Significance of positive semen culture in relation to male infertility and the assisted reproductive technology process
Introduction
Positive semen cultures are common in in vitro fertilization (IVF) clinics, with significant differences in sperm quality and cultured pathogens seen among men. It is unclear whether this is a sign of male infertility or more relevant for the IVF process. Limited data without any unifying sperm abnormalities between studies has kept the indication and significance of semen culture a highly debated topic. To compound the situation, there are currently no guidelines by the World Health Organization (WHO) or American Urological Association (AUA) on the indications of semen culture and the details of treatment.
The current WHO guidelines regarding sterile preparation prior to semen collection are rudimentary and listed in Table 1 (1). Most studies follow these WHO guidelines or add additional sterilization precautions. Although the WHO provides a simplified preparation technique, it does not provide “normal” levels of organisms for semen culture. Inconsistent control groups, definitions of “positive culture,” interpretations of multiple pathogen groups, and recordings of “nonpathogenic organisms” among studies has made comparisons and conclusions difficult.

Full table
We present the pathogenic mechanisms of several organisms, significance of each organism for impaired semen parameters, possible routes of semen sample contamination, and methods to reduce contamination. We also discuss the association with leukocytospermia and mechanisms of damaging sperm.
Relationship between bacteriospermia and leukocytospermia
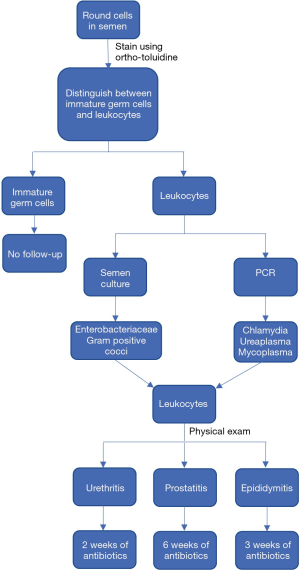
The presence of leukocytes in sperm is associated with the production of ROS. ROS destroys mitochondrial DNA and inhibits intracellular ATP production to affect sperm function and motility (2-5). The resultant oxidative stress has been shown to decrease the success of both IVF and ICSI (6,7). The WHO defines leukocytospermia as more than 1×106 WBC/mL, but seminal oxidative stress has been demonstrated at lower leukocyte levels (8). The WHO does not provide a reference range for peroxidase-positive cells in a fertile man (1). The terms leukospermia and pyospermia may be used interchangeably with leukocytospermia (1). Figure 1 outlines the workup once leukocytes have been seen within semen.
Instead of protecting sperm, the presence of leukocytes may actually decrease semen quality. A retrospective study found genetic sequence homologies between human B-tubulin and proteins of different bacterial species, suggesting that infection may induce immunomediated damage (9). In spite of the proposed mechanism, the genetic similarities may be coincidental and more a result of functional similarities. After all, the study that suggested this mechanism found no significant differences between bacteria positive and negative fertile men (9). In contrast, a retrospective study concluded that leukocytospermia has little diagnostic value in the detection of bacteriospermia and impaired semen quality (10). Rather than simply being a response to bacteria, leukocytospermia has also been attributed to cigarette smoking, heavy alcohol use, and age (11-13).
Potentially pathogenic bacteria
Chlamydia trachomatis
Besides urethritis, conjunctivitis and Reiter’s syndrome, serotypes A-C may cause epididymitis, orchitis, prostatitis, and sperm tract obstruction (14). Despite the diverse presentation in symptoms and disease, many patients may also be asymptomatic (14). The suggested mechanism of male infertility is by the induction of anti-sperm antibodies by semen IgA antibodies or serum IgG antibodies against C. trachomatis (14). Chlamydia is an intracellular organism and difficult to culture, so the diagnosis is usually made through nucleic acid amplification tests (NAAT) or polymerase chain reaction (PCR). In the evaluation of male infertility by Chlamydia, two prospective studies found significant difference in sperm quality among Chlamydia positive and negative men that were fertile (15,16). Both Liu et al. and Al-Sweih et al. found that sperm vitality was significantly different between the Chlamydia positive and negative men (15,16). Al-Sweih et al. also found that rapid progressive motility and total progressive motility differed between these two groups (16). Among infertile men, Al-Sweih found that Chlamydia positive men had significantly more leukocytes than Chlamydia negative men (16). Despite several differences in semen parameters, both studies found no significant difference in the prevalence of Chlamydia between fertile and infertile men (15,16).
