsten T, Elkin S, Arthur E, et al. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin Cancer Res 2016;22:259-67. [Crossref] [PubMed]
- Nogova L, Sequist LV, Perez Garcia JM, et al. Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Kinase Inhibitor, in Patients With Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I, Dose-Escalation and Dose-Expansion Study. J Clin Oncol 2017;35:157-65. [Crossref] [PubMed]
- Seiler R, Ashab HAD, Erho N, et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur Urol 2017;72:544-54. [Crossref] [PubMed]


