The Society for Translational Medicine: clinical practice guidelines for sperm DNA fragmentation testing in male infertility
Introduction
Male factors are responsible in approximately half of all infertility cases (1). The remarkable evolution of assisted reproductive techniques (ART) has significantly impacted the development of clinical andrology (2). The value of proper male evaluation is overlooked since intracytoplasmic sperm injection (ICSI) possibly gives the couple a baby without explaining the nature or cause of underlying male infertility. In contrast to the rapid development in ART, conventional semen analysis (SA) remained the cornerstone for evaluation of infertile men. However, despite its ability in providing an overall assessment of male fertility potential, SA fails as an accurate predictor of fecundity due to variations in sperm quantity and quality (3). In addition, the live birth rate utilizing ICSI as treatment of male factor infertility does not exceed 30% (4). As a result, there is a need to search for additional diagnostic tools which improve prediction of fertility and direct management decisions of infertile men.
Infertility researchers have turned their attention to sperm function tests in the last few decades. Sperm DNA fragmentation (SDF) is perhaps the only investigation that has withstood the test of time with increasing availability among andrology laboratories worldwide. The importance of sperm DNA integrity in fertilization, early embryo development, implantation, and pregnancy has been supported by various in vitro and in vivo studies (5). Protamination of sperm DNA is essential in compaction of paternal genome during transportation to female genital tract (6). Some DNA damage or breaks may occur during the process, which can be repaired by oocytes. However, infertility can ensue when the damage exceeds the threshold of oocyte repair machinery (7).
While the implication of SDF on male infertility is increasingly understood, the role of SDF testing in clinical practice remains poorly defined. Although there is insufficient evidence to support the routine use of SDF testing in evaluation of infertile men (8), the value of SDF testing has been acknowledged in the latest American Urological Association (AUA) and European Association of Urology (EAU) guidelines (8,9). A recently published practice recommendation on clinical utility of SDF testing represents the first attempt to suggest specific indications for SDF testing in clinical practice (10).
This clinical practice guideline aims to present the most updated information on clinical utility and illustrate the principle of SDF testing. Management of increased SDF in each indication is reviewed. The most widely available methodologies for SDF testing are briefly discussed. The guidelines are intended to serve as a reference for fertility specialists in identifying the circumstances in which SDF testing should be of greatest clinical value.
SDF tests
SDF tests are generally classified into direct and indirect assays. Direct assays measure the extent of SDF by using probes and dyes directly. On the other hand, indirect assays measure both the existing breaks and the susceptibility of DNA to denaturation which occurs more commonly in fragmented DNA. There are eight described methods to assess SDF (10). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), the sperm chromatin structure assay (SCSA), and the sperm chromatin dispersion test (SCD) are the three most commonly used and reported tests (11).
TUNEL utilizes fluorescent nucleotides in detection of “nicks” or free ends of DNA (12). The assay quantifies the incorporation of dUTP into single-stranded or double-stranded DNA breaks through an enzymatic reaction by using flow cytometry or fluorescence microscopy. The SCSA is a flow cytometry-based assay that evaluates acridine orange staining to DNA breaks in a large number of cells (5). Following a mild acid denaturation of sperm DNA, the nucleic acid-selective cationic fluorescent dye binds to double-stranded DNA producing green fluorescence or to single-stranded DNA producing red fluorescence. The extent of DNA denaturation is determined by measuring the metachromatic shift from green to red fluorescence (13). The SCD, also known as the Halo test (14), is based on the concept that sperm with fragmented DNA do not produce the characteristic halo of dispersed DNA loops that are observed in sperm with non-fragmented DNA following acid denaturation and removal of nuclear proteins. Halos can be observed under microscopes for SDF quantification of a specimen.
Generally, SDF measurement provides a more accurate representation of a male’s fertility status (15-17). Numerous studies illustrated the prognostic value of SDF tests in the assessment of sperm DNA damage and fertility potential of an individual irrespective of the testing method used (18). In fact, various SDF tests including TUNEL, SCSA and SCD have correlation coefficients ranging from 0.3 to 0.7 signifying a moderate correlation (19,20). However, the true nature of sperm DNA damage and exactly what it is that each test measures is unclear. Refinement in methodology is required before its widespread use among andrology laboratories and incorporation into male fertility evaluation. Multiple factors, including sperm preparation and quality control, are important in ensuring accuracy and precision of SDF tests.
Preparation of semen sample
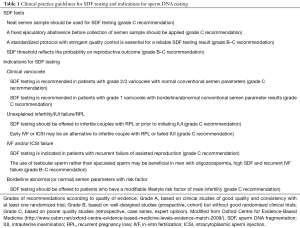
- Neat semen sample should be used for SDF testing (Table 1, grade C recommendation);
- A fixed ejaculatory abstinence before collection of semen sample should be applied (Table 1, grade C recommendation).
Full table
Neat sample is most commonly used for SDF testing as reported in the literature. Indeed, there is concern about the negative implication of sperm preparation procedures for in vitro fertilization (IVF)/ICSI on sperm DNA damage. Density gradient centrifugation (DGC) is a common sperm preparation procedure before ART and has been shown to increase SDF in approximately 50% of patients leading to lower pregnancy rate (21). In contrast to sperm motility which improves after DGC, there is a significant increase in SDF from 15% to 25% in spermatozoa recovered after DGC in infertile patients (22). DGC has been reported to result in increased SDF especially when higher centrifugation force, longer duration and Percoll gradients were used (22,23). On the other hand, several reports provided an opposing view and demonstrated a reduction in the proportion of sperm with damaged DNA ranging from 22% to 47% after DGC (24,25). The implication of sperm processing on SDF remains inconclusive and further study in the area is required.
