Clinical scenarios for neoadjuvant chemotherapy of squamous penile cancer that is clinically node positive
Background
Metastatic squamous penile cancer can be very challenging to manage, even just for planning surgical management. There are a variety of contexts for which chemotherapy could impact on the disease course, but arguably neoadjuvant therapy to convert a patient with local spread to a curative outcome is the most compelling.
Consistent with most of the experiences in penile cancer therapy, there are no prospective randomized comparative trials from which to base treatment choices for up-front initial chemotherapy in the setting of squamous penile cancer with clinically node positive disease. The largest series, for patients who could then be treated surgically with curative intent, described 59 patients treated over 15 years at the MD Anderson Cancer Center, with three different treatment plans. The authors make the keystone observation that after chemotherapy some patients have pathologic negative lymph nodes (pN0) and most importantly, long term disease-free survivorship (1). That retrospective study was followed by a 30-patient prospective format, single arm trial, using the paclitaxel-ifosfamide-cisplatin (TIP) regimen, described in more detail below (2). Working from these studies, and a generally narrow base of experiences, there is a basis to say that a significant debulking and downstaging of this regional spread, as well as of the disease at the phallus can be realistic goals for clinically node positive squamous penile cancer.
Neoadjuvant systemic chemotherapy can be a key tool, in coordination with surgical and external beam radiotherapy consolidation (generally also with concurrent chemotherapy), and with potential use of post-operative, adjuvant chemotherapy. Surgical approaches may require coordination of urologic oncology, plastic surgery, vascular surgery, and other specialties.
Heterogeneity of disease and patient features
Those selected for a neoadjuvant approach are acknowledged to be in a gray zone. The boundaries of this gray zone can be described (albeit without an empiric precision) in anatomic terms as clinically identified inguinal, superficial or deep pelvic nodes, which are too numerous by CT, too large by exam or CT, or active on PET scan, but still with the patient not having identified distant disease. Overall, more spread of the cancer than is expected by the surgeon to be resectable for cure, in an up-front, limited surgical approach, but less spread of the cancer than obviates a curative intent.
Despite some unifying anatomic attributes at presentation, the incident penile cancer population present heterogeneity on several axes: age, tumor grade; association or independence of human papilloma virus (HPV); nodal, dermal, or visceral spread. That heterogeneity is barrier to generalized assumptions about response patterns as are used for presenting expectations of clinical trials, but that heterogeneity is not the issue for the patient presenting in isolation. His issue is a tailored approach incorporating an individualized disease- and comorbid-assessment. To explore some of these contexts, differing scenarios for neoadjuvant chemotherapy in the context of node-positive disease are illustrated.
Cases with small volume adenopathy
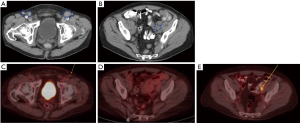
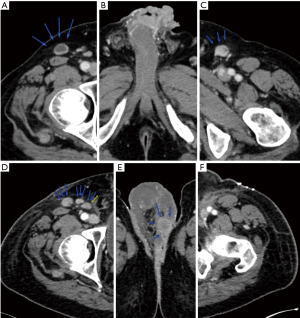
In the first case, small volume adenopathy had a transient complete nodal response to TIP. A 72-year-old man, who a decade earlier had had penile CIS (carcinoma in situ) resected, then developed a meatal ulcer showing squamous cancer, that then was refractory to topical 5-fluorouracil. Preoperative scans showed bilateral superficial inguinal adenopathy, and left iliac node enlargement (Figure 1A,B). He was referred for neoadjuvant chemotherapy, and completed 3 cycles of TIP, the adenopathy appeared resolved (Figure 1C). Then he did well with the surgery on the primary alone; the total penectomy and urethrostomy specimen pathology showed 2.5 cm dominant mass, pT3 (urethral invasion) but without invasion of corpora spongiosum [staging citation reference: (3)]. He continued on follow up, with no findings through +12 months (Figure 1D), but then at +15 months a single PET-positive left, deep lymph node (Figure 1E) was observed. He then had resection (right superficial, left superficial and deep) and that specimen showed single 2 cm left external iliac squamous cancer positive node, and 15 other negative nodes. He then completed two additional (now adjuvant) TIP cycles and remains in complete remission at 18+ months from the second surgery. This example shows a case where combined modality management of the groins was initially just using chemotherapy (and surgery on the primary penile lesion), but nodal surgery was later needed anyhow.