A different prospective study evaluating the clinical significance of Chlamydia within semen cultures found that 3/11 Chlamydia positive patients within an IVF treatment group had azoospermia and that 3/7 of the study’s azoospermic patients were Chlamydia positive (17). Although nearly half of the azoospermic patient cultures were positive for Chlamydia, no significant differences in bacteria were found between fertile and IVF treatment men (17). Similarly, the presence of bacterial growth was not found to be related to sperm abnormalities (17). Despite long-standing assumptions and a potential mechanism, the absence of a clear causative effect suggests that another factor may influence an interplay between Chlamydia presence and infertility. Perhaps the duration of Chlamydia trachomatis infection correlates with the degree of semen abnormalities and reproductive system damage. Unfortunately, the effect of infection duration will be difficult to assess since these organisms are treated when detected. Without more robust data, the presence of Chlamydia trachomatis within semen culture cannot be interpreted as a sign of male infertility.
Ureaplasma
Ureaplasma is found frequently within the genital tract and semen of males (14) and is thought to cause infertility through the production of reactive oxygen species (ROS) (14) and antisperm antibodies (18). Ureaplasma is generally considered to be an extracellular pathogen, although intracellular localization is possible. Cell culture is difficult, so enzyme-linked immunosorbent assays (ELISA) and PCR are usually used to diagnosis this organism. A prospective study showed that in fertile males, sperm concentration and total motile count significantly differed between Ureaplasma positive and negative patients (15). However, in infertile men, those who were Ureaplasma positive had significantly lower mean sperm concentration and vitality compared to those who were negative (15). The clinical significance of these differences in semen quality were evaluated by a retrospective study specifically measuring the effect on IVF outcomes. Pregnancy rates did not significantly differ based on semen culture positivity, or if positive cultures were treated prior to the IVF attempt (19). Even if Ureaplasma positivity affects mean sperm concentration, this difference does not appear to have clinical significance.
Mycoplasma
Mycoplasma is also found frequently in the genital tract and semen of men, (14) but is highly associated with female infertility factors, such as cervicitis, endometritis, and pelvic inflammatory disease (14). Although a specific mechanism has not been proposed, M. genitalium has been found to attach to human spermatozoa, making this pathogen very relevant throughout the entire fertilization process (20). Mycoplasma is primarily diagnosed through ELISA and PCR, but may also be detected using cell culture.
In a prospective study of men at an IVF clinic, low frequencies of Mycoplasmas were observed in first-catch urine samples, midstream urine samples, and semen samples (21). This suggests that Mycoplasma is likely a part of the normal urethral flora, rather than a pathogen harming the sites of gamete production. In two prospective studies, Al-Sweih et al. and Liu et al. found no overall significant difference in the presence of Mycoplasma and other suspected pathogens in fertile compared to infertile men (15,16). Among fertile and infertile men, Al-Sweih found that those positive for M. hominis had semen samples that showed significantly higher mean sperm pH and leukocyte count than those negative for M. hominis (16). In contrast, Liu found no significant differences in sperm quality among Mycoplasma positive and negative men (15). Significantly different semen parameters were once again seen to vary by fertility status for bacterial species and M. morganii (9). Although the means of the fertility index score were significantly lower for infertile men within each bacterial species, all fertility index scores were greater than the WHO cut-off except for the M. morganii group (9). Despite being a unique genus of bacteria, Mycoplasma has not demonstrated any unifying effect on semen quality or relationship with male infertility.
General bacteria
Enterobacteriaceae is a family of gram negative bacteria that includes organisms such as E. coli, Klebsiella, Salmonella, Proteus, and Pseudomonas. These bacteria are associated with epididymitis, orchitis, and prostatitis, suggesting that they may have a role in infertility (14). Although few mechanisms have been proposed for Enterobacteriaceae, E. coli have been found to have a special effect on sperm motility and acrosomal function (9).
Gram positive cocci include Enterococci, Streptococci, and Staphylococci. Prostatitis and epididymitis have been associated with these organisms, suggesting that they may have a role in infertility too (14). In in vitro studies, gram positive cocci have demonstrated a negative influence on sperm morphology, probably mediated by its virulence factor hemolysin (9). These organisms are very prevalent in semen cultures and have even been found to be the most common bacteria (19). In one retrospective study, 80/342 cultures grew more than 10,000 colony forming units of Enterococci (19). However, this study did not find that pregnancy rates significantly differed with the semen culture positivity before or during the IVF attempt (19). Pregnancy rates also did not significantly correlate with the successful treatment of the semen culture prior to the IVF attempt (19). Despite the potential for inflammatory disease, Enterobacteriaceae and gram positive cocci do not appear to have significant correlation with male infertility.