It is noted that SDF of DGC-processed sperm measured by SCSA did not predict ART outcomes in contrast to neat samples, despite the observation of a reduction in SDF after DGC (26). Also, no association between sperm SDF after swim-up and fertilization, implantation and pregnancy rates could be demonstrated (27). Therefore, the use of processed sample in SDF testing for prediction of ART outcome is not supported by current evidence.
A fixed ejaculatory abstinence before collection of neat semen sample is suggested to be influential of SDF result. Quick repetitive ejaculation with short abstinence has been proposed as a treatment for high SDF (28,29). Short abstinence has resulted in a significantly lower SDF in patients with 1 day of abstinence compared to those with longer abstinence (29). Although the optimal time of ejaculatory abstinence is yet to be defined, a recommended abstinence of 2–3 days before sample collection probably reduce the impact of intra- and inter-individual variation in SDF on the interpretation and comparison of test result.
The susceptibility to stress in sperm from infertile men with high baseline SDF may be explained by the higher content of reactive oxygen species (ROS) in the semen samples compared to normal controls (30). Several strategies have been proposed to reduce the deleterious impact of sperm preparation on SDF including: short incubation time (31), storage at room temperature (32), and addition of antioxidants/cryoprotectants to culture media (33,34).
Cryopreservation of sperm may exert deleterious effect on SDF. However, data from large studies are lacking. While some studies suggested negative impact of freeze/thaw on SDF (35-38), others failed to demonstrate such a relationship (39-41).
Grades of recommendations according to quality of evidence: Grade A, based on clinical studies of good quality and consistency with at least one randomized trial; Grade B, based on well-designed studies (prospective, cohort) but without good randomised clinical trials; Grade C, based on poorer quality studies (retrospective, case series, expert opinion). Modified from Oxford Centre for Evidence-Based Medicine (http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/). SDF, sperm DNA fragmentation; IUI, intrauterine insemination; RPL, recurrent pregnancy loss; IVF, in-vitro fertilization; ICSI, intracytoplasmic sperm injection.
Standardization of SDF tests
- A standardized protocol with stringent quality control is essential for a reliable SDF testing result (Table 1, grade B–C recommendation).
Currently, all SDF tests share the common pitfall that the nature and type of DNA damage are unknown (42). Therefore, the results of these assays are generally not comparable due to the different aspects of SDF measured. Nonetheless, the tests are interrelated to a greater or lesser extent by reflecting the overall quality of the specimen and the properties of the sperm DNA, and may point to a common origin of damage (43). As a result, the reliability of the assays can only be ascertained with standard protocol and quality control in specialized andrology laboratories. Among the SDF assays, SCSA (44), TUNEL (45), SCD (46) and COMET (47) have refined and standardized protocols reported in the literature. A recently published study has reported a high correlation in TUNEL results between two laboratories when the same set of semen samples was independently analyzed under standardized assay conditions by using identical instrument (48). Comparative measurement of semen samples by technicians experienced in SCSA with standardized protocol also revealed highly reliable results between laboratories (49).
The application of flow cytometry allows thousands of cells to be analyzed in a relatively short time which significantly increase the reliability of SDF tests (13,50). Incorporation of flow cytometry in TUNEL and SCSA decrease the intra- and inter-observer variability compared with the use of optical or fluorescence microscopy in other techniques (49,50). Indeed, SDF of a semen sample can only be assessed accurately by studying a large number of cells since each spermatozoon in an ejaculate is unique and variable in its DNA integrity.
Cut-off values
- SDF threshold reflects the probability on reproductive outcome (Table 1, grade B–C recommendation).
The lack of standard cut-off values for SDF testing is a common criticism for routine application of the test in evaluation of infertile men (8,9). However, it is unlikely that a single laboratory test with clear cut-off values can completely assess the complex human reproductive system involving multiple factors from both partners. Multiple cut-off values for various SDF assays in predicting outcomes of natural conception and ART have been proposed and summarized (42). While SCSA DNA fragmentation index (DFI) >30% is consistently associated with negative pregnancy outcome in natural conception and intrauterine insemination (IUI), the reported cut-off values in predicting ART outcome varies (42). Infertility problem may occur when SCSA DFI reaches 20–25% and success of ART decreases as DFI rises (51,52). However, a high DFI does not exclude the possibility of successful conception. Therefore, SDF testing result should be regarded as a statistical value in reflecting the probability of pregnancy. It is rational to adjust the cut-off according to specific clinical scenarios and taking into account other confounding factors. The acceptable threshold level for each infertile couple may vary and probably no absolute cut-off value is available.
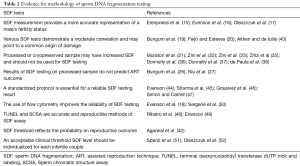
Evidence on sample preparation, test standardization, and cut-off values of SDF tests are summarized in Table 2.
Full table
Indications for SDF testing
Clinical varicocele
- SDF testing is recommended in patients with grade 2/3 varicocele with normal conventional semen parameters (Table 1, grade C recommendation);
- SDF testing is recommended in patients with grade 1 varicocele with borderline/abnormal conventional semen parameter results (Table 1, grade C recommendation).
Varicocele is a clinical condition associated with considerable debate. Varicocele is the most common cause of primary and secondary infertility in men (53,54). While the benefit of varicocelectomy has been proven with 60–80% improvement in semen parameters and 20–60% improvement of natural pregnancy in couples (55), only 20% of adult men with varicocele have difficulties conceiving (56). Selection of patients who will benefit from surgical intervention remains challenging and the use of conventional semen parameters as the laboratory indicator for treatment decision is flawed (57). Efforts were made to search for adjunct laboratory tests that would help identify those who would benefit most after surgery. Interest in SDF testing began after a significantly positive association with varicocele was detected in early reports (58).