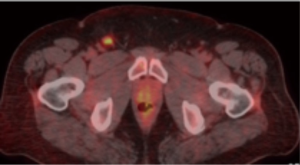
In the second case illustration, a 42-year-old man had had treated congenital hypospadias, had had a T1, well differentiated tumor resected from glans, and then 5-fluorouracil topical treatment; but then a year later a separate lesion, and that latter biopsy showed poorly differentiated squamous cancer. Preoperative PET scan showed a single bright right inguinal node (Figure 2). With the apparently small volume inguinal disease, surgery was the initial treatment. This was penectomy, perineal urethrostomy, and bilateral superficial and deep inguinal lymph node dissection. That pathology showed 3.0 cm moderately to poorly differentiated primary squamous cancer, negative surgical margins, angiolymphatic invasion present and also a 2.8 cm right inguinal lymph node with extranodal extension of cancer and 3 positive left inguinal lymph nodes the larger of which were 2.8 and 1.6 cm, also with extranodal extension of cancer; and four other negative nodes. So the stage was pT2 N3 cM0 (IIIb), which overall was a higher stage than had been anticipated. Following the uneventful surgical recovery, an adjuvant chemotherapy and radiation treatment plan will be implemented (no long term follow up yet). As discussed below, the InPACT study may help us to understand quantitatively the frequency to which a neoadjuvant chemotherapy approach could be expected to downstage adenopathy, for cases where either surgery or chemotherapy could be initial treatments.
Cases where skin condition is a driving factor
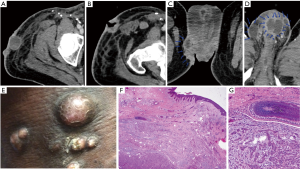
Skin integrity is a practical factor that could impede use of conventional chemotherapy because of neutropenic infection risks. If there are already open, erosive tumors or wounds on the phallus, scrotum, or groin, that could tip the planning back to an up-front surgery, so as to get to a point of intact skin. In the third example, a 60-year-old man had presented with 6 months’ history of enlarging, and then draining groin mass; it initially had closed up after some antibiotics, then again opened; biopsies then showed squamous cancer. The open ulcer precluded conventional chemotherapy neoadjuvant treatment (Figure 3). He did well with the extensive surgery, which included partial penectomy, excision of bulky left ilioinguinal mass, skin and soft tissue, en bloc resection of matted superficial and deep left lymph nodes, dissection of left femoral artery and vein, left pelvic lymph node dissection, and complex abdominal fascial closure; recovery was uneventful, but leg edema persists. Pathology showed on the partial penectomy multifocal, invasive squamous cell carcinoma, moderately to poorly differentiated (pT1b) and the left ilioinguinal tumor mass and en bloc resection 9 cm ulcerated tumor mass and additionally 7/11 lymph nodes, and vascular invasion, and a single deep margin positive, and additional 20 negative lymph nodes. Adjuvant chemoradiation treatment was intensity-modulated radiation therapy (IMRT) to bilateral groins (39.6 gray in 22 fractions) then boost to left groin (25.2 gray in 14 fractions), with concurrent cisplatin 40 mg/m2/dose, weekly ×8 doses. Despite the large specimen size, and with adjuvant chemoradiotherapy used, a durable complete response has been maintained 2+ years out.

Although the primary tumors of squamous penile cancer clearly originate in the skin, metastatic disease may be limited to adenopathy, or may have a dermal-infiltrative pattern. The latter may ultimately involve a perineal field beyond any practical resection, with the appearance of satellite nodules of cancer on scrotum, thighs, buttocks or abdomen. Among those with this pattern, getting to negative surgical margins is a major concern, and may not be possible. Growth in the surgical wounds themselves also may be observed. In the case illustrated in Figure 4, a 46-year-old man had pT1b (resection) N1 (biopsy) cancer, 2 months later he had bilateral deep inguinal femoral lymphadenectomy with right sartorius muscle flap; pathology showed 5.5 cm right inguinal mass, and additionally 7/12 (right) and 3/28 (left) positive lymph nodes. Following that, therapies included external beam radiation therapy; two cycles TIP; three cycles cisplatin/5-fluorouracil; however he succumbed to the disease. Some partial regressions were observed during chemotherapy, but there was no point at which the evident cutaneous disease was completely gone. The images are from later in the disease course, but the skin pattern was seen earlier as well, starting in the scrotum.