One retrospective study found significant sperm quality differences between fertile and infertile men within each bacterial positive group, but no significant differences between fertile men with bacteria positive and negative semen cultures (9). Within each bacteria positive group, the mean sperm concentration was significantly lower among infertile men, but was always higher than the WHO normal value of 20×106 (9). This suggests that potential differences in sperm composition by the presence of general bacteria may not have clinical significance. This same study also excluded 171/417 males because cultures contained two or more bacteria species or a non-significant bacteria colony count (9). The extent of excluded cultures highlights the potential burden of general bacteria as urethral flora and/or contamination, as well as the possible selection biases from differing reproduction times between bacterial species.
Skin flora
Despite many reports of contamination with bacteria commonly associated with skin flora, the WHO only gives the general recommendations of strict hygiene and passage of urine before producing semen sample by masturbation (1). One study suggests that skin flora contamination may be unavoidable, since as much as 71% of the bacterial strains colonizing the coronal sulcus are also present in the distal urethra (22). The ability of skin flora to inhabit the urethra was also suggested by a prospective study that found that the majority of positive cultures contained gram positive bacteria from first-catch urine, midstream urine, and semen samples (21). This study found no significant difference in the presence or absence of bacteriospermia or leukocytospermia in achieving pregnancy (21). Similarly, a retrospective study found no significant difference in the IVF rates between cultures that grew skin contaminants and those that were growth free (23). The presence of skin flora within semen cultures may be unavoidable and appears to lack clinical significance.
A summarization of the mode of detection and effect on semen parameters for each group of organisms is presented in Table 2.

Full table
Treatment of bacteriospermia
Although skin flora may colonize the urethra, a skin preparation that is more thorough than the WHO guidelines of soap and water may be beneficial. The use of multiple antibacterial skin preparations was found to significantly reduce total bacterial isolates and enteric organisms (24). However, the extra preparation did not significantly reduce skin flora (24), suggesting that skin flora contamination persists through urethral colonization or through specimen handling by the patient or semen analyzer. The significance of post-production contamination was underscored by observations that the presence of enterococci significantly increased when semen samples were not processed within the same day (25). This same study also found that samples taking two or more days to process had significantly increased presence of Gram-negative bacilli and other “potentially pathogenic organisms (25)”.
The WHO recommendations to urinate prior to semen collection may not be effective in reducing culture contamination. One study evaluated its efficacy by comparing semen cultures to urine cultures during various points of urination. Among men who had negative midstream urine samples, corresponding semen cultures were negative in over half of the men (21). Similarly, only 12/22 men with a positive first-catch urine culture also had a positive midstream culture (21). Unfortunately, this study did not publish the culture results of men who had positive midstream cultures (21). Even without this data, the incongruity between urine and semen culture results suggests that urination prior to semen collection may not aid contamination prevention.
Although the presence of bacteria has not been shown to affect assisted reproductive technology (ART) outcomes, the use of antibiotics has been shown to eliminate all non-specific bacteria without having any effect on sperm quality or pregnancy rates (26). However, antibiotic use may increase the likelihood of inoculating antibiotic-resistant pathogenic bacteria from the vagina into the embryo culture system during oocyte collection (25). Even if antibiotics have a negative effect on the IVF process, classic centrifuge techniques (density gradient and swim-up) have been shown to effectively separate bacteria from sperm and select higher quality sperm (27). New technologies, like microfluidics, electrophoresis, motile sperm organelle morphology examination (MSOME), and birefringence can have similar results and without the ROS production derived from centrifugation (27).
Among the bacteria discussed, men within the studies lacked classic symptoms of infection. The presence of leukocytospermia, bacteriospermia, and a positive physical exam warrant treatment in the evaluation of male infertility, as summarized by Figure 1.
Summary
The incidence of positive semen culture does not consistently differ between fertile and infertile men. Semen quality parameters between men positive and negative for each bacteria species differ between studies, with no unifying trend or clinical significance. Attempts to reduce bacteria in sperm from skin flora contamination or urethral flora contamination through increased skin preparation or urination have been unsuccessful. Although bacteriospermia has no significance in the ART process, there are new techniques to isolate sperm from bacteria. Leukocytospermia can be detrimental to sperm quality, but is not related to bacteriospermia. Overall, there does not appear to be an indication for routine semen culture prior to IVF or cryopreservation. However, leukocytospermia and a positive semen culture need to be treated to account for the symptoms of patients.
Acknowledgements
The authors would like to thank Dr. Juergen Eisermann for his question that stimulated the discussion of this topic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization. WHO laboratory manual for the Examination and processing of human semen. Available online: http://apps.who.int/iris/bitstream/10665/44261/1/9789241547789_eng.pdf, accessed April 25, 2017.