The prevalence of SDF in varicocele patients has been reported by many studies. In a literature review including 16 case-control studies, Zini et al. reported that both fertile and infertile men with varicocele tend to have higher SDF than controls, thus suggesting that varicocele is associated with DNA damage even when fertility has not been compromised (59). A significantly higher SDF was observed in patients with varicoceles than controls, with a mean difference of 9.84% (95% CI, −4.09 to −2.65; P<0.001) (60). A multicenter study by Esteves et al. evaluated SDF in various etiologic conditions and found that the highest SDF rate was observed in men with varicocele (35.7±18.3%) (61). Smith et al. examined semen samples from patients with clinical varicocele. Increase in SDF was observed in 49% and 58% in this group of patients who have normal and abnormal semen parameters respectively (62).
The association between varicocele and increased SDF was further validated by examining the effect of varicocelectomy on sperm DNA damage. A reduction of SDF after varicocelectomy was reported in 511 patents in 12 studies in a review by Zini et al. (59). A meta-analysis also showed that varicocelectomy improves sperm DNA integrity, with a mean difference of −3.37% (95% CI, −4.09 to −2.65; P<0.00001) compared to no treatment (60). More recent studies further reported increase in pregnancy rate after varicocelectomy in addition to reduction in SDF. Smit et al. examined 49 patients who had a 1-year history of infertility and underwent varicocelectomy. Significant decrease in SDF from 35.2% to 30.2% (P=0.019) measured by SCSA was observed postoperatively. Natural pregnancy was observed in 37% of patients who had a significantly lower SDF than patients who did not conceive naturally or who conceived with assisted reproduction (63). Similar finding was reported by Ni et al. in their study evaluating 43 infertile men with varicocele and 10 normozoospermic fertile controls. Marked improvement in semen parameters and sperm DNA integrity was noted after varicocele repair. Notably, the SDF result in patients who achieved pregnancy after varicocelectomy (20.6±3.5%) was not significantly different from controls (11.5±3.9%), but was lower than both preoperative values (27.4±6.3%; P<0.01) and the results of non-pregnant patients (24.7±6.5%; P<0.01) (64). The beneficial effect of varicocelectomy on SDF was present in 78–90% of patients with high SDF and clinical varicocele 3 to 6 months after surgery (65,66).
While the association between SDF and clinical grade 2/3 varicocele is supported by evidence from various studies, fewer studies have investigated SDF levels in lower grade varicocele. Only grade 3 varicocele patients had a statistically significant reduction in SDF after surgery as reported by Sadek et al. (67). Similarly, Ni et al. reported reduction in SDF in grades 2 and 3 varicocele after surgical repair (64). There is currently insufficient evidence to highlight the clinical utility of SDF testing in low grade varicocele and additional research is needed to elucidate the significance of SDF in grades 1 and 2 disease. Nonetheless, varicocelectomy could result in desirable outcome in low-grade disease. A recent study evaluated 482 infertile patients with varicocele who underwent surgical ligation. There was a statistically significant improvement in semen parameters after surgery in all three grades of varicocele. More importantly, lower grade varicocele patients had achieved natural pregnancies that were similar to those of grade 3 varicocele patients after surgery (68).
The result of SDF testing may affect management decision of adolescent varicocele by objectively demonstrating testicular dysfunction which may predict possible progression to infertility. Studies have shown that sperm nuclear DNA fragmentation was increased in adolescent with varicocele despite the lack of difference in semen parameters compared to non-varicocele group (69) and the beneficial effect of varicocelectomy in adolescents was also suggested by increased sperm DNA integrity and mitochondrial activity after operation (70).
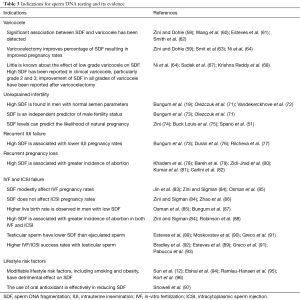
Collectively, the association between SDF and varicocele, and the reversible nature of high SDF in the majority of patients after varicocelectomy support the utility of SDF testing in better selection of varicocelectomy candidates. While further studies are required in ascertaining the role of SDF testing in grade 1 varicocele patients, the correlation between high SDF and grade 2/3 varicocele is more clear (Table 3).
Full table
Unexplained infertility/IUI failure/recurrent pregnancy loss (RPL)
- SDF testing should be offered to infertile couples with RPL or prior to initiating IUI (Table 1, grade C recommendation);
- Early IVF or ICSI may be an alternative to infertile couple with RPL or failed IUI (Table 1, grade C recommendation).
Unexplained infertility is used to define couples with conventional semen parameters within reference values and in whom definitive male and female infertility factors have not been identified (98). It constitutes about 10–30% of couples seeking evaluation of infertility (99,100) and perhaps demonstrates the limitations of conventional SA.
Men with unexplained infertility may have impaired sperm DNA integrity with otherwise normal semen parameters (73). As a matter of fact, 25–40% of infertile men with normal semen parameters present with SDF rates >20–30% (19). Olezczuk et al. compared 119 men with unexplained infertility to 95 men with proven fertility. It was found that SDF index was above 30% in 17.7% of men with unexplained infertility compared to 10.5% of men with proven fertility (P=0.005) (71). A recent prospective study enrolling 25 infertile couples with unexplained infertility demonstrated that 43% and 29% of patients had SDF levels above 20% and 30% measured by SCD test respectively (72).