Cases where rapid tumor growth drives decision
The apparent tumor growth rate of penile cancer is variable. In planning therapies for squamous penile cancer, one should understand the potential for a rapid growth kinetic. This could be so, even if preceded by a distinctly slower, more orderly pattern that could mislead patient and physician expectations. Insightful patients without barriers to medical access could present with tumors with obvious and large appearance, for which a question of neglect might be raised in an inexperienced setting. Rapid growth cuts both sides of the issue of patient selection for neoadjuvant chemotherapy—a rapid growth pattern could indicate more sensitivity to conventional chemotherapy—but also concern that the penile primary or adenopathy becoming anatomically unresectable. The specific mutational events that drive a fast growth pattern change are not defined, but one may certainly speculate that molecular characterization will be feasible at some point.
The case illustrated Figure 5 is of a 50-year-old who presented with a 3.5 cm tip of penis mass, 9 cm left, and 4 cm right inguinal masses, but no distant disease. He was treated with initial penile resection, the pathology for which showed well to moderately differentiated squamous penile cancer on glans and foreskin, involving corpus spongiosum, with perineural and lymphovascular invasion present. This then was immediately followed by concurrent chemoradiotherapy, to bilateral inguinal fields (5,040 cGy in 28 fractions of 180 cGy), with weekly cisplatin; then immediately by TIP °¡3 cycles; then surgery, with bilateral inguinal superficial and deep resection, with sartorial rotational flaps. That specimen showed left superficial 7.5 cm metastatic necrotic keratinizing squamous cell carcinoma with therapy related effects, infiltrating connective tissue and no right side cancer. There were, altogether 4 months between the surgeries. Surgical recovery was uneventful, except two 1.5 cm areas, which healed. He remains in complete remission, 7+ years.
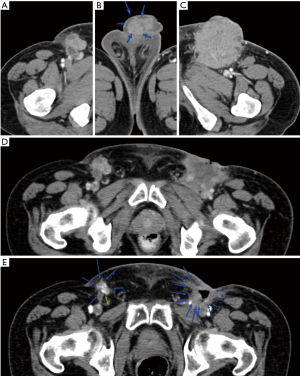
Rapid growth could be also after surgery. In the case with images illustrated in Figure 6, an 80-year-old man with history of treated genital warts a decade earlier, had had 1 year of symptoms at the glans, which then progressed to bleeding. The initial CT scan shows the primary tumor and bilateral small adenopathy. Thirteen days later, the first surgery on the primary, showed pT1a, 3.7 cm, invasive squamous cell carcinoma keratinizing type, moderately differentiated, 4mm depth of invasion; no lymphovascular invasion; negative margins; pT1a. Forty two days further along, bilateral lymph dissection showed on the left, 3/13 lymph nodes largest were 15 and 30 mm, and 0/3 on the right. Then 44 days later, the first postoperative scan shows 2–3 right inguinal LN and new left perineal nodule. In a case such as this, which initially looked like a moderately differentiated, T1b, is actually a high stage IIIb cancer. Each repeat evaluation (that is surgery, then CT scan) shows measurably larger disease than the prior evaluation. The potential for an initial neoadjuvant chemotherapy plan to yield a surgical downstaging can be understood. Chemotherapy TIP treatment is planned at this point; there is no long term follow-up yet.
The observation of the very large masses and skin infiltration in some of the cases illustrated suggest rapid growth. Similarly, after debulking, nodal and dermal lesions may grow faster than even the healing time of routine surgical wounds, or the more protracted healing time that must be anticipated from a combined superficial and deep inguinal resection. It would be speculative to guess for a particular case whether from an overall survival will change with sequencing the treatment with neoadjuvant chemotherapy, then resection then an option for chemoradiotherapy consolidation, on the one hand, or on the other hand, surgery first (staged surgeries in this case history) followed by healing and then active (as opposed to nominally adjuvant) chemotherapy, and an option for chemoradiotherapy consolidation. Under an assumption that there even is a generalizable answer a prospective trial may address this.