- Villegas J, Schulz M, Soto L, et al. Influence of reactive oxygen species produced by activated leukocytes at the level of apoptosis in mature human spermatozoa. Fertil Steril 2005;83:808-10. [Crossref] [PubMed]
- de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl 1992;13:368-78. [PubMed]
- de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J Androl 1992;13:379-86. [PubMed]
- Pentyala S, Lee J, Annam S, et al. Current perspectives on pyospermia: a review. Asian J Androl 2007;9:593-600. [Crossref] [PubMed]
- Henkel R, Kierspel E, Hajimohammad M, et al. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod Biomed Online 2003;7:477-84. [Crossref] [PubMed]
- Barraud-Lange V, Pont J-C, Ziyyat A, et al. Seminal leukocytes are Good Samaritans for spermatozoa. Fertil Steril 2011;96:1315-9. [Crossref] [PubMed]
- Agarwal A, Mulgund A, Alshahrani S, et al. Reactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermia. Reprod Biol Endocrinol 2014;12:126. [Crossref] [PubMed]
- Moretti E, Capitani S, Figura N, et al. The presence of bacteria species in semen and sperm quality. J Assist Reprod Genet 2009;26:47-56. [Crossref] [PubMed]
- Lackner J, Schatzl G, Horvath S, et al. Value of counting white blood cells (WBC) in semen samples to predict the presence of bacteria. Eur Urol 2006;49:148-52; discussion 152-3. [Crossref] [PubMed]
- Saleh RA, Agarwal A, Sharma RK, et al. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril 2002;78:491-9. [Crossref] [PubMed]
- Maneesh M, Dutta S, Chakrabarti A, et al. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J Physiol Pharmacol 2006;50:291-6. [PubMed]
- Cocuzza M, Athayde KS, Agarwal A, et al. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology 2008;71:490-4. [Crossref] [PubMed]
- Pellati D, Mylonakis I, Bertoloni G, et al. Genital tract infections and infertility. Eur J Obstet Gynecol Reprod Biol 2008;140:3-11. [Crossref] [PubMed]
- Liu J, Wang Q, Ji X, et al. Prevalence of Ureaplasma Urealyticum, Mycoplasma Hominis, Chlamydia Trachomatis Infections, and Semen Quality in Infertile and Fertile Men in China. Urology 2014;83:795-9. [Crossref] [PubMed]
- Al-Sweih NA, Al-Fadli AH, Omu AE, et al. Prevalence of Chlamydia trachomatis, Mycoplasma hominis, Mycoplasma genitalium, and Ureaplasma urealyticum infections and seminal quality in infertile and fertile men in Kuwait. J Androl 2012;33:1323-9. [Crossref] [PubMed]
- Ombelet W, Bosmans E, Janssen M, et al. Semen parameters in a fertile versus subfertile population: a need for change in the interpretation of semen testing. Hum Reprod 1997;12:987-93. [Crossref] [PubMed]
- Günyeli I, Abike F, Dünder I, et al. Chlamydia, Mycoplasma and Ureaplasma infections in infertile couples and effects of these infections on fertility. Arch Gynecol Obstet 2011;283:379-85. [Crossref] [PubMed]
- Shalika S, Dugan K, Smith RD, et al. The effect of positive semen bacterial and Ureaplasma cultures on in-vitro fertilization success. Hum Reprod 1996;11:2789-92. [Crossref] [PubMed]
- Taylor-Robinson D, Furr PM. Update on sexually transmitted mycoplasmas. Lancet 1998;351 Suppl 3:12-5. [Crossref] [PubMed]
- Cottell E, Harrison RF, McCaffrey M, et al. Are seminal fluid microorganisms of significance or merely contaminants? Fertil Steril 2000;74:465-70. [Crossref] [PubMed]
- Willén M, Holst E, Myhre EB, et al. The bacterial flora of the genitourinary tract in healthy fertile men. Scand J Urol Nephrol 1996;30:387-93. [Crossref] [PubMed]
- Hewitt J, Cohen J, Fehilly CB, et al. Seminal bacterial pathogens and in vitro fertilization. J In Vitro Fert Embryo Transf 1985;2:105-7. [Crossref] [PubMed]
- Kim FY, Goldstein M. Antibacterial skin preparation decreases the incidence of false-positive semen culture results. J Urol 1999;161:819-21. [Crossref] [PubMed]
- Liversedge NH, Jenkins JM, Keay SD, et al. Antibiotic treatment based on seminal cultures from asymptomatic male partners in in-vitro fertilization is unnecessary and may be detrimental. Hum Reprod 1996;11:1227-31. [Crossref] [PubMed]
- Dissanayake DM, Amaranath KA, Perera RR, et al. Antibiotics supplemented culture media can eliminate non-specific bacteria from human semen during sperm preparation for intra uterine insemination. J Hum Reprod Sci 2014;7:58-62. [Crossref] [PubMed]
- Rappa KL, Rodriguez HF, Hakkarainen GC, et al. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnol Adv 2016;34:578-87. [Crossref] [PubMed]