Despite its relative paucity, good quality evidence on the correlation of SDF with natural pregnancy and IUI outcomes is not lacking (42). A meta-analysis involving 3 studies and 616 couples suggested high SCSA SDF index was associated with failure to achieve natural pregnancy with an odds ratio of 7.01 (95% CI, 3.68–13.36) (74). The prospective LIFE study (75) and the Danish First Pregnancy Planner study (51) provided solid evidence by using time-to-pregnancy to assess fertility. The studies illustrated the correlation between infertility and SCSA SDF index >30% in an unselected population of unknown fertility capability (51). Although the association between high SDF and poor IUI outcomes is not without debate, several studies had demonstrated a strong relationship. A higher probability, with an odds ratio 7.0 to 8.7, of successful pregnancy was observed in couples whose male partner has low SDF (42). Duran et al. evaluated semen sample from 154 IUI cycles. The SDF level was significantly higher among the failed cycles, where no pregnancy was achieved with sperm having a SDF >12% measured by TUNEL (76). Another study by Bungum et al. reported significantly lower biochemical pregnancy (3% vs. 24%), clinical pregnancy (3% vs. 23.7%) and delivery rates (1% vs. 19%) in patients with an SDF index >30% vs. <30%, respectively (73). A recent study concurred with the above findings by showing that SCSA SDF index >27% has negative impact on IUI pregnancy rate (77).
Furthermore, RPL, which is defined by three consecutive pregnancy losses prior to 20-week gestation (101), has been linked to high SDF. Khadem et al. compared 30 couples with RPL to another 30 controls and found that SDF measured by SCD was higher in RPL group (43.3% vs. 16.7%, P=0.024) (78). Using TUNEL, Bareh et al. also demonstrated higher SDF rates in 26 couples with RPL compared to controls (36.8±5.0% vs. 9.4±2.7%; P<0.001) (79). The finding was corroborated by Zidi-Jrad et al. who reported higher SDF rates by TUNEL in 22 couples with RPL compared to fertile controls (17.1% vs. 10.2%; P=0.01) (80). Kumar et al. evaluated 45 couples with RPL and found that SCSA SDF index was 1.2-fold higher than controls (28.1±4.9% vs. 21.7±4.7%; P<0.05). A ROC curve analysis demonstrated that RPL couples and controls are discriminated at SDF index of 26% with a 73% sensitivity, 90% specificity, and accuracy of 83% (81). The association of high SDF and RPL is further supported by Carlini et al. Despite normal semen parameters, TUNEL SDF was higher in the RPL group (18.8±7.0%) than fertile controls (12.8±5.3%), and similar to those of infertile couples (20.8±8.9%). A significant positive relationship between the number of RPL events and elevated SDF was also reported (r =0.20; P<0.05) (82).
In summary, SDF testing should be offered to couples with unexplained infertility, IUI failure, and idiopathic RPL. A high SDF index would provide a possible explanation for the adverse reproductive outcome, though the exact cut-off values for prediction of IUI success and RPL are still debated. Possible interventions to reduce SDF should be implemented. The application of IVF or ICSI sooner rather than later may be indicated in couples with RPL or prior to initiating IUI (Table 3).
IVF and/or ICSI failure
- SDF testing is indicated in patients with recurrent failure of assisted reproduction (Table 1, grade C recommendation);
- The use of testicular sperm rather than ejaculated sperm may be beneficial in men with oligozoospermia, high SDF and recurrent IVF failure (Table 1, grade B–C recommendation).
The relationship between SDF and IVF/ICSI outcome has been extensively investigated. Controversies persist in view of the involvement of multiple confounding factors and heterogenous nature of the studies (42). Female factor is particularly influential in this area as demonstrated by Jin et al. The study reported that SDF significantly affect outcomes of ART only in patients with reduced ovarian reserve (83).
Systematic reviews have reported a significant relationship between sperm DNA damage and pregnancy rates with IVF (84,85). Zini et al evaluated 9 IVF studies and reported lower pregnancy rates in patients with a high SDF with a combined odds ratio of 1.57 (95% CI, 1.18–2.07; P<0.05) (84). In contrary, existing evidence suggests SDF has a negligible effect on fertilization rate during ICSI cycles. The systematic review by Zini et al. failed to find a significant association between SDF and ICSI pregnancy rates with a combined odds ratio of 1.14 (95% CI, 0.86–1.54) (84). Zhao et al., in their meta-analysis of 2,756 couples, revealed a lower pregnancy rate in the context of high SDF was noted only in patients undergoing IVF but not ICSI (86). The difference may be explained by the technical differences between the two procedures. Both gametes in IVF are subjected to prolonged culture and the resultant oxidative stress may impact the outcome (102). Conversely, ICSI sperm is injected directly to oocyte soon after ejaculation. The potentially better quality oocyte with less in vitro damage may be more capable to repair the damage in the sperm which are also subjected to less oxidative stress during ICSI (103).
The association between SDF and live birth rates in patients undergoing IVF and ICSI has been reported recently. A meta-analysis including 6 studies and 998 couples summarized that men with low SDF had a higher live birth rate than those with high SDF (RR 1.17, 95% CI, 1.07–1.28; P=0.0005) (85). Another study showed a significantly higher live birth rate in men with low SDF with risk ratio 1.27 (95% CI, 1.05–1.52; P=0.01) and 1.11 (95% CI, 1.00–1.23; P=0.04) for IVF and ICSI respectively (87).
Several studies have reported a relationship between SDF and pregnancy loss after IVF and ICSI (42). In one study evaluating 5 IVF and 6 ICSI studies and 1,549 treatment cycles, the combined OR of 2.48 (95% CI, 1.52–4.04; P<0.0001) indicates that high SDF is predictive of pregnancy loss after ART (79). In another study pooling 16 papers and 2,969 couples, the risk of early pregnancy loss was increased by 2.16-fold when semen specimens with high SDF were used for IVF or ICSI (95% CI, 1.54–3.03; P<0.001) (88).