Regimen selection
The recently opened Eastern Cooperative Oncology Group (ECOG) trial EA8134 NCT02305654 (International Penile Cancer Adjuvant Chemotherapy Trial, “InPACT”) will be a cooperative group prospective, open label, phase III randomized trial using neoadjuvant TIP combination treatment (two schedule options) versus initial surgery, and may inform on some aspects of the issue of for whom, among clinically node positive penile cancer patients, to use initial surgery versus initial neoadjuvant chemotherapy. For the patient not on-study, a few regimens may bear consideration. The largest retrospective series of Bermejo et al. (1) describes experiences using several different regimens. These included TIP; carboplatin and paclitaxel; and bleomycin, methotrexate and cisplatin. The one we have used most often in our group here has been based on the experience described by Pagliaro (2):
- Day 1, paclitaxel 175 mg/m2/dose, over 3 hours;
- Day 1, 2, 3, cisplatin 25 mg/m2/dose over 2 hours;
- Day 1, 2, 3, ifosfamide 1,200 mg/m2/dose, over 2 hours. Mesna is given 400 mg/m2/dose right before ifosfamide, and then 200 mg/m2/dose at +4 and +8 hours.
For concurrent chemotherapy and radiation therapy, the regimen we have used most frequently has been cisplatin 30–40 mg/m2/dose, weekly, during treatment weeks, but generally not on Monday or Friday, so that a radiation dose will both precede and follow each chemotherapy dose. This is generally similar to protocol “P2” for bladder cancer organ preservation therapy described by Zapatero et al. (4) or as has been developed for cervical cancer combined modality treatment (5). Radiation therapy is the subject of the second (post-pathology and surgical convalescence) randomization of the InPACT trial, for those in whom the resection specimen meets high risk features.
Realistically, a rational basis for any regimen that is active in the wider field of squamous cancers could additionally bear consideration—there are no directly comparative studies. This is particularly germane in the setting of relative or absolute contraindications to cisplatin use, such as pre-existing neuropathy, partial deafness, or renal insufficiency beyond a threshold. The ideal threshold level of creatinine clearance for use of cisplatin remains a subject of debate (6,7), with a lower value, such as 50 mL/min instead of 60 mL/min considered for regimens such as TIP in which the cisplatin doses are smaller, daily doses instead of a single cisplatin dose of 60–100 mg/m2/dose. Of note, cisplatin-ineligible patients will not be included in the planned accrual of the InPACT phase III trial
In contrast to the drugs in TIP, which have been foundational conventional chemotherapies for decades, newer agents are in wide development across oncology; however the acknowledged rare status for penile cancer presents a limitation for the rate at which they can be tested. Screening tests for these targets, such as the products offered by Foundation One (www.foundationmedicine.com) or Caris (www.carislifesciences.com), or in the NCI MATCH trial (NCT 02465060 https://clinicaltrials.gov/ct2/show/NCT02465060) are among the available modalities to try to select for targeted therapy. It can be anticipated that as targeted therapies find niche application in infrequent, genetic situations, probably initially in squamous penile cancer patients with advanced disease, that efficient testing may find opportunities for neoadjuvant application. Among these newer agents, checkpoint inhibitors, for which there are on-label uses in other squamous histology cancers, such as head and neck cancers and squamous lung cancers, can be particularly highlighted.
While the focus of this article is squamous penile cancer in which there is nodal spread, another neoadjuvant approach, in some small series directed at downstaging primary tumors may be mentioned. The technique of intra-arterial chemotherapy infusion involves placement of an arterial infusion catheter, above the bifurcation of the aorta but below the inferior mesenteric artery, in a way that first-pass exposure of chemotherapy is to the tumor (8-10). Development of the technique remains limited.
Disease and response assessment
Disease evaluation, before addressing chemotherapy, surgery, radiation and coordinated plans should include a careful physical exam, and radiologic evaluations both of the regional situation, and for evaluation of out-of-field, extrapelvic metastatic disease. Surgical planning may encompass multiple surgical subspecialties. Attention to skin-infiltrative growth patterns warrants mention again.