Several strategies are proposed to minimize the impact of abnormal SDF on ART outcomes. The intake of oral antioxidants, varicocele repair, frequent ejaculation, sperm selection techniques (such as magnetic cell sorting, physiological ICSI or intracytoplasmic morphologically selected sperm injection), and the use of testicular sperm for ICSI have been attempted with varying success. However, the routine application of SDF testing before ART is debated partly due to the uncertain effect of the treatment strategies on ART outcomes (104). Nonetheless, evidence supporting the effectiveness of intervention in patients with high SDF is evolving. Bradley et al. found that live birth rates were higher with testicular sperm extraction/aspiration (49.8%), intracytoplasmic morphologically selected sperm (28.7%) and physiological ICSI (38.5%) compared with no intervention (24.2%) (92).
The use of sperm harvested from testis instead of ejaculated sperm in ICSI is seemingly a plausible maneuver based on the belief that most sperm DNA damage occurs during the epididymal transit (105,106). The hypothesis is supported by the finding of significantly higher SDF levels in ejaculated compared with testicular sperm (89-91). The use of testicular sperm is further solicited by Esteves et al. who demonstrated a higher clinical pregnancy rate (51.9% vs. 40.2%), a lower miscarriage rate (10.0% vs. 34.3%) and higher birth rate (46.7% vs. 26.4%) in association with significantly lower SDF in testicular sperm compared to ejaculated sperm (8.3% vs. 40.7%) in men with oligozoospermia (89). In another study of similar design but involving normozoospermic men with high SDF, the fertilization and miscarriage rates did not differ; however, a significantly higher pregnancy rate was reported in testicular sperm cycles (93).
While further research in this area is warranted, SDF testing in patients with ART failure is indicated as it can provide prognostic information on subsequent ART cycles (Table 3). The existing evidence revealed that the use of testicular sperm rather than ejaculated sperm in men with oligozoospermia, high SDF and recurrent IVF failure may be potentially beneficial (Table 1). A high SDF result in patients with IVF/ICSI failure may prompt early application of ICSI with or without the use of testicular sperm in improving ART outcomes.
Borderline abnormal (or normal) semen parameters with risk factors
- SDF testing should be offered to patients who have a modifiable lifestyle risk factor of male infertility (Table 1, grade C recommendation).
An imbalance between ROS and antioxidants triggers a state of oxidative stress which may damage sperm DNA. Indeed, oxidative stress-induced SDF is the underlying pathology associated with a number of lifestyle factors in male infertility (42).
Smoking has a detrimental effect on conventional semen parameters (107), sperm fertilizing capacity (108), and risk of infertility (109). It is shown that SDF in smokers is consistently higher than that in non-smokers (12). A study by Elshal et al. categorized patients with idiopathic infertility into fertile non-smokers, infertile non-smokers, and infertile smokers. SDF was significantly higher in infertile smokers than infertile non-smokers. In addition, significant negative associations were reported between the degree of SDF and worsening of semen parameters (94).
Obesity has been linked to subfecundity and a dose-response relationship between increasing body mass index (BMI) and subfecundity has been reported (95). Kort et al. evaluated 520 male partners of infertile couples and found a positive correlation between BMI and SDF, with the mean SDF rising from 19.9% in men with a normal BMI to 27.0% in obese men (96). Few emerging studies revealed that weight loss in severely obese men leads to improved semen parameters and reproductive hormonal profile; however, no change in SDF was observed (110,111).
While the negative implication of smoking and obesity has been consistently reported by several studies, further research is needed to confirm the role of lifestyle modifications in improving sperm DNA integrity and how these changes possibly translate into better reproductive outcomes. On the other hand, a recent Cochrane review suggested the possible effect of antioxidant therapy in increasing live birth rate. Two trials including 100 patients found that oral antioxidant significantly reduces SDF compared to placebo (mean difference −13.8%; 95% CI, 10.4–17.7%; P<0.00001) (97).
In summary, SDF should be offered during evaluation of infertile men who have a potentially modifiable lifestyle factor (Table 1). The clear association between SDF and the lifestyle factors makes SDF testing an ideal tool in identification of at risk patients and monitoring the response to intervention. SDF testing can also help in reinforcement of lifestyle modification (Table 3).
Conclusions
Sperm DNA integrity is essential in the success of human reproduction. There is fair evidence indicating that SDF testing is useful in evaluation of infertile men. While it has been extensively researched over the past two decades, further studies help us in identifying the role of SDF testing in clinical practice. SDF testing should be included in the evaluation of male infertility in selected patients along with SA.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod 1991;6:811-6. [Crossref] [PubMed]
- Schlegel PN, Girardi SK. Clinical review 87: in vitro fertilization for male factor infertility. J Clin Endocrinol Metab 1997;82:709-16. [Crossref] [PubMed]
- Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol 2014;40:443-53. [Crossref] [PubMed]
- Neri QV, Tanaka N, Wang A, et al. Intracytoplasmic sperm injection. Accomplishments and qualms. Minerva Ginecologica 2004;56:189-96. [PubMed]
- Lewis SE, Aitken JR, Conner SJ, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online 2013;27:325-37. [Crossref] [PubMed]
- Erenpreiss J, Spano M, Erenpreisa J, et al. Sperm chromatin structure and male infertility: biological and clinical aspects. Asian J Androl 2006;8:11-29. [Crossref] [PubMed]
- Evenson DP, Jost LK, Marshall D, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999;14:1039-49. [Crossref] [PubMed]
- Jarrow J, Sigman M, Kolettis PN, et al. The optimal evaluation of the infertile male: best practice statement reviewed and validity confirmed 2011. Available online: https://www.auanet.org/education/guidelines/male-infertility-d.cfm
- Jungwirth A, Diemer T, Dohle GR, et al. Guidelines on male infertility. Available online: https://uroweb.org/guideline/male-infertility/
- Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. [Crossref] [PubMed]
- Majzoub A, Agarwal A, Cho CL, et al. Sperm DNA fragmentation testing: a cross sectional survey on current practices of fertility specialists. Transl Androl Urol 2017;6:S710-9.
- Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod 1997;56:602-7. [Crossref] [PubMed]
- Darzynkiewicz Z, Traganos F, Sharpless T, et al. Thermal denaturation of DNA in situ as studied by acridine orange staining and automated cytofluorometry. Exp Cell Res 1975;90:411-28. [Crossref] [PubMed]
- Fernández JL, Muriel L, Rivero MT, et al. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl 2003;24:59-66. [PubMed]
- Erenpreiss J, Bungum M, Spano M, et al. Intra-individual variation in sperm chromatin structure assay parameters in men from infertile couples: clinical implications. Hum Reprod 2006;21:2061-4. [Crossref] [PubMed]
- Evenson DP, Jost LK, Baer RK, et al. Individuality of DNA denaturation patterns in human sperm as measured by the sperm chromatin structure assay. Reprod Toxicol 1991;5:115-25. [Crossref] [PubMed]
- Oleszczuk K, Giwercman A, Bungum M. Intra-individual variation of the sperm chromatin structure assay DNA fragmentation index in men from infertile couples. Hum Reprod 2011;26:3244-8. [Crossref] [PubMed]
- Gosalvez J, Lopez-Fernandez C, Fernandez JL, et al. Unpacking the mysteries of sperm DNA fragmentation. Ten frequently asked questions. J Reprod Biotechnol Fertil 2015;4:1-16. [Crossref]
- Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl 2011;13:69-75. [Crossref] [PubMed]
- Feijó CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril 2014;101:58-63.e3. [Crossref] [PubMed]
- Muratori M, Tarozzi N, Cambi M, et al. Variation of DNA fragmentation levels during density gradient sperm selection for assisted reproduction techniques: a possible new male predictive parameter of pregnancy? Medicine (Baltimore) 2016;95:e3624. [Crossref] [PubMed]
- Zini A, Nam RK, Mak V, et al. Influence of initial semen quality on the integrity of human sperm DNA following semen processing. Fertil Steril 2000;74:824-7. [Crossref] [PubMed]
- Zini A, Finelli A, Phang D, et al. Influence of semen processing technique on human sperm DNA integrity. Urology 2000;56:1081-4. [Crossref] [PubMed]
- Gosálvez J, de la Torre J, Lopez-Fernandez C, et al. DNA fragmentation dynamics in fresh versus frozen thawed plus gradient-isolated human spermatozoa. Syst Biol Reprod Med 2010;56:27-36. [Crossref] [PubMed]
- Xue X, Wang WS, Shi JZ, et al. Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet 2014;31:1161-6. [Crossref] [PubMed]
- Bungum M, Spano M, Humaidan P, et al. Sperm chromatin structure assay parameters measured after density gradient centrifugation are not predictive for the outcome of ART. Hum Reprod 2008;23:4-10. [Crossref] [PubMed]
- Niu ZH, Shi HJ, Zhang HQ, et al. Sperm chromatin structure assay results after swim-up are related only to embryo quality but not to fertilization and pregnancy rates following IVF. Asian J Androl 2011;13:862-6. [Crossref] [PubMed]
- Gosálvez J, Gonzalez-Martinez M, Lopez-Fernandez C, et al. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil Steril 2011;96:1083-6. [Crossref] [PubMed]
- Agarwal A, Gupta S, Du Plessis S, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology 2016;94:102-10. [Crossref] [PubMed]
- Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl 1988;9:367-76. [Crossref] [PubMed]
- Nabi A, Khalili MA, Halvaei I, et al. Prolonged incubation of processed human spermatozoa will increase DNA fragmentation. Andrologia 2014;46:374-9. [Crossref] [PubMed]
- Matsuura R, Takeuchi T, Yoshida A. Preparation and incubation conditions affect the DNA integrity of ejaculated human spermatozoa. Asian J Androl 2010;12:753-9. [Crossref] [PubMed]
- Agarwal A, Durairajanayagam D, du Plessis SS. Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrinol 2014;12:112. [Crossref] [PubMed]
- Li ZL, Lin QL, Liu RJ, et al. Reducing oxidative DNA damage by adding antioxidants in human semen samples undergoing cryopreservation procedure. Zhonghua Yi Xue Za Zhi 2007;87:3174-7. [PubMed]
- Zribi N, Feki Chakroun N, El Euch H, et al. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril 2010;93:159-66. [Crossref] [PubMed]
- Donnelly ET, Steele EK, McClure N, Lewis SE. Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Hum Reprod 2001;16:1191-9. [Crossref] [PubMed]
- Donnelly ET, McClure N, Lewis SEM, et al. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril 2001;76:892-900. [Crossref] [PubMed]
- de Paula TS, Bertolla RP, Spaine DM, et al. Effect of cryopreservation on sperm apoptotic deoxyribonucleic acid fragmentation in patients with oligozoospermia. Fertil Steril 2006;86:597-600. [Crossref] [PubMed]
- Duru NK, Morshedi M, Oehninger S. Cryopreservation-thawing of fractionated human spermatozoa is associated with membrane phosphatidylserine externalisation and not DNA fragmentation. J Androl 2001;22:646-51. [PubMed]
- Isachenko V, Isachenko E, Katkov II, et al. Cryoprotectant-free cryopreservation of human spermatozoa by vitrification and freezing in vapour: effect on motility, DNA integrity, and fertilization ability. Biol Reprod 2004;71:1167-73. [Crossref] [PubMed]
- Isachenko E, Isachenko V, Katkov II, et al. DNA integrity and motility of human spermatozoa after standard slow freezing versus cryoprotectant-free vitrification. Hum Reprod 2004;19:932-9. [Crossref] [PubMed]
- Agarwal A, Cho CL, Esteves SC. Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol 2016;28:164-71. [Crossref] [PubMed]
- Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod 2010;16:3-13. [Crossref] [PubMed]
- Evenson D. Sperm Chromatin Structure Assay (SCSA): detailed protocol. In: Zini A, Agarwal A. editors. Sperm chromatin: biological and clinical applications in male infertility and assisted reproduction. Springer Sciences, 2011:487-97.