As some of the cases illustrated, an integrated timeline to plan the surgical and chemotherapy portions of the combined treatment is advantageous to avoid delays that could be clinically costly. Besides possible impact on overall survival, at the local level, organ preservation, the potential to minimize post-surgical lymphatic obstruction, and decrease potential need for post-resection irradiation could improve. The surgical date should be set in a way that chemotherapy-associated neutropenia or thrombocytopenia should be resolved. Use of leukocyte growth factors should be applied as needed, based on observed cytopenias. The most frequent plan will be, for TIP regimen 3 cycles (9 weeks) and then surgery about week 10–12, or 4 cycles (12 weeks) and surgery about week 13–15. Certainly, for eligible patients, enrollment in InPACT may be of interest.
While curative-intent approach is always a conceptual goal, it will be recognized there are contexts of potential for debulking and general perineal closure requiring surgery even if other disease appears to be persistent. Palliative impact may be substantial. While some of the example cases showed rapid growth, there are also clinical patterns of metastatic squamous cancer that have an indolent course, despite being anatomically extra-pelvic, so a deferred commitment to address the other areas may work to the patient’s advantage.
Conclusions
Among squamous penile cancers, those that are clinically node positive could be slow or fast. Since the intrinsic growth rate of squamous penile cancer may be rapid, an integrated multimodality plan should be organized and implemented with minimal delays. Practical generalized treatment recommendations are made recognizing the limitations of a critique of levels of empiric evidence, because large prospective or randomized squamous penile cancer treatment trials simply do not exist. It is incumbent for the treatment team to be prepared to capitalize on the opportunities to make a real impact.
Recognizing that among clinically node positive squamous penile cancer patients there will be those who are initially operable, but could also have neoadjuvant chemotherapy, there is an ongoing phase III randomized trial, which may inform on what is the best sequential use of coordinated multimodality treatment plan for that population. There will be other cases of squamous penile cancer with clinically node positive disease that will not appear to even potentially be candidates for initial surgical approaches, and as the series of Bermejo (1), of Pagliaro (2), or others and some examples here describe, long term complete response can still be a realistic goal for some. Some patients may need non-cisplatin containing approaches; expansion of therapies beyond classical chemotherapies remains an area for development.
Acknowledgements
The author would like to thank Dr. Wade Sexton and Dr. Jasreman Dhillon.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Bermejo C, Busby JE, Spiess PE, et al. Neoadjuvant chemotherapy followed by aggressive surgical consolidation for metastatic penile squamous cell carcinoma. J Urol 2007;177:1335-8. [Crossref] [PubMed]
- Pagliaro LC, Williams DL, Daliani D, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol 2010;28:3851-7. [Crossref] [PubMed]
- Staging reference for penile cancer. Available online: https://www.cancer.gov/types/penile/hp/penile-treatment-pdq#section/_6
- Zapatero A, Martin De Vidales C, Arellano R, et al. Long-term results of two prospective bladder-sparing trimodality approaches for invasive bladder cancer: neoadjuvant chemotherapy and concurrent radio-chemotherapy. Urology 2012;80:1056-62. [Crossref] [PubMed]
- Strauss HG, Kuhnt T, Laban C, et al. Chemoradiation in cervical cancer with cisplatin and high-dose rate brachytherapy combined with external beam radiotherapy. Results of a phase-II study. Strahlenther Onkol 2002;178:378-85. [Crossref] [PubMed]
- Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 2006;107:506-13. [Crossref] [PubMed]
- Ahn MJ, D'Cruz A, Vermorken JB, et al. Clinical recommendations for defining platinum unsuitable head and neck cancer patient populations on chemoradiotherapy: a literature review. Oral Oncol 2016;53:10-6. [Crossref] [PubMed]
- Roth AD, Berney CR, Rohner S, et al. Intra-arterial chemotherapy in locally advanced or recurrent carcinomas of the penis and anal canal: an active treatment modality with curative potential. Br J Cancer 2000;83:1637-42. [Crossref] [PubMed]
- Chen CH, Kang CH, Chiang PH. Intra-arterial infusion of chemotherapy in the treatment of penile cancer. Jpn J Clin Oncol 2009;39:825-8. [Crossref] [PubMed]
- Huang XY, Kubota Y, Nakada T, et al. Intra-arterial infusion chemotherapy for penile carcinoma with deep inguinal lymph node metastasis. Urol Int 1999;62:245-8. [Crossref] [PubMed]