- Sharma R, Ahmad G, Esteves SC, et al. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values, and quality control. J Assist Reprod Genet 2016;33:291-300. [Crossref] [PubMed]
- Gosalvez J, Lopez-Fernandez C, Fernandez JL. Sperm chromatin dispersion test: technical aspects and clinical applications. In: Zini A, Agarwal A. editors. Sperm chromatin: biological and clinical applications in male infertility and assisted reproduction. Springer Sciences, 2011:151-70.
- Simon L, Carrell DT. Sperm DNA damage measured by comet assay. Methods Mol Biol 2013;927:137-46. [Crossref] [PubMed]
- Ribeiro S, Sharma R, Gupta S, et al. Inter- and intra-laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Andrology 2017;5:477-85. [Crossref] [PubMed]
- Evenson DP. The Sperm Chromatin Structure Assay (SCSA) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci 2016;169:56-75. [Crossref] [PubMed]
- Sergerie M, Laforest G, Boulanger K, et al. Longitudinal study of sperm DNA fragmentation as measured by terminal uridine nick end-labelling assay. Hum Reprod 2005;20:1921-7. [Crossref] [PubMed]
- Spanò M, Bonde JP, Hjollund HI, et al. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril 2000;73:43-50. [PubMed]
- Oleszczuk K, Giwercman A, Bungum B. Sperm chromatin structure assay in prediction of in vitro fertilization outcome. Andrology 2016;4:290-6. [Crossref] [PubMed]
- Dubin L, Amelar RD. Etiologic factors in 1294 consecutive cases of male infertility. Fertil Steril 1971;22:469-74. [Crossref] [PubMed]
- Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril 1993;59:613-6. [Crossref] [PubMed]
- Schlesinger MH, Wilets IF, Nagler HM. Treatment outcome after varicocelectomy. A critical analysis. Urol Clin North Am 1994;21:517-29. [PubMed]
- Diamond DA, Gargollo PC, Caldmone AA. Current management principles for adolescent varicocele. Fertil Steril 2011;96:1294-8. [Crossref] [PubMed]
- Agarwal A, Sharma R, Harlev A, et al. Effect of varicocele on semen characteristics according to the new 2010 World Health Organization criteria: a systematic review and meta-analysis. Asian J Androl 2016;18:163-70. [Crossref] [PubMed]
- Saleh RA, Agarwal A, Sharma RK, et al. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril 2003;80:1431-6. [Crossref] [PubMed]
- Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril 2011;96:1283-7. [Crossref] [PubMed]
- Wang YJ, Zhang RQ, Lin YJ, et al. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online 2012;25:307-14. [Crossref] [PubMed]
- Esteves SC, Gosalvez J, Lopez-Fernandez C, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol 2015;47:1471-7. [Crossref] [PubMed]
- Smith R, Kaune H, Parodi D, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod 2006;21:986-93. [Crossref] [PubMed]
- Smit M, Romijn JC, Wildhagen MF, et al. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol 2013;189:S146-50. [Crossref] [PubMed]
- Ni K, Steger K, Yang H, et al. Sperm protamine mRNA ratio and DNA fragmentation index represent reliable clinical biomarkers for men with varicocele after microsurgical varicocele ligation. J Urol 2014;192:170-6. [Crossref] [PubMed]
- Werthman P, Wixon R, Kasperson K, et al. Significant decrease in sperm deoxyribonucleic acid fragmentation after varicocelectomy. Fertil Steril 2008;90:1800-4. [Crossref] [PubMed]
- Moskovtsev SI, Lecker I, Mullen JB, et al. Cause-specific treatment in patients with high sperm DNA damage result in significant DNA improvement. Syst Biol Reprod Med 2009;55:109-15. [Crossref] [PubMed]
- Sadek A, Almohamdy AS, Zaki A, et al. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil Steril 2011;95:1705-8. [Crossref] [PubMed]
- Krishna Reddy SV, Shaik AB, Sailaja S, et al. Outcome of varicocelectomy with different degrees of clinical varicocele in infertile men. Advances in Andrology 2015;9.
- Bertolla RP, Cadenha AP, Hassun Filho PA, et al. Sperm nuclear DNA fragmentation in adolescents with varicocele. Fertil Steril 2006;85:625-8. [Crossref] [PubMed]
- Lacerda JI, Del Giudice PT, da Silva BF, et al. Adolescent varicocele: improved semen function after varicocelectomy. Fertil Steril 2011;95:994-9. [Crossref] [PubMed]
- Oleszczuk K, Augustinsson L, Bayat N, et al. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013;1:357-60. [Crossref] [PubMed]
- Vandekerckhove FW, De Croo I, Gerris J, et al. Sperm chromatin dispersion test before sperm preparation is predictive of clinical pregnancy in cases of unexplained infertility treated with intrauterine insemination and induction with clomiphene citrate. Front Med (Lausanne) 2016;3:63. [Crossref] [PubMed]
- Bungum M, Humaidan P, Axmon A, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 2007;22:174-9. [Crossref] [PubMed]
- Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med 2011;57:78-85. [Crossref] [PubMed]
- Buck Louis GM, Sundaram R, Schisterman EF, et al. Semen quality and time to pregnancy: the Longitudinal Investigation of Fertility and the Environment Study. Fertil Steril 2014;101:453-62. [Crossref] [PubMed]
- Duran EH, Morshedi M, Taylor S, et al. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 2002;17:3122-8. [Crossref] [PubMed]
- Rilcheva VS, Ayvazova NP, Ilieva LO, et al. Sperm DNA integrity test and assisted reproductive technology (Art) outcome. J Biomed Clin Res 2016;9:21-9. [Crossref]
- Khadem N, Poorhoseyni A, Jalali M, et al. Sperm DNA fragmentation in couples with unexplained recurrent spontaneous abortions. Andrologia 2014;46:126-30. [Crossref] [PubMed]
- Bareh GM, Jacoby E, Binkley P, et al. Sperm deoxyribonucleic acid fragmentation assessment in normozoospermic male partners of couples with unexplained recurrent pregnancy loss: a prospective study. Fertil Steril 2016;105:329-36.e1. [Crossref] [PubMed]
- Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril 2016;105:58-64. [Crossref] [PubMed]
- Kumar K, Deka D, Singh A, et al. Predictive value of DNA integrity analysis in idiopathic recurrent pregnancy loss following spontaneous conception. J Assist Reprod Genet 2012;29:861-7. [Crossref] [PubMed]
- Carlini T, Paoli D, Pelloni M, et al. Sperm DNA fragmentation in Italian couples with recurrent pregnancy loss. Reprod Biomed Online 2017;34:58-65. [Crossref] [PubMed]
- Jin J, Pan C, Fei Q, et al. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril 2015;103:910-6. [Crossref] [PubMed]
- Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl 2009;30:219-29. [Crossref] [PubMed]
- Osman A, Alsomait H, Seshadri S, et al. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online 2015;30:120-7. [Crossref] [PubMed]
- Zhao J, Zhang Q, Wang Y, et al. Whether sperm deoxyribonucleic acid fragmentation has effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril 2014;102:998-1005.e8. [Crossref] [PubMed]
- Bungum M, Humaidan P, Spano M, et al. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod 2004;19:1401-8. [Crossref] [PubMed]
- Robinson L, Gallos ID, Conner SJ, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908-17. [Crossref] [PubMed]
- Esteves SC, Sanchez-Martin F, Sanchez-Martin P, et al. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. [Crossref] [PubMed]
- Moskovtsev SI, Jarvi K, Mullen JB, et al. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril 2010;93:1142-6. [Crossref] [PubMed]
- Greco E, Scarselli F, Iacobelli M, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod 2005;20:226-30. [Crossref] [PubMed]
- Bradley CK, McArthur SJ, Gee AJ, et al. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology 2016;4:903-10. [Crossref] [PubMed]
- Pabuccu EG, Caglar GS, Tangal S, et al. Testicular versus ejaculated spermatozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous ART failures. Andrologica 2016. [Epub ahead of print].
- Elshal MF, El-Sayed IH, Elsaied MA, et al. Sperm head defects and disturbances in spermatozoal chromatin and DNA integrities in idiopathic infertile subjects: association with cigarette smoking. Clin Biochem 2009;42:589-94. [Crossref] [PubMed]
- Ramlau-Hansen CH, Thulstrup AM, Nohr EA, et al. Subfecundity in overweight and obese couples. Hum Reprod 2007;22:1634-7. [Crossref] [PubMed]
- Kort HI, Massey JB, Elsner CW, et al. Impact of body mass index values on sperm quality and quantity. J Androl 2006;27:450-2. [Crossref] [PubMed]
- Showell MG, Mackenzie-Proctor R, Brown J, et al. Antioxidants for male subfertility. Cochrane Database Syst Rev 2014.CD007411. [PubMed]
- Esteves SC, Schattman GL, Agarwal A. Definitions and relevance of unexplained infertility in reproductive medicine. In: Schattman G, Esteves SC, Agarwal A. editors. Unexplained infertility: pathophysiology, evaluation and treatment. 1st Edition. New York: Springer, 2015:3-5.
- Isaksson R, Tiitinen A. Present concept of unexplained infertility. Gynecol Endocrinol 2004;18:278-90. [Crossref] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Effectiveness and treatment for unexplained infertility. Fertil Steril 2006;86:S111-4. [Crossref] [PubMed]
- Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol 2009;2:76-83. [PubMed]
- Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int 2005;95:503-7. [Crossref] [PubMed]
- Lewis SE. The place of sperm DNA fragmentation testing in current day fertility management. Middle East Fertil Soc J 2013;18:78-82. [Crossref]
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2015;103:e18-25. [Crossref] [PubMed]
- Alvarez JG. DNA fragmentation in human spermatozoa: significance in the diagnosis and treatment of infertility. Minerva Ginecol 2003;55:233-9. [PubMed]
- Ollero M, Gil-Guzman E, Lopez MC, et al. Characterization of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis and treatment of male infertility. Hum Reprod 2001;16:1912-21. [Crossref] [PubMed]
- Esteves SC, Agarwal A, Sharma R, et al. Reply to Eugenio Ventimiglia, Montorsi Francesco, and Andrea Salonia’s letter to the editor Re: Reecha Sharma, Avi Harlev, Ashok Agarwal, Sandro C. Esteves. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur Urol 2017;71:e21-e22. [PubMed]
- Soares SR, Melo MA. Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol 2008;20:281-91. [Crossref] [PubMed]
- Yang F, Li L, Chen JP, et al. Couple’s infertility in relation to male smoking in a Chinese rural area. Asian J Androl 2017;19:311-5. [Crossref] [PubMed]
- Håkonsen LB, Thulstrup AM, Aggerholm AS, et al. Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reprod Health 2011;8:24. [Crossref] [PubMed]
- El Bardisi H, Majzoub A, Arafa M, et al. Effect of bariatric surgery on semen parameters and sex hormone concentrations: a prospective study. Reprod Biomed Online 2016;33:606-11. [Crossref] [PubMed]